Abstract
The environmental obesogen hypothesis proposes that exposure to endocrine disruptors during developmental ‘window’ contributes to adipogenesis and the development of obesity. MEHP [mono-(2-ethylhexyl) phthalate], a metabolite of the widespread plasticizer DEHP [di-(2-ethylhexyl) phthalate], has been found in exposed organisms and identified as a selective PPARγ (peroxisome-proliferator-activated receptor γ) modulator. However, implication of MEHP on adipose tissue development has been poorly investigated. In the present study, we show the dose-dependent effects of MEHP on adipocyte differentiation and GPDH (glycerol-3-phosphate dehydrogenase) activity in the murine 3T3-L1 cell model. MEHP induced the expression of PPARγ as well as its target genes required for adipogenesis in vitro. Moreover, MEHP perturbed key regulators of adipogenesis and lipogenic pathway in vivo. In utero exposure to a low dose of MEHP significantly increased b.w. (body weight) and fat pad weight in male offspring at PND (postnatal day) 60. In addition, serum cholesterol, TAG (triacylglycerol) and glucose levels were also significantly elevated. These results suggest that perinatal exposure to MEHP may be expected to increase the incidence of obesity in a sex-dependent manner and can act as a potential chemical stressor for obesity and obesity-related disorders.
Keywords: adipogenesis, mono(2-ethylhexyl) phthalate (MEHP), obesity, perinatal exposure, peroxisome proliferator-activated receptor γ
Abbreviations: aP2, adipocyte-specific fatty acid binding protein; BPA, bisphenol A; b.w., body weight; C/EBPα, CCAAT/enhancer-binding protein α; DEHP, di-(2-ethylhexyl) phthalate; DMEM, Dulbecco’s modified Eagle’s medium; EDC, endocrine disrupting chemical; FAS, fatty acid synthase; GPDH, glycerol-3-phosphate dehydrogenase; LPL, lipoprotein lipase; MEHP, mono(2-ethylhexyl)phthalate; NRIFP, National Research Institute for Family Planning; PND, postnatal day; PPAR, peroxisome-proliferator-activated receptor; PVC, poly(vinyl chloride); Srebf1, sterol-regulatory-element-binding factor 1; TAG, triacylglycerol
INTRODUCTION
The prevalence of obesity is increasing throughout the world. This has resulted in a significant increase in morbidity and mortality associated with metabolic syndrome [1–3]. The number of children and adolescents who are overweight, or at risk for being overweight, has risen in parallel with that reported in adults [4]. Although the imbalance between caloric intake and expenditure is the central mechanism of obesity, the reason for its surge might not be solely related to excessive food intake and/or decreased physical activity [5]. Recently, there has been increased exposure to EDCs (endocrine disrupting chemicals) in daily life and these chemicals have incidentally been taken by humans. Epidemiological studies indicate that the serum/urine concentrations of some of these chemicals have been found to be associated with the onset and incidence rate of obesity and diabetes [6–15]. This has led to the hypothesis that some of the numerous environmental pollutants that interfere with various aspects of metabolism might add one more risk factor for obesity and could be considered as ‘environmental obesogens’ [5]. The ‘obesogens’ inappropriately regulate lipid metabolism and adipogenesis, and further promote the development of obesity [16].
Phthalates are widespread environmental micro-pollutants used in a variety of products, including cosmetics, shampoos, soaps, lubricants, pesticides and paints; they are also used as a softener of PVC [poly(vinyl chloride)]. Human exposure to phthalates mainly occurs through food, because of the use of PVC in wrapping materials and food processing [17]. Once ingested through food contamination, DEHP [di-(2-ethylhexyl) phthalate], one of the most widely used and studied phthalates, are quickly metabolized to its monoester equivalent MEHP [mono-(2-ethylhexyl)phthalate], which is then preferentially absorbed [18–20]. The biological effects of MEHP are hence of major concern but so far elusive. In the past, research on endocrine disruption caused by MEHP has mainly focused on reproductive defects [21–23]. Previous studies have shown that MEHP is a selective PPARγ (peroxisome-proliferator-activated receptor γ) modulator [24]. PPARγ belongs to the nuclear receptor superfamily of ligand-activated transcription factors, and PPARγ induced during adipocyte differentiation is both necessary and sufficient [25]. However, it is unclear whether low doses of MEHP exposure will disrupt adipose tissue homoeostasis and promote development of obesity and obesity-associated disease.
In the present study, we investigated the dose-dependent effects of MEHP on adipocyte differentiation using the murine 3T3-L1 cell model. During differentiation, MEHP induced the expression of PPARγ as well as its target genes required for adipogenesis. Consistent with in vitro results, we observed that MEHP activated PPARγ and its target genes in vivo. To determine whether perinatal exposure to MEHP could have an impact on filial adipogenesis, we defined adipose tissue deposition, serum lipids and glucose levels in offspring at PND (postnatal day) 60. Our results suggested that perinatal exposure to MEHP might be expected to increase the incidence of obesity in male offspring and could act as a potential chemical stressor for obesity and obesity-related disorders.
MATERIALS AND METHODS
Cell culture and treatment
3T3-L1 mouse embryo preadipocytes, purchased from Institute of Biochemistry and Cell Biology, SIBS, CAS were maintained in standard DMEM (Dulbecco's modified Eagle's medium; Hyclone) supplemented with 10% BCS (bovine calf serum; Invitrogen) and 1% penicillin/streptomycin. Cells were maintained as subconfluent cultures at 37°C in a humidified 5% CO2 atmosphere with media changes every 2–3 days. For differentiation assays, cells were seeded at 6×104 cells per well into polylysine-coated six-well cell culture plates in 10% FBS (fetal bovine serum; Invitrogen)/DMEM, after which cultures were grown for 2 days and then treated with different concentrations of MEHP (Xiyu) or troglitazone (Sigma–Aldrich) with 10 μg/ml insulin (Sigma–Aldrich) for 8 days. An 8-day treatment with 10 μg/ml insulin only served as a control. Media and drug treatments were renewed every 2 days. After 8 days, cells were stained with Oil Red O for lipid droplet accumulation as described below.
Oil Red O staining
Cells were washed with sterile PBS, fixed with 10% formaldehyde for 15 min at room temperature (25°C) [26], washed with distilled water, and then stained with filtered Oil Red O solution (4 g/l, 60% propan-2-ol) for 15 min. Excess stain was removed by washing three times with distilled water.
GPDH (glycerol-3-phosphate dehydrogenase) activity
Cells were rinsed with ice-cold PBS, scraped into 0.2 ml extraction buffer (GPDH assay kit; TaKaRa Bio Inc.), and centrifuged for 10 min at 4°C. GPDH activity was assayed in the supernatant by monitoring the decrease in absorbance at 340 nm of NADH in the presence of dihydroxyacetone phosphate [27].
Quantitative real-time PCR analysis
Total RNA from 3T3-L1 cells or C57BL/6J mouse tissues (liver and epididymal adipose tissue) was isolated using RNeasy mini kit (Qiagen) and reversed-transcribed using iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer's instructions. Triplicate cDNA samples (15 ng per reaction) were analysed by quantitative real-time PCR on an ABI prism 7000 thermal cycler (Applied Biosystems) using FastStart universal SYBR Green master (Roche). Fold changes in expression levels were calculated after normalization to 18s rRNA or β-actin. Gene-specific primers are shown in Table 1.
Table 1. Primer sequences for real-time PCR.
| Gene name | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| 18s rRNA | CTCTGTTCCGCCTAGTCCTG | AATGAGCCATTCGCAGTTTC |
| PPARγ | TGGGTGAAACTCTGGGAGATTC | AATTTCTTGTGAAGTGCTCATAGGC |
| PPARα | TTTCCCTGTTTGTGGCTGCTA | CCCTCCTGCAACTTCTCAATG |
| C/EBPα | CCAAGAAGTCGGTGGACAAGA | CGGTCATTGTCACTGGTCAACT |
| aP2 | GAATTCGATGAAATCACCGCA | CTCTTTATTGTGGTCGACTTTCCA |
| FAS | TCGGGTGTGGTGGGTTTGGTGAAT | ACTTGGGGGCGTGAGATGTGTTGC |
| Srebf1 | GCCCCTGCCCACCTCAAACCT | ACTGGCACGGGCATCCTTCCTC |
| LPL | GTGTTGCTTGCCATTCTC | TCTCCTGATGACGCTGAT |
| β-Actin | CAGAAGGAGATTACTGCTCTGGCT | GGAGCCACCGATCCACACA |
Animal care and MEHP exposure
C57BL/6J mice were bred in the animal facility of NRIFP (National Research Institute for Family Planning) (mouse protocol SYXK 2009-0033) and housed in a room with a 12 h light/12 h dark cycle (lights on at 7:30 h and off at 19:30 h) with access to food and water ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of NRIFP. Six-week-old male mice received an intraperitoneal injection of MEHP (0.5 mg/kg of b.w.), troglitazone (0.5 mg/kg of b.w.) or vehicle (olive oil) for 24 h. Animals were killed by decapitation. Liver and epididymal adipose tissue were collected for total RNA extraction as described above. Pregnant mice were dosed by gavage with MEHP (0.5, 0.25 or 0.05 mg/kg of b.w.) or vehicle (olive oil) from embryonic day 12 (E12) every 24 h until the 7th day of lactation (PND 7 of offspring). Pups were weaned at 3 weeks of age (PND 21) and maintained on standard rodent chow.
Measurement of obesity parameters
After 8-week-old pups were weighed, animals were killed by asphyxia. Liver, epididymal/parametrial and perirenal fat pads were harvested and weighed. Blood was collected by cardiac puncture after an overnight fasting. Blood glucose, serum cholesterol and TAGs (triacylglycerols) were measured by colorimetric kit assays (Leadman) and analysed using a fully automatic biochemistry analyser (Hitachi).
Data analysis
For GPDH activity assay and quantitative real-time PCR assay, data were analysed using one-way ANOVA, followed by a Tukey's test. For studies examining obesity parameters in mice, data were analysed statistically using ANOVA, and subsequent comparisons were performed using the Tukey–Kramer test, which allows for unequal sample sizes. All statistical procedures were carried out using SPSS software (version 16.0). Results are represented as means±S.E.M.; a value of P<0.05 was considered statistically significant.
RESULTS
MEHP promotes 3T3-L1 preadipocyte differentiation
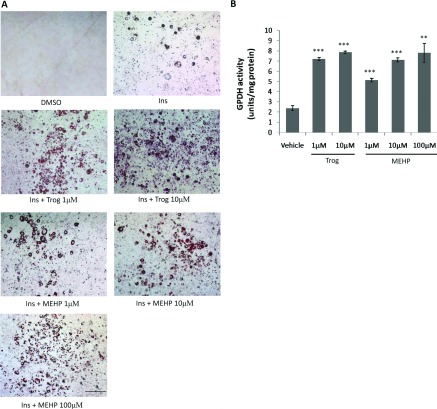
In the murine 3T3-L1 preadipocyte cell model, adipogenic signals induce the differentiation markers PPARγ and C/EBPα (CCAAT/enhancer-binding protein α), which drive terminal adipocyte differentiation and lipid accumulation [28–31]. We therefore examined the effects of MEHP on the differentiation of 3T3-L1 preadipocyte and compared its effect with PPARγ agonist troglitazone. An 8-day treatment of 3T3-L1 preadipocyte with the PPARγ agonist troglitazone in the presence of insulin strongly induced adipogenesis in a dose-dependent manner. Insulin alone had only a mild effect, whereas the combination of insulin and MEHP induced dose-dependent adipogenic effects, as evidenced by Oil Red O staining (Figure 1A). The ability of MEHP to induce adipogenesis was maximal at 100 μM, with no further enhancement at higher doses (results not shown). The actions of MEHP on adipocyte differentiation were further confirmed by quantification of cellular GPDH activity. When insulin alone was present, the cultures contained 2.37±0.24 units of GPDH activity/mg of protein. The combination of MEHP together with insulin caused a 2–3-fold increase in a dose-dependent manner; likewise, the presence of troglitazone also caused a 3-fold increase (Figure 1B). Thus, MEHP significantly induces adipocyte differentiation, although it has a reduced adipogenic potential compared with troglitazone.
Figure 1. MEHP induces adipogenesis in 3T3-L1 cells.
Confluent 3T3-L1 cells were treated for 8 days in the presence of DMSO, 10 μg/ml insulin alone or insulin plus ligands at the indicated concentrations. (A) Differentiated adipocytes were evidenced by Oil Red O staining (representative images from three individual experiments). Scale bar represents 200 μm. (B) GPDH activity was determined in cell lysates. Values are means±S.E.M (n=3). **P<0.01, ***P<0.001. Ins, insulin; Trog, troglitazone.
MEHP induces adipogenic regulators and markers in 3T3-L1 adipocytes
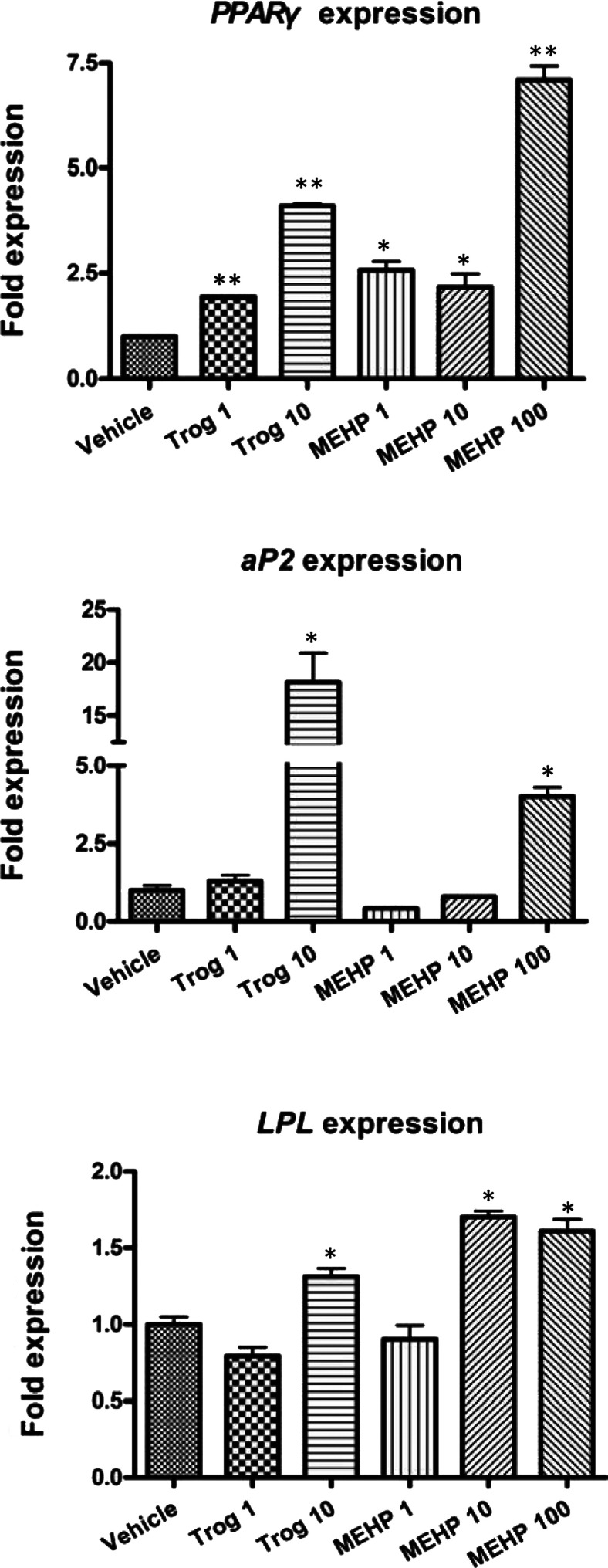
In order to characterize the adipogenic actions of MEHP, we performed gene expression analysis on adipogenic regulators by quantitative RT–PCR (reverse transcription–PCR). Expression levels from cells treated with insulin alone and from cells differentiated with troglitazone or MEHP in the presence of insulin were compared. Expression of PPARG (encoding PPARγ), a transcription factor that not only is critical for adipogenesis, but also represents a specific marker of fat cell differentiation [31,32], was strikingly increased by troglitazone or MEHP respectively. And even MEHP at dose of 100 μM caused a 7-fold increase of PPARG gene expression compared with insulin alone, and its downstream target gene aP2 (adipocyte-specific fatty acid binding protein) was activated by MEHP at 100 μM or troglitazone at 10 μM. MEHP at 10–100 μM or troglitazone at 10 μM also clearly up-regulated the level of LPL (lipoprotein lipase) transcript, thus confirming that the newly formed PPARγ was functionally active (Figure 2).
Figure 2. Effects of MEHP on mRNA expression of adipogenic modulators in mature 3T3-L1 adipocytes.
3T3-L1 cells were treated for 8 days with 10 μg/ml insulin alone or a combination of 10 μg/ml insulin and ligands at the indicated concentrations (1–10 μM of Trog and 1–100 μM of MEHP). mRNA expression levels of PPARG, aP2 and LPL were detected by quantitative real-time PCR. Expression levels were standardized to levels of 18s rRNA mRNA by calculating threshold cycle values (Ct). The data shown are representative of three independent experiments. Values are means±S.E.M (n=3). *P<0.05, **P<0.01. Trog, troglitazone.
Overall, the results show that MEHP combined with insulin activates transcriptional factors PPARG as well as its target genes, thus promoting 3T3-L1 preadipocytes into differentiate into mature adipocytes.
MEHP induces adipogenic regulators and markers in vivo
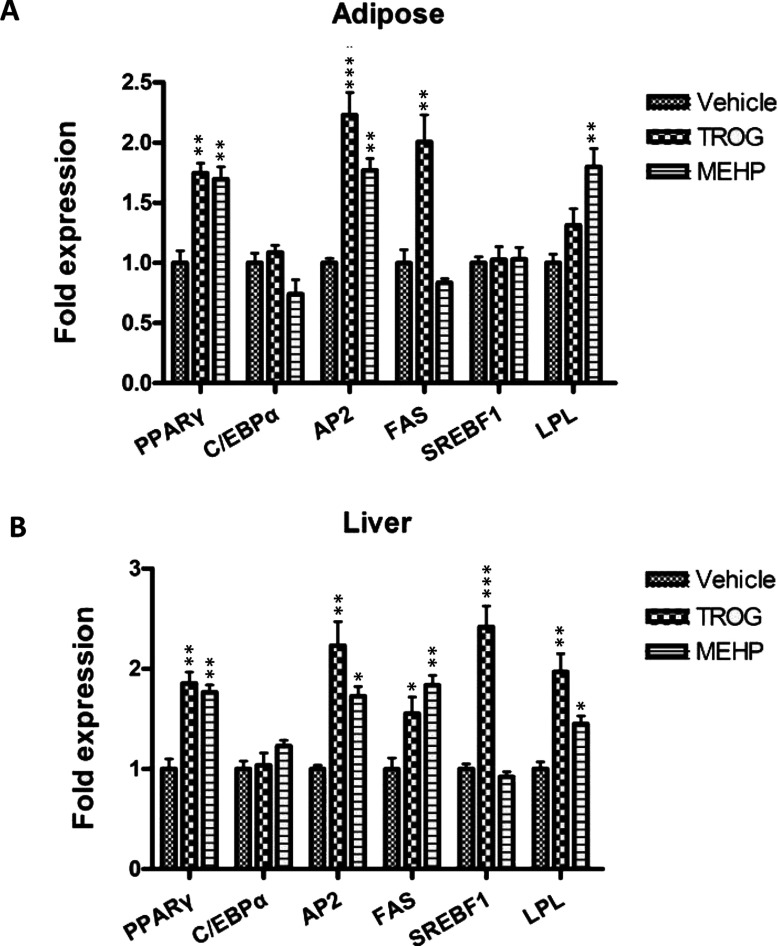
The ability of MEHP to regulate key modulators of adipogenesis and lipid homoeostasis in vivo has not been previously examined. Therefore we next asked whether MEHP could perturb expression of critical regulators, including transcriptional mediators PPARG, CEBPA and Srebf1 (sterol-regulatory-element-binding factor 1) as well as known target genes of PPARγ signalling. Liver and epididymal adipose tissue of 6-week-old male mice dosed for 24 h with MEHP (0.5 mg/kg of b.w.), troglitazone (0.5 mg/kg of b.w.) or vehicle (olive oil) were dissected for total RNA extraction and target gene analysis.
A significant expression increase induced by MEHP was observed for adipogenic markers such as PPARG, aP2 and LPL, whereas troglitazone significantly induced expression of PPARG as well as the late adipogenic markers such as aP2 and FAS (fatty acid synthase) in adipose tissue (Figure 3A). Liver is a key organ involved in metabolism, as it controls synthesis of many nutrients including lipids and carbohydrates. The results showed that troglitazone significantly promoted PPARG transcription and induced the expression of PPARγ target genes such as aP2, LPL and FAS in liver. In addition, troglitazone was also able to clearly up-regulate the expression level of Srebf1 transcript, another critical transcriptional mediator during adipogenesis, which regulates transcription of many lipid genes and participates in the generation of endogenous PPARγ ligands [32,33]. Similarly, MEHP significantly induced expression of PPARG, aP2, LPL and FAS in liver (Figure 3B). Therefore the co-ordinate increased expression of PPARG as well as its downstream target genes in liver suggests that MEHP stimulates fatty acid uptake and TAG synthesis.
Figure 3. In vivo induction of adipogenic modulators.
Six-week-old C57BL/6J male mice were dosed with vehicle (olive oil), MEHP (0.5 mg/kg of b.w.) or troglitazone (0.5 mg/kg of b.w.) by intraperitoneal injection. Animals were killed after 24 h and cDNA was prepared from epididymal adipose tissue or liver for quantitative real-time PCR analysis. Expression levels were standardized to levels of 18s rRNA mRNA by calculating threshold cycle values (Ct). The data shown are representative of three independent experiments. Values are means±S.E.M (n=3). *P<0.05, **P<0.01. Trog, troglitazone.
Taken together, these data indicate that MEHP exposure activates lipogenic gene expression, and MEHP may be a potential adipogenc agent in vivo.
Perinatal exposure to MEHP leads to obesity of offspring in mice model
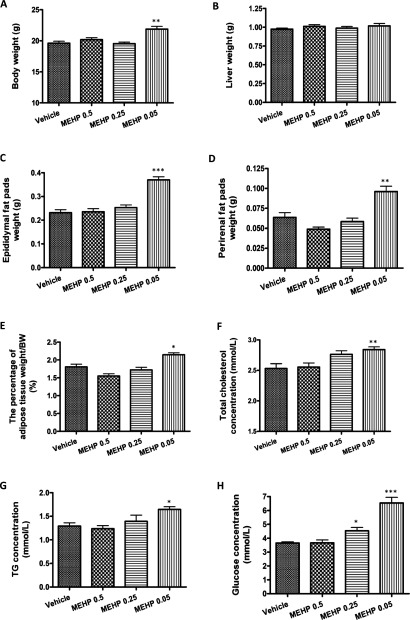
Based on its ability to induce 3T3-L1 adipocyte differentiation, and in vivo activation responses, we reasoned that perinatal exposure to MEHP, which is a sensitive period of adipose tissue development [34], would disrupt normal endocrine controls and adipogenesis. Therefore pregnant mice were dosed by gavage with MEHP (0.05, 0.25 or 0.5 mg/kg of b.w.) or vehicle (olive oil) from embryonic day 12 (E12) every 24 h until the 7th day of lactation (PND 7 of offspring). Eight-week-old pups were weighed.
In male offspring, the mean b.w. increased by 12% at 0.05 mg/kg of b.w. (21.88±0.95 g) compared with the control group (19.62±1.25 g). In contrast, higher concentration of MEHP (0.5 or 0.25 mg/kg of b.w.) did not increase b.w. gain in male offspring (Figure 4A).
Figure 4. Perinatal exposure to MEHP induces obese phenotype in male offspring.
Pregnant mice were dosed by gavage with vehicle (olive oil) or MEHP (0.5, 0.25 or 0.05 mg/kg of b.w.) from E12 until the 7th day of lactation (PND 7 of offspring). Obesity parameters of 8-week-old male offspring were measured. (A) b.w. (B) Liver weight. (C) Epididymal fat pad weight. (D) Perirenal fat pad weight. (E) The percentage of adipose tissue weight to b.w. (F) Serum levels of total cholesterol. (G) Serum levels of TAGs. (H) Serum glucose levels. Values are means±S.E.M. (n=16 vehicle-treated male pups from five litters; n=12 MEHP-treated (0.05 mg/kg of b.w.) male pups from three litters; n=14 MEHP-treated (0.25 mg/kg of b.w.) male pups from three litters; n=13 MEHP-treated (0.5 mg/kg of b.w.) male pups from three litters). *P<0.05, **P<0.01, ***P<0.001.
To determine whether the increased b.w. reflects body composition changes, we weighed liver and two distinct fat pads (epididymal and perirenal). The results showed that MEHP treatment did not alter liver weight in male offspring (Figure 4B). However, both epididymal (0.37±0.01 g) and perirenal fat mass (0.10±0.01 g) at the dose of 0.05 mg/kg of b.w. significantly increased compared with controls (epididymal: 0.23±0.01 g; perirenal: 0.06±0.01 g). In contrast, the adipose tissue weight at higher doses (0.5 or 0.25 mg/kg of b.w.) did not differ significantly from the control group (Figures 4C and 4D). The percentage of adipose tissue weight to b.w. was significantly higher at 0.05 mg/kg of b.w. (2.15±0.06%), but not the other two groups (0.5 mg/kg of b.w.: 1.55±0.06%; 0.25 mg/kg of b.w.: 1.72±0.08%), than that in the control group (1.81±0.07%) (Figure 4E).
To assess whether these alterations in b.w. and fat pad weight were related to changes in circulating levels of nutrients, we measured the levels of serum total cholesterol, TAG and blood glucose. Consistent with the increased fat mass, serum cholesterol (2.84±0.05 mmol/l) and TAG (1.65±0.06 mmol/l) levels at 0.05 mg/kg of b.w. were increased approximately 12 and 28% compared with the controls (total cholesterol: 2.53±0.08 mmol/l; TAG: 1.29±0.07 mmol/l) (Figures 4F and 4G). Blood glucose levels in MEHP-treated male offspring were increased approximately 79% (Figure 4H). The higher (0.5 or 0.25 mg/kg of b.w.) doses of MEHP did not lead to the increase in serum levels of total cholesterol and TAG (Figures 4F and 4G). Glucose levels, while higher at the dose of 0.25 mg/kg of b.w. (4.53±0.24 mmol/l) than those in the controls (3.65±0.09 mmol/l), remained within the physiological range (Figure 4H).
In female offspring, MEHP treatment at different doses did not significantly alter b.w., body composition, serum lipids and glucose levels (Table 2). Although the glucose level at the dose of 0.25 mg/kg of b.w. was increased compared with the control group, it remained within the physiological range (Table 2).
Table 2. Measurements of obesity parameters, serum lipids and glucose in female offspring.
Pregnant mice were dosed by gavage with vehicle (olive oil) or MEHP (0.5, 0.25 or 0.05 mg/kg of b.w.) from day 12 of gestation until day 7 of lactation (PND 7 of offspring). The b.w., liver weight, fat pads weight, the percentage of adipose tissue weight to b.w., and serum lipids and glucose levels were measured in 8-week-old female offspring. Values are means±S.E.M. (n=16 vehicle-treated female pups from five litters; n=10 MEHP-treated (0.5 mg/kg of b.w.) female pups from three litters; n=12 MEHP-treated (0.25 mg/kg of b.w.) female pups from three litters; n=10 MEHP-treated (0.05 mg/kg of b.w.) female pups from three litters). *P<0.05.
| MEHP dose (mg/kg of b.w.) | ||||
|---|---|---|---|---|
| Parameter | Vehicle | 0.5 | 0.25 | 0.05 |
| b.w. (g) | 15.86±0.16 | 15.48±0.24 | 15.51±0.13 | 16.05±0.84 |
| Liver weight (g) | 0.82±0.03 | 0.77±0.02 | 0.77±0.01 | 0.84±0.02 |
| Parametrial fat pad weight (g) | 0.15±0.01 | 0.15±0.01 | 0.14±0.01 | 0.15±0.01 |
| Perirenal fat pad weight (g) | 0.05±0.01 | 0.06±0.00 | 0.06±0.01 | 0.08±0.02 |
| Adipose tissue weight/b.w. (%) | 1.37±0.09 | 1.36±0.10 | 1.31±0.11 | 1.38±0.12 |
| Cholesterol (mmol/l) | 2.23±0.06 | 2.36±0.09 | 2.18±0.06 | 2.10±0.13 |
| TAG (mmol/l) | 0.87±0.05 | 0.87±0.06 | 0.93±0.04 | 0.77±0.05 |
| Glucose (mmol/l) | 3.35±0.08 | 3.61±0.16 | 4.01±0.21* | 3.84±0.22 |
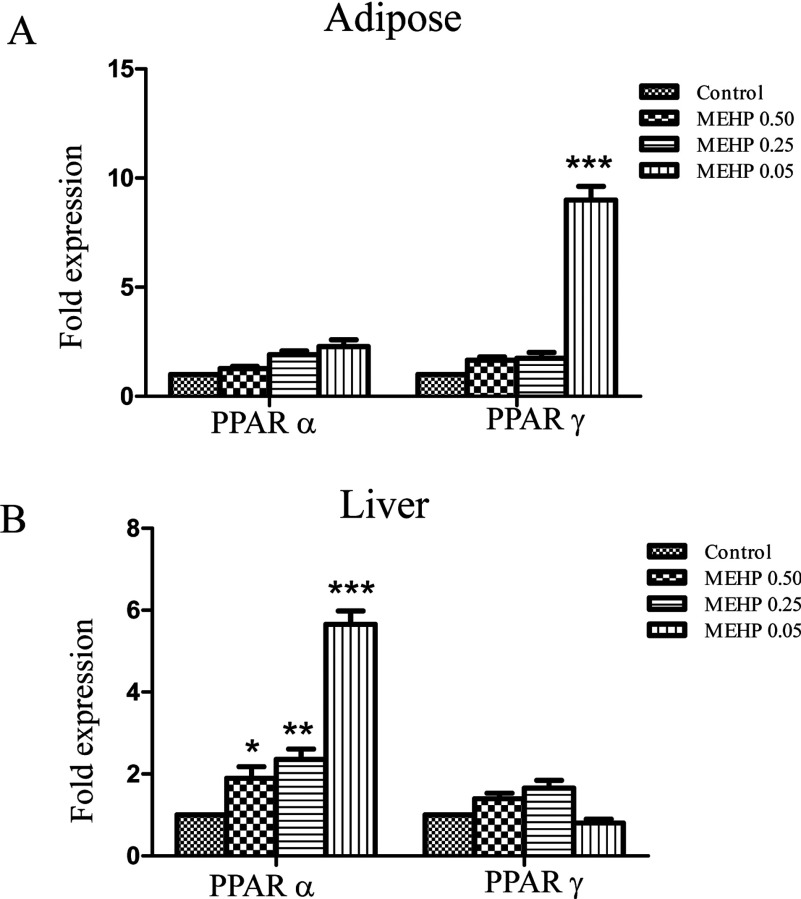
To analyse the possible mechanisms of the alteration in b.w. and fat pad weight, we detected the expression of PPARγ and PPARα in adipose tissues and liver of male offspring in MEHP-treated mice. The expression of PPARγ was significantly increased by MEHP treatment (0.05 mg/kg of b.w.; P<0.001) and PPARα was modestly activated by MEHP treatment in adipose tissues (Figure 5A). In liver, a significant expression increase of PPARα was observed in mice treated by MEHP of 0.5 (P<0.05), 0.25 (P<0.01) or 0.05 mg/kg of b.w. (P<0.001), but the activity of PPARγ was not significantly affected by MEHP treatment (Figure 5B).
Figure 5. The induction of PPARγ and PPARα in obese male offspring.
The expression of PPARγ and PPARα was detected in adipose tissues (A) and liver (B) in obese male offspring in MEHP-treated maternal mice by quantitative real-time PCR analysis. Expression levels were standardized to levels of β-actin mRNA by calculating threshold cycle values (Ct). The data shown are representative of three independent experiments. Values are means±S.E.M (n=3). *P<0.05, **P<0.01, ***P<0.001.
Overall, these results suggest that perinatal exposure to MEHP increases the susceptibility to obesity in offspring, and male offspring tend to be more sensitive than females at the specific dose.
DISCUSSION
In the present study, we have shown the concentration-dependent effects of MEHP on adipocyte differentiation using the murine 3T3-L1 cell model. MEHP induced the expression of PPARγ as well as its target genes required for adipogenesis. Consistent with in vitro results, MEHP also activated PPARγ and its target genes in vivo. Furthermore, perinatal exposure to MEHP increased b.w., fat mass and serum cholesterol, TAG and glucose levels in male offspring. Thus, our results suggested that perinatal exposure to MEHP might be expected to increase the incidence of obesity in male offspring and could act as a potential chemical stressor for obesity and obesity-related disorders.
The effects of MEHP on the differentiation of 3T3-L1 preadipocytes have been previously reported [24,35,36]. We showed herein that 3T3-L1 preadipocytes were induced into mature adipocytes by MEHP in a dose-dependent manner, which is consistent with the previous studies [24]. Furthermore, the results of GPDH activity, which is the late-stage differentiation marker, reflected the differentiation state of 3T3-L1 preadipocytes at the quantitative level. In the present study, we tried to induce 3T3-L1 preadipocytes at the dose of 1 mM MEHP combined with insulin. Nevertheless, the observation results showed the presence of cell debris, we therefore infer that higher dose of MEHP than 100 μM might possess cytotoxic activity and lead to cell death.
During adipogenesis, preadipocyte-like preadipocytes differentiate into lipid-laden and insulin-responsive adipocytes. The process occurs in several stages and involves a cascade of transcriptional factors, among which PPARγ is considered the crucial determinant of adipocyte fate [32,33]. The previous studies demonstrated that MEHP activated PPARγ in transactivation assays [24,35,36]. In the present study, the effect of MEHP on adipocyte differentiation was confirmed by molecular evidence. MEHP between 1 and 100 μM effectively induced the expression of PPARG in 3T3-L1 cells. The newly formed PPARγ was functionally active, since its increase stimulated the expression of LPL and aP2 genes, two of its specific targets [32,33]. Furthermore, MEHP activated the expression of PPARG as well as its downstream target genes such as LPL and aP2 in vivo, which is consistent with the results in 3T3-L1 preadipocytes model.
Previous studies mainly focused on the effects on reproductive toxicity at high dose of phthalate exposure [21–23]. Although much proof has demonstrated that a low dose of EDCs during development can interfere with the normal function of the endocrine system, consequently inducing overweight and obesity later in life [37–41], the effects on obesity in offspring have not been elucidated at low dose of in utero MEHP exposure. In the present study, pregnant mice were dosed by gavage with MEHP (0.05, 0.25 or 0.5 mg/kg of b.w.) or vehicle (olive oil) during the critical stages of differentiation (developmental ‘window’). Our results demonstrated for the first time that the b.w. and fat storage of 8-week-old male offspring was significantly increased at the dose of 0.05 mg/kg of b.w., but a 5–10-fold higher dose of MEHP was inefficient in inducing the development of obesity. A key concept in the field of endocrine disruption is that there may be non-traditional dose-response curves such as an inverted ‘U’ or even multiple ‘U’ shaped curves, i.e. the response is not proportional to the dose [42]. So far, there have been some reports on the differences in response to endocrine disruptors. For example, prenatal and postnatal exposure to BPA [bisphenol A; 1 mg/l in drinking water, corresponding to a maximal 70 μg/kg of b.w. per day exposure), an oestrogenic EDC, induced the increase in offspring b.w. [39], but higher doses of BPA administered to gestating dams (50–600 mg/kg of b.w. per day) reduced b.w. in growing Sprague–Dawley rat and CD-1 mouse pups [43,44]. Consistent with the finding, Miyawaki et al. [40] observed that a 10-fold higher dose (10 mg/l in drinking water) was inefficient in inducing overstorage of fat, but the lower exposure (1 mg/l) dose effectively induced obesity and hyperlipidaemia in female offspring. In addition, a recent study showed that genistein, one of the most abundant phyto-oestrogens in the human diet, at pharmacologically high doses did indeed inhibit adipose tissue fat deposition, while at low doses, similar to those found in Western and Eastern diets, in soya milk or in food supplements containing soya, it induced increased adipose tissue fat deposition, especially in males. Further, this increase in adipose tissue fat deposition by genistein was correlated with mild peripheral insulin resistance [45]. In our studies, lower dose (0.05 mg/kg of b.w.) of MEHP induced the increase of b.w. and fat mass in male offspring, but the higher doses (0.25 or 0.5 mg/kg of b.w.) did not. This suggests that the development of obesity induced by MEHP exposure follows the non-traditional dose–response curves, making it impossible to predict response to environmental exposure range. Therefore the susceptibility to endocrine disruptors might be highly specific for a given dose and timing of exposure, and the kind of endocrine disruptors used.
Although low dose of MEHP induced overstorage of fat in male offspring, it is currently unknown whether the increased adiposity in vivo results from an increase in adipocyte precursor cell number, enhanced adipocyte differentiation from the same number of precursors, an increase in adipocyte size without an increase in number, or some combination of these. Liver weight was not significantly different between the two groups; we thus infer that a statistical significance continues to be further studied at older ages. Consistent with the increased fat pad weight, serum cholesterol, TAG and glucose levels were elevated in a sex-dependent manner. It may be helpful to explain the previous study results that illustrated that concentration of urinary phthalates metabolites were associated with increased waist circumference and insulin resistance in adult males in the United States [13,14].
Our present data are not in accordance with previous publications [46,47]. Feige et al. [46] found that the treatment with 1000 mg of DEHP/kg of b.w. per day from 3 weeks of age until 13 weeks induced less weight than controls, while the treatment with 100 mg/kg of b.w. per day did not alter the b.w. Hayashi et al. [47] reported that DEHP did not change the b.w. gain in adult mice exposed to a range of 10–145 mg of DEHP/kg of b.w. per day from 12 weeks of age until 16 weeks [47]. We infer that the difference may be caused by the precise dose and timing of exposure. First, in our study maternal mice was exposed to MEHP from GD 12 (day 12 of gestation) until PND 7, which is the critical period of cellular differentiation of reproductive tract, immune and adipocyte. The developing fetus and neonate are particularly sensitive to perturbation by EDCs because the placenta does not completely protect the unborn fetus from its external environment and the organism undergoes periods of extremely rapid cell division and differentiation thus resulting in cells that can differentiate abnormally and pass altered programming to subsequent generation. However, in previous toxicity studies adult mice exposed to DEHP (3–13 weeks of age, or 12–16 weeks of age) were developed to be experimental mouse models [46,47], although Hayashi et al. showed prenatal exposure to DEHP led to the decrease of the maternal b.w. on GD 18 but not offspring, which may be attributable to the decrease in the total or live number of fetuses [47]. Secondly, the difference of phenotype may result from the different exposure dose. Just as the inverted ‘U’ shaped curves described above, a high dose of DEHP treatment (1000 mg/kg of b.w. per day) induced the lean phenotype in adult mice, while medium dose (100 mg/kg of b.w. per day) had no effect [46]. Similarly, DEHP treatment in a range of 10–145 mg/kg of b.w. per day did not also alter the b.w. gain for 4 weeks exposure in adult mice. In addition, perinatal exposure to the medium dose (10–145 mg/kg of b.w. per day) of DEHP had no effect on body and organ weight gain in fetuses and neonates [47]. This suggests that the phenotype alteration resulted from DEHP exposure may follow the inverted ‘U’ shaped curves.
Feige et al. [46] and Hayashi et al. [47] found that DEHP-treated mice were protected from diet-induced obesity via PPARα-dependent activation of hepatic fatty acid catabolism, whereas the activity of neither PPARβ nor PPARγ was affected. MEHP, not DEHP, is an exogenous ligand of PPARγ and PPARα that is primarily activated through ligand binding [36,48]. Therefore we want to know whether the expression of PPARγ and PPARα was altered by MEHP treatment. In the present study, we found that the expression of PPARγ was significantly enhanced and the activity of PPARα was modestly affected by MEHP treatment in adipose tissues of male offspring in MEHP-treated mice. In liver, MEHP induced the expression PPARα, not PPARγ, which was consistent with the reports of Feige et al. [46] and Hayashi et al. [47]. However, adult male mice dosed for 24 h with MEHP (0.5 mg/kg of b.w.) significantly promoted the expression of PPARγ in liver, which was different from the reports of Feige et al. [46]. There were many reports to show that different doses of MEHP or DEHP caused different phenotype: development defects at high dose and obesity at low doses [46,49,50]. Therefore we consider that this discrepancy may be due to following reasons. First, the phenotypes of mice induced by DEHP or MEHP were distinguishing because of the difference of route of administration. In Feige et al.'s study [46], mice was given a diet containing 0, 100 or 1000 mg of DEHP/kg of b.w. per day at 3 weeks of age. At week 13, the b.w. was decreased in mice treated with 1000 mg of DEHP/kg of b.w. per day. In the present study, 6-week-old male mice received an intraperitoneal. injection of MEHP (0.5 mg/kg of b.w.) for 24 h to observe the adipocyte differentiation. We found that MEHP induced a significant increase for adipogenic markers, implying that MEHP might promote the differentiation of adipocyte. Moreover, perinatal in utero exposure to a low dose of MEHP does induce obesity in mice. Next, although PPARα and PPARγ are members of the nuclear receptor family that regulates the expression of genes that control proliferation of peroxisomes, they have different roles in a variety of biological processes. PPARγ is predominantly expressed in adipose tissue and essential for adipocyte differentiation, adipogenesis and lipid storage [51,52], whereas PPARα is mainly expressed in liver and promotes fatty acid oxidation to inhibit adipocyte accumulation [53,54]. Taken together, the increase of PPARα in liver in mice treated with high doses of DEHP, which causes low b.w., may mean a significantly increase in fatty acid oxidation to inhibit obesity. The increase in PPARγ in liver in mice treated with lower doses of MEHP for 24 h may imply the adipocyte differentiation in liver to promote lipid accumulation. Also, the activation of PPARα in liver of obese male offspring in MEHP-treated mice may be conducive to the inhibition of the accumulation of liver adipocyte and prevent the increase of liver weight, and PPARγ enhancement in adipose tissues may contribute to lipid storage and the maintenance of the adipocyte phenotype.
In conclusion, the perinatal exposure of mice to MEHP increases adipose storage, serum lipid and glucose levels in offspring. The effect of MEHP on the development of obesity is more pronounced in males than in females. Furthermore, because exposure to MEHP is ubiquitous and does not cease during human life, further studies are urgently needed to better understand the long-term consequences of permanent MEHP exposure on obesity and obesity-related disorders.
AUTHOR CONTRIBUTION
Chanjuan Hao and Xu Ma designed the experiments. Chanjuan Hao and Xuejia Cheng performed the assays. Chanjuan Hao and Hongfei Xia analysed the data. Chanjuan Hao wrote the paper, and all the authors approved the submission.
FUNDING
This work was supported by the National Basic Research Program of China [grant numbers 2007CB511905] and the National Research Institute for Family Planning [grant numbers 2009GJSSJKA06].
References
- 1.Collins S. Overview of clinical perspectives and mechanisms of obesity. Birth Defects Res. A Clin. Mol. Teratol. 2005;73:470–471. doi: 10.1002/bdra.20140. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad A. H., Ford E. S., Bowman B. A., Dietz W. H., Vinicor F., Bales V. S., Marks J. S. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA, J. Am. Med. Assoc. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 3.Mokdad A. H., Serdula M. K., Dietz W. H., Bowman B. A., Marks J. S., Koplan J. P. The spread of the obesity epidemic in the United States, 1991–1998. JAMA, J. Am. Med. Assoc. 1999;282:1519–1522. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- 4.Ogden C. L., Flegal K. M., Carroll M. D., Johnson C. L. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA, J. Am. Med. Assoc. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 5.Desvergne B., Feige J. N., Casals-Casas C. PPAR-mediated activity of phthalates: a link to the obesity epidemic? Mol. Cell. Endocrinol. 2009;304:43–48. doi: 10.1016/j.mce.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Goncharov A., Haase R. F., Santiago-Rivera A., Morse G., Akwesasne Task Force on the Environment, McCaffrey R. J., Rej R., Carpenter D. O. High serum PCBs are associated with elevation of serum lipids and cardiovascular disease in a Native American population. Environ. Res. 2008;106:226–239. doi: 10.1016/j.envres.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gladen B. C., Ragan N. B., Rogan W. J. Pubertal growth and development and prenatal and lactational exposure to polychlorinated biphenyls and dichlorodiphenyl dichloroethene. J. Pediatr. 2000;136:490–496. doi: 10.1016/s0022-3476(00)90012-x. [DOI] [PubMed] [Google Scholar]
- 8.Vasiliu O., Cameron L., Gardiner J., Deguire P., Karmaus W. Polybrominated biphenyls, polychlorinated biphenyls, body weight, and incidence of adult-onset diabetes mellitus. Epidemiology. 2006;17:352–359. doi: 10.1097/01.ede.0000220553.84350.c5. [DOI] [PubMed] [Google Scholar]
- 9.Lee D. H., Lee I. K., Jin S. H., Steffes M., Jacobs D. R., Jr Association between serum concentrations of persistent organic pollutants and insulin resistance among non-diabetic adults: results from the National Health and Nutrition Examination Survey 1999–2002. Diabetes Care. 2007;30:622–628. doi: 10.2337/dc06-2190. [DOI] [PubMed] [Google Scholar]
- 10.Lee D. H., Steffes M. W., Jacobs D. R., Jr Can persistent organic pollutants explain the association between serum γ-glutamyltransferase and type 2 diabetes? Diabetologia. 2008;51:402–407. doi: 10.1007/s00125-007-0896-5. [DOI] [PubMed] [Google Scholar]
- 11.Pelletier C., Doucet E., Imbeault P., Tremblay A. Associations between weight loss-induced changes in plasma organochlorine concentrations, serum T3 concentration, and resting metabolic rate. Toxicol. Sci. 2002;67:46–51. doi: 10.1093/toxsci/67.1.46. [DOI] [PubMed] [Google Scholar]
- 12.Smink A., Ribas-Fito N., Garcia R., Torrent M., Mendez M. A., Grimalt J. O., Sunyer J. Exposure to hexachlorobenzene during pregnancy increases the risk of overweight in children aged 6 years. Acta Paediatr. 2008;97:1465–1469. doi: 10.1111/j.1651-2227.2008.00937.x. [DOI] [PubMed] [Google Scholar]
- 13.Stahlhut R. W., van Wijngaarden E., Dye T. D., Cook S., Swan S. H. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ. Health Perspect. 2007;115:876–882. doi: 10.1289/ehp.9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatch E. E., Nelson J. W., Qureshi M. M., Weinberg J., Moore L. L., Singer M., Webster T. F. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environ. Health. 2008;7:27. doi: 10.1186/1476-069X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeuchi T., Tsutsumi O., Ikezuki Y., Takai Y., Taketani Y. Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocr. J. 2004;51:165–169. doi: 10.1507/endocrj.51.165. [DOI] [PubMed] [Google Scholar]
- 16.Grün F., Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147:S50–S55. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- 17.Wams T. J. Diethylhexylphthalate as an environmental contaminant – a review. Sci. Total Environ. 1987;66:1–16. doi: 10.1016/0048-9697(87)90072-6. [DOI] [PubMed] [Google Scholar]
- 18.Hauser R., Calafat A. M. Phthalates and human health. Occup. Environ. Med. 2005;62:806–818. doi: 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koch H. M., Bolt H. M., Angerer J. Di(2-ethylhexyl)phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium-labelled DEHP. Arch. Toxicol. 2004;78:123–130. doi: 10.1007/s00204-003-0522-3. [DOI] [PubMed] [Google Scholar]
- 20.Rusyn I., Peters J. M., Cunningham M. L. Modes of action and species-specific effects of di-(2-ethylhexyl)phthalate in the liver. Crit. Rev. Toxicol. 2006;36:459–479. doi: 10.1080/10408440600779065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arcadi F. A., Costa C., Imperatore C., Marchese A., Rapisarda A., Salemi M., Trimarchi G. R., Costa G. Oral toxicity of bis(2-ethylhexyl) phthalate during pregnancy and suckling in the Long-Evans rat. Food Chem. Toxicol. 1998;36:963–970. doi: 10.1016/s0278-6915(98)00065-9. [DOI] [PubMed] [Google Scholar]
- 22.Li L. H., Jester W. F., Jr, Orth J. M. Effects of relatively low levels of mono-(2-ethylhexyl) phthalate on cocultured Sertoli cells and gonocytes from neonatal rats. Toxicol. Appl. Pharmacol. 1998;153:258–265. doi: 10.1006/taap.1998.8550. [DOI] [PubMed] [Google Scholar]
- 23.Grande S. W., Andrade A. J., Talsness C. E., Grote K., Chahoud I. A dose–response study following in utero and lactational exposure to di(2-ethylhexyl)phthalate: effects on female rat reproductive development. Toxicol. Sci. 2006;91:247–254. doi: 10.1093/toxsci/kfj128. [DOI] [PubMed] [Google Scholar]
- 24.Feige J. N., Gelman L., Rossi D., Zoete V., Métivier R., Tudor C., Anghel S. I., Grosdidier A., Lathion C., Engelborghs Y., et al. The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor γ modulator that promotes adipogenesis. J. Biol. Chem. 2007;282:19152–19166. doi: 10.1074/jbc.M702724200. [DOI] [PubMed] [Google Scholar]
- 25.Tontonoz P., Spiegelman B. M. Fat and beyond: the diverse biology of PPARγ. Annu. Rev. Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 26.Hanlon P. R., Ganem L. G., Cho Y. C., Yamamoto M., Jefcoate C. R. AhR- and ERK-dependent pathways function synergistically to mediate 2,3,7,8-tetrachlorodibenzo-p-dioxin suppression of peroxisome proliferator-activated receptor-γ 1 expression and subsequent adipocyte differentiation. Toxicol. Appl. Pharmacol. 2003;189:11–27. doi: 10.1016/s0041-008x(03)00083-8. [DOI] [PubMed] [Google Scholar]
- 27.Wise L. S., Green H. Participation of one isozyme of cytosolic glycerophosphate dehydrogenase in the adipose conversion of 3T3 cells. J. Biol. Chem. 1979;254:273–275. [PubMed] [Google Scholar]
- 28.Lane M. D., Tang Q. Q., Jiang M. S. Role of the CCAAT enhancer binding proteins (C/EBPs) in adipocyte differentiation. Biochem. Biophys. Res. Commun. 1999;266:677–683. doi: 10.1006/bbrc.1999.1885. [DOI] [PubMed] [Google Scholar]
- 29.Tang Q. Q., Otto T. C., Lane M. D. CCAAT/enhancer binding protein beta is required for mitotic clonal expansion during adipogenesis. Proc. Natl. Acad. Sci. U.S.A. 2003;100:850–855. doi: 10.1073/pnas.0337434100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang Q. Q., Lane M. D. Activation and centromeric localization of CCAAT/enhancer-binding proteins during the mitotic clonal expansion of adipocyte differentiation. Genes Dev. 1999;13:2231–2241. doi: 10.1101/gad.13.17.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ntambi J. M., Young-Cheul K. Adipocyte differentiation and gene expression. J. Nutr. 2000;130:3122S–3126S. doi: 10.1093/jn/130.12.3122S. [DOI] [PubMed] [Google Scholar]
- 32.Lefterova M. I., Lazar M. A. New developments in adipogenesis. Trends Endocrinol. Metab. 2009;20:107–114. doi: 10.1016/j.tem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Farmer S. R. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newbold R. R. Impact of environmental endocrine disrupting chemicals on the development of obesity. Hormones (Athens) 2010;9:206–217. doi: 10.14310/horm.2002.1271. [DOI] [PubMed] [Google Scholar]
- 35.Bility M. T., Thompson J. T., McKee R. H., David R. M., Butala J. H., Vanden Heuvel J. P., Peters J. M. Activation of mouse and human peroxisome proliferator-activated receptors (PPARs) by phthalate monoesters. Toxicol. Sci. 2004;82:170–182. doi: 10.1093/toxsci/kfh253. [DOI] [PubMed] [Google Scholar]
- 36.Hurst C. H., Waxman D. J. Activation of PPARα and PPARγ by environmental phthalate monoesters. Toxicol. Sci. 2003;74:297–308. doi: 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- 37.Grün F., Watanabe H., Zamanian Z., Maeda L., Arima K., Cubacha R., Gardiner D. M., Kanno J., Iguchi T., Blumberg B. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol. Endocrinol. 2006;20:2141–2155. doi: 10.1210/me.2005-0367. [DOI] [PubMed] [Google Scholar]
- 38.Arsenescu V., Arsenescu R. I., King V., Swanson H., Cassis L. A. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ. Health Perspect. 2008;116:761–768. doi: 10.1289/ehp.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Somm E., Schwitzgebel V. M., Toulotte A., Cederroth C. R., Combescure C., Nef S., Aubert M. L., Hüppi P. S. Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ. Health Perspect. 2009;117:1549–1555. doi: 10.1289/ehp.11342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyawaki J., Sakayama K., Kato H., Yamamoto H., Masuno H. Perinatal and postnatal exposure to bisphenol a increases adipose tissue mass and serum cholesterol level in mice. J. Atheroscler. Thromb. 2007;14:245–252. doi: 10.5551/jat.e486. [DOI] [PubMed] [Google Scholar]
- 41.Newbold R. R., Padilla-Banks E., Snyder R. J., Jefferson W. N. Developmental exposure to estrogenic compounds and obesity. Birth Defects Res. A Clin. Mol. Teratol. 2005;73:478–480. doi: 10.1002/bdra.20147. [DOI] [PubMed] [Google Scholar]
- 42.vom Saal F. S., Akingbemi B. T., Belcher S. M., Birnbaum L. S., Crain D. A., Eriksen M., Farabollini F., Guillette L. J., Jr, Hauser R., Heindel J. J., et al. Chapel Hill Bisphenol A Expert Panel Consensus Statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod. Toxicol. 2007;24:131–138. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tyl R. W., Myers C. B., Marr M. C., Thomas B. F., Keimowitz A. R., Brine D. R., Veselica M. M., Fail P. A., Chang T. Y., Seely J. C., et al. Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague–Dawley rats. Toxicol. Sci. 2002;68:121–146. doi: 10.1093/toxsci/68.1.121. [DOI] [PubMed] [Google Scholar]
- 44.Tyl R. W., Myers C. B., Marr M. C., Sloan C. S., Castillo N. P., Veselica M. M., Seely J. C., Dimond S. S., Van Miller J. P., Shiotsuka R. N., et al. Two-generation reproductive toxicity study of dietary bisphenol A in CD-1 (Swiss) mice. Toxicol. Sci. 2008;104:362–384. doi: 10.1093/toxsci/kfn084. [DOI] [PubMed] [Google Scholar]
- 45.Penza M., Montani C., Romani A., Vignolini P., Pampaloni B., Tanini A., Brandi M. L., Alonso-Magdalena P., Nadal A., Ottobrini L., et al. Genistein affects adipose tissue deposition in a dose-dependent and gender-specific manner. Endocrinology. 2006;147:5740–5751. doi: 10.1210/en.2006-0365. [DOI] [PubMed] [Google Scholar]
- 46.Feige J. N., Gerber A., Casals-Casas C., Yang Q., Winkler C., Bedu E., Bueno M., Gelman L., Auwerx J., Gonzalez F. J., et al. The pollutant diethylhexyl phthalate regulates hepatic energy metabolism via species-specific PPARalpha-dependent mechanisms. Environ. Health Perspect. 2010;118:234–241. doi: 10.1289/ehp.0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashi Y., Ito Y., Yamagishi N., Yanagiba Y., Tamada H., Wang D., Ramdhan D. H., Naito H., Harada Y., Kamijima M., et al. Hepatic peroxisome proliferator-activated receptor α may have an important role in the toxic effects of di(2-ethylhexyl)-phthalate on offspring of mice. Toxicology. 2011;289:1–10. doi: 10.1016/j.tox.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Maloney E. K., Waxman D. J. trans-Activation of PPARα and PPARγ by structurally diverse environmental chemicals. Toxicol. Appl. Pharmacol. 1999;161:209–218. doi: 10.1006/taap.1999.8809. [DOI] [PubMed] [Google Scholar]
- 49.Dobrzyńska M. M., Tyrkiel E. J., Derezińska E., Pachocki K. A., Ludwicki J. K. Two generation reproductive and developmental toxicity following subchronic exposure of pubescent male mice to di(2-ethylhexyl)phthalate. Ann. Agric. Environ. Med. 2012;19:31–37. [PubMed] [Google Scholar]
- 50.Ellero-Simatos S., Claus S. P., Benelli C., Forest C., Letourneur F., Cagnard N., Beaune P. H., de Waziers I. Combined transcriptomic-1H NMR metabonomic study reveals that mono-ethylhexyl-phthalate stimulates adipogenesis and glyceroneogenesis in human adipocytes. J. Proteome Res. 2011;10:5493–5502. doi: 10.1021/pr200765v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lowell B. B. PPARγ: an essential regulator of adipogenesis and modulator of fat cell function. Cell. 1999;99:239–242. doi: 10.1016/s0092-8674(00)81654-2. [DOI] [PubMed] [Google Scholar]
- 52.Park B. H., Kim D. S., Won G. W., Jeon H. J., Oh B. C., Lee Y., Kim E. G., Lee Y. H. Mammalian ste20-like kinase and SAV1 promote 3T3-L1 adipocyte differentiation by activation of PPARγ. PLoS ONE. 2012;7:e30983. doi: 10.1371/journal.pone.0030983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Raalte D. H., Li M., Pritchard P. H., Wasan K. M. Peroxisome proliferator-activated receptor (PPAR)-α: a pharmacological target with a promising future. Pharm. Res. 2004;21:1531–1538. doi: 10.1023/b:pham.0000041444.06122.8d. [DOI] [PubMed] [Google Scholar]
- 54.Yu S., Matsusue K., Kashireddy P., Cao W. Q., Yeldandi V., Yeldandi A. V., Rao M. S., Gonzalez F. J., Reddy J. K. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor γ1 (PPARγ1) overexpression. J. Biol. Chem. 2003;278:498–505. doi: 10.1074/jbc.M210062200. [DOI] [PubMed] [Google Scholar]