Abstract
Symbiotic relationships are widespread in nature and are fundamental for ecosystem functioning and the evolution of biodiversity. In marine environments, photosymbiosis with microalgae is best known for sustaining benthic coral reef ecosystems. Despite the importance of oceanic microbiota in global ecology and biogeochemical cycles, symbioses are poorly characterized in open ocean plankton. Here, we describe a widespread symbiotic association between Acantharia biomineralizing microorganisms that are abundant grazers in plankton communities, and members of the haptophyte genus Phaeocystis that are cosmopolitan bloom-forming microalgae. Cophylogenetic analyses demonstrate that symbiont biogeography, rather than host taxonomy, is the main determinant of the association. Molecular dating places the origin of this photosymbiosis in the Jurassic (ca. 175 Mya), a period of accentuated marine oligotrophy. Measurements of intracellular dimethylated sulfur indicate that the host likely profits from antioxidant protection provided by the symbionts as an adaptation to life in transparent oligotrophic surface waters. In contrast to terrestrial and marine symbioses characterized to date, the symbiont reported in this association is extremely abundant and ecologically active in its free-living phase. In the vast and barren open ocean, partnership with photosymbionts that have extensive free-living populations is likely an advantageous strategy for hosts that rely on such interactions. Discovery of the Acantharia–Phaeocystis association contrasts with the widely held view that symbionts are specialized organisms that are rare and ecologically passive outside the host.
Keywords: protists, Radiolaria, DMSP, eukaryote biodiversity
Symbiosis, whereby different biological species live together in close and long-term interaction, plays key ecological roles in terrestrial and marine ecosystems and is regarded as a major source of evolutionary innovation (1). Photosymbiosis, whereby microalgae live intracellularly within a heterotrophic host organism, is typically considered mutually beneficial for the two partners: the symbiont provides photosynthetically derived products to the host, which in turn maintains a sheltered and relatively nutrient-rich environment for the symbiont (2, 3). Combining heterotrophy, autotrophy, and often biomineralization, photosymbioses profoundly impact food webs and ecosystem functioning in shallow coastal waters, particularly in oligotrophic settings (4). The most familiar case of photosymbiosis is the association of reef-building corals with “zooxanthellae” (dinoflagellates of the genus Symbiodinium), but other hosts include molluscs, anemones, acoel flatworms, sponges, and various protists (4–7). Despite early recognition of the existence of photosymbioses in the plankton realm (8) and more recent emphasis on the fundamental ecological and evolutionary significance of symbiosis, remarkably little attention has been given to the diversity, function, and ecological impact of symbioses in open ocean plankton. In most known photosymbioses, the algal symbiont must be acquired from the surrounding environment during each host generation (horizontal transmission), implying the existence of a free-living phase of the symbiont. In shallow waters, free-living populations of known symbionts are typically elusive and often undetectable in the water column, but in the case of Symbiodinium are rather concentrated in surface sediments surrounding the coral hosts (9, 10). In open ocean surface waters, where microbiotia are typically very diluted, it is not clear what strategy hosts use to ensure repeated capture of appropriate symbionts in successive generations.
Large heterotrophic amoeboid protists, such as Radiolaria and Foraminifera from the eukaryote supergroup Rhizaria, are known to host endosymbiotic microalgae in oligotrophic oceanic surface waters (11, 12). Molecular investigations associated with culturing methods have shown that symbionts can be dinoflagellates, prasinophytes, or haptophytes (13–15), but in most cases their precise identity, host specificity, and biogeography are unknown. The fact that photosymbiosis gave rise to light-harvesting chloroplasts and the spread of photosynthesis across the eukaryotic tree of life (16) means that investigation of modern photosymbioses can help understanding these key evolutionary processes.
The Acantharia are radiolarians characterized by possessing an endoskeleton of barium-enriched strontium sulfate (celestite). They are widely distributed throughout the world’s oceans and are particularly abundant in oligotrophic open ocean waters. Contributing up to 40% of total zooplankton biomass (17), they typically outnumber other biomineralizing protists like Foraminifera and Polycystinea (18). Classically known as grazers in marine ecosystems (19), certain acantharians have long been recognized to also participate, sometimes significantly (20), in primary production by harboring endosymbiotic microalgae. Symbiotic Acantharia are abundant in surface waters, whereas their nonsymbiotic relatives typically inhabit deeper, mesopelagic, waters (21, 22). Photosymbiosis in Acantharia is associated with significant morphological innovation and an increased complexity of the celestite skeleton, with symbiotic species having thicker spicules, a tighter central association, and a more robust shell than nonsymbiotic species (23). Acantharian symbionts were taxonomically assigned three decades ago to the division Haptophyta on the basis of an in hospite study of their ultrastructure (24), but their exact identity has never been elucidated.
Results and Discussion
Diversity and Specificity of the Acantharia–Phaeocystis Symbiosis.
We isolated individual symbiont-bearing acantharian cells from seven oceanic regions (Fig. 1) and subsequently PCR amplified genetic markers from the host (18S and 28S rDNA) and the symbionts (18S and 28S rDNA, rbcL and psbA). For more than 100 acantharian specimens analyzed, representing 25 distinct morphospecies and the majority of known symbiotic families, we found that symbionts consistently belonged to the well-known haptophyte genus Phaeocystis. The microalga Phaeocystis is one of the most extensively studied taxa of marine phytoplankton, but has never been reported to occur in symbiosis. Free-living Phaeocystis are ubiquitous from poles to tropics and from coastal to open ocean waters (25). They are recognized both as harmful algae and as one of the few keystone phytoplankton genera that shape the structure and functioning of marine ecosystems (26). Phaeocystis not only are major contributors to the global carbon budget (27), but also impact sulfur cycling by producing substantial amounts of dimethylsulfoniopropionate (DMSP) and its volatile catabolite dimethylsulfide (DMS), a climatically active trace gas emitted from the ocean (28). In coastal areas, blooms of Phaeocystis are detrimental to the growth and reproduction of shellfish and zooplankton and strongly impact human activities such as fisheries, aquaculture, and tourism (25).
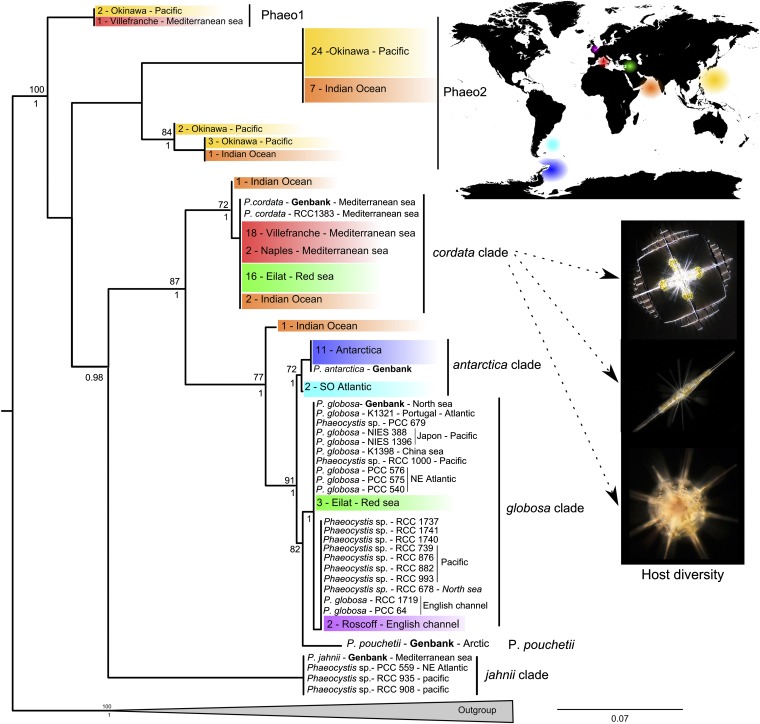
Fig. 1.
Genetic diversity of Phaeocystis found worldwide in symbiosis with Acantharia. The figure shows a RAxML phylogenetic reconstruction of the genus Phaeocystis based on concatenation of the ribosomal 18S and 28S rDNA genes and the plastidial psbA and rbcL genes (131 taxa and 3.1 kb aligned positions). Sequences of Phaeocystis in symbiosis are colored according to their geographic origin, and the number of host specimens examined (representing different species) is indicated. Cultures of free-living Phaeocystis and their corresponding geographic origin are in black. All sequences were produced in this study except for those indicated in boldface type (from GenBank). Bootstrap values ≥60% and Bayesian posterior probability ≥0.7 are given above and beneath the node, respectively. The outgroup contains five sequences from other members of the Haptophyta. See SI Materials and Methods for one-gene phylogenies (Figs. S1–S4).
Multigene phylogenetic analyses including sequences from both symbiotic and cultured free-living Phaeocystis revealed that symbionts belong to different Phaeocystis species (Fig. 1). Most Phaeocystis species known as free-living forms were found in association with Acantharia including species such as Phaeocystis globosa and Phaeocystis antarctica that are known to form massive blooms consisting of colonies of cells maintained in a gelatinous matrix. In contrast to the vast majority of terrestrial and marine symbiotic associations described to date, the genetic footprint of Phaeocystis symbionts was strictly identical or very similar to species that are known to be very abundant in the free-living state. Results from our multigene analysis confirmed previously reported phylogenetic relationships between described Phaeocystis species (29), but also revealed additional putative cryptic species. Notably, two Phaeocystis clades (named “Phaeo1” and “Phaeo2”) contained only sequences retrieved from symbionts or culture-independent environmental surveys in the <5-μm size fraction (Figs. S2 and S3), demonstrating a broader genetic diversity than previously reported for this extensively studied microalgal genus. Different acantharian species from a single location typically live in symbiosis with the same Phaeocystis genotype, whereas the same host species living in distinct habitats can harbor different Phaeocystis genotypes (Fig. 1). For instance, most acantharians from the Antarctic live with the symbiont P. antarctica, whereas those from the Mediterranean Sea and the Red Sea mostly associate with Phaeocystis cordata. The strong influence of symbiont biogeography on the association was confirmed by a Mantel test for each genetic marker (Table S1), which revealed a highly significant correlation between the genetic and geographical distances for symbionts (Pearson’s R > 0.40; P = 0.001), whereas no such correlation was found for hosts (Pearson’s R < 0.03; P > 0.28).
Taxonomically unrelated organisms that interact closely in a symbiotic relationship can mutually influence each other’s evolution and thus exhibit congruent phylogenies. In the case of the Acantharia–Phaeocystis symbiosis, cophylogenetic analyses based on 94 associations showed that symbiont phylogeny does not mirror host phylogeny (Fig. S5). In addition, reconstruction of putative coevolutionary events (such as cospeciation or host switch) onto host phylogeny did not reveal a strong cospeciation pattern (Fig. S6). The absence of a cophylogenetic signal can be attributed to the lack of species-level host specificity in the interaction and to the high level of dispersal of the extensive free-living population of Phaeocystis outside the host. Parallel speciation of symbiotic partners is rare in cases such as this where the symbiont is transmitted horizontally from the environment (Fig. 2C) through host generations (30). In summary, biogeography, rather than strict taxonomic specificity, is the main determinant in this flexible symbiotic relationship.
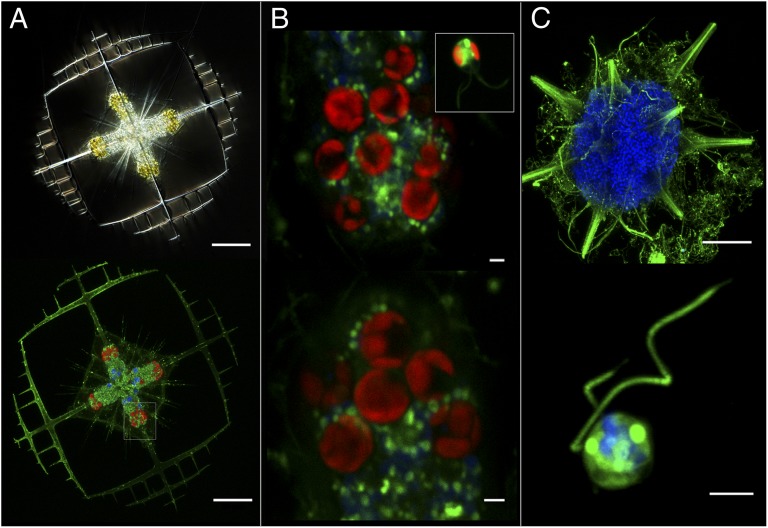
Fig. 2.
Acquisition and maintenance of Phaeocystis in symbiosis. (A) Adult Acantharia harboring golden endosymbiotic Phaeocystis cells in light microscopy (Upper) and red autofluorescence of chlorophyll-containing cells in fluorescence microscopy (Lower). Blue and green staining show the nuclei and membranes, respectively. (Scale bars: 50 μm.) (B) Detail of the phenotypic transformation of the symbiont in hospite: the size and the number of plastids of Phaeocystis cells increase from the free-living (Inset) to the symbiotic form (Upper and Lower). (Scale bars: 2 μm.) (C) The thousands of blue nuclei in the adult Acantharia (Upper) represent individual “swarmers” (detail, Lower) that are released in the environment at the end of the life cycle. The absence of symbionts at this stage indicates that Phaeocystis are acquired de novo at each host generation (horizontal transmission). [Scale bars: 50 μm (Upper) and 2 μm (Lower).] See SI Materials and Methods for details on fluorescence image acquisition.
Evolutionary Origin of the Symbiosis.
We used the exceptional fossil records of Radiolaria and Haptophyta to calibrate Bayesian relaxed molecular clock analyses on both host and symbiont phylogenies, allowing assessment of the evolutionary origin of the Acantharia–Phaeocystis symbiosis. The molecular dating analysis revealed that Phaeocystis originated between 288 and 178 Mya (Fig. 3A) and that the symbiosis, indicated by the emergence of the monophyletic group of symbiotic Acantharia (clades E and F in Fig. 3B), arose once between 175 and 93 Mya. The earliest possible origin of the Acantharia–Phaeocystis symbiosis corresponds to the first diversification that led to extant Phaeocystis species (∼178 Mya). Coemergence has been reported in well-known plant–fungi associations (31) and coral–microalgae symbioses (4), allowing the colonization of terrestrial and reef ecosystems, respectively. Between 190 and 100 Mya, when the Acantharia–Phaeocystis partnership arose, continental weathering (as indicated by the 87Sr/86Sr ratio) was lower than at any point during the last 550 My (Fig. 3C) and markedly lower than at present (32). Oligotrophy was undoubtedly a constant feature in ancient oceanic gyre water masses and the lower continental nutrient flux to the marine systems in the Jurassic may have resulted in generalized oligotrophy in surface waters. We hypothesize that the biological novelty of this symbiosis, resulting from the combination of the metabolic capacities of a heterotroph and an autotroph, was initially selected because it allowed the holobiont to cope with drastically low nutrient settings in transparent open oceans.
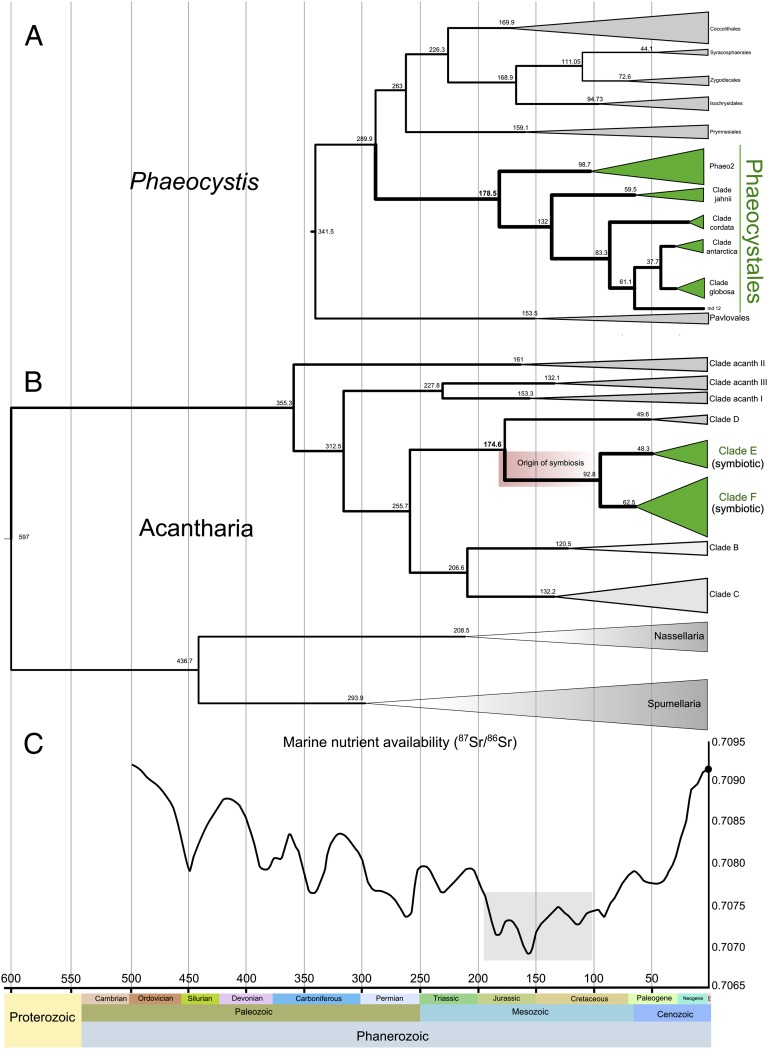
Fig. 3.
Fossil-calibrated molecular clock dating the origin of the Acantharia–Phaeocystis photosymbiosis. (A and B) Chronograms resulting from the Bayesian relaxed molecular clock analysis of haptophytes including Phaeocystis (A) and Acantharia including symbiotic species from clades E and F in green (B). Details of the fossil-based calibrations and the 95% credibility intervals [highest posterior density (HPD)] for the nodes are provided in SI Materials and Methods and in Figs. S7 and S8. (C) Ratio values (87Sr/86Sr) throughout the Phanerozoic eon (550 Mya to present), reflecting nutrient availability in the ocean (modified from ref. 32).
DMSP–Dimethyl Sulfoxide Measurements in the Holobiont.
Symbiotic Acantharia are abundant and widespread in the sunlit oligotrophic waters of modern oceans (18). The ecological success of this photosymbiosis implies the existence of physiological mechanisms to overcome constraints inherent to intracellular photosynthesis in high-irradiance environments. Phaeocystis produces relatively high amounts of DMSP, which can represent up to 10% of total cell carbon (28). To assess the potential role of DMSP, its catabolite DMS, and its oxidation product dimethyl sulfoxide (DMSO) in the symbiosis, we measured total DMSP + DMS (referred to here as DMSPt; SI Materials and Methods) and total DMSO (DMSOt) in cultured free-living P. cordata cells and in field-sampled Acantharia–P. cordata holobionts. In cultured P. cordata, DMSPt concentrations were 12 ± 1 fmol⋅cell−1 (358 ± 28 mM on a cell volume basis), within the range reported for other Phaeocystis species (28). The holobionts had a DMSPt per cell content three orders of magnitude higher (Table 1), yet they harbored only 29 ± 27 symbionts each. If all holobiont DMSPt were contained in the symbionts, it would represent an inconceivable proportion (ca. 300%) of Phaeocystis cell carbon. Thus, the major part of DMSP and DMS must be transferred from the endosymbiont to the host. Furthermore, we found that holobionts have a much larger proportion of DMSO than cultured Phaeocystis, as shown by sixfold lower DMSPt/DMSOt ratios (Table 1). DMSP, DMS, and DMSO have been suggested to serve as antioxidants in phytoplankton cells, with DMS and DMSO having, respectively, 60- and 20-fold greater oxidant scavenging efficiencies than DMSP (33). We therefore suggest that the transfer of these three dimethylated sulfur compounds from the symbiont to the host cell confers antioxidant protection to the holobiont to cope with oxidants produced by photosynthesis and high irradiance. This might also explain the high DMSP concentrations that have been reported in various hosts involved in other photosymbiotic associations, such as corals, anemones, clams, and flatworms (34, 35). In each of these cases, DMSP is produced by symbiotic dinoflagellates that, like haptophytes, are important DMSP producers in their free-living state (28). The overall picture suggests that in UV-transparent oligotrophic waters, like those where the Acantharia–Phaeocystis symbiosis likely originated and is still abundant, antioxidant mechanisms are important factors for photosymbiotic associations.
Table 1.
DMSPt content and DMSPt/DMSOt ratios in field-sampled holobionts and cultured Phaeocystis cells
| DMSPt (pmol⋅cell−1) | DMSPt/DMSOt | |
| Acantharia–P. cordata holobionts | 37.4 ± 10.7 | 5.1 ± 0.9 |
| P. cordata in culture | 0.012 ± 0.001 | 31.6 ± 0.9 |
Holobiont values are the average of four samples prepared from western Mediterranean coastal waters collected on two consecutive days. Each sample contained 50–200 Acantharia individually picked, rinsed, and transferred to filtered seawater. Phaeocystis values are the average of three subsamples from a P. cordata culture containing 2.5 × 10−5 cells⋅ml−1. All samples were analyzed in two to four replicates.
Mutualism or Enslavement?
Whereas the benefits of this association for the acantharian host are manifest, for the symbiont it is not clear where the cursor lies along the gradient of symbiotic interactions from mutualism to parasitism. To gain benefit from the association over an evolutionary timescale (i.e., one generation or more), Phaeocystis cells would need to be released to the environment in a viable condition following the symbiotic phase. If this is the case, the symbiosis likely plays a key role in the ecology of Phaeocystis, for example by protecting cells from grazing or viral attack (36) and/or by providing inocula for establishment of free-living populations. The present-day ecological success of the microalga Phaeocystis may thus partly result from this ancient and persistent symbiosis with Acantharia. Alternatively, the association may be an ecological (and hence evolutionary) cul-de-sac for the symbionts. To our knowledge, attempts to isolate the symbionts of Acantharia into culture (using mechanical or chemical disruption of the host cell or natural disruption during gametogenesis) have never been successful, despite the relative ease with which free-living Phaeocystis can be cultured. Within the host, the phenotype of Phaeocystis symbionts can be extensively modified. The cell volume of symbionts can increase massively (up to 10-fold compared with the free-living form), with formation of large vacuoles, several mitochondria, and numerous large plastids (Fig. 2B and ref. 24). This suggests that the host is able to exert a high degree of control over the symbiont to block cytokinesis and maximize photosynthetic capacity. Contrary to other photosymbioses where viable symbionts can be released (37–39), Acantharia may thus enslave and exploit Phaeocystis cells over a period before either digesting them or shedding nonviable cells. Sequestration of prey organelles, such as plastids (kleptoplastidy) and nuclei (karyoklepty), is well documented (11, 40), including a report that a heterotrophic dinoflagellate from Antarctic waters is capable of temporarily retaining functional Phaeocystis plastids (41). Acquisition of phototrophy in Acantharia seems rather to occur by irreversible sequestration of whole cells (“cytoklepty”), a rare biological phenomenon that has been described for the ciliate Mesodinium rubrum and the sand-dwelling flagellate Hatena arenicola that maintain cells of a cryptophyte (42) and a prasinophyte (43), respectively. The observation that the host Hatena can transfer the phenotypically modified symbiont to a daughter cell during cell division led to the suggestion that the interaction is representative of an intermediate evolutionary step in plastid acquisition via secondary endosymbiosis. Vertical transmission of symbionts is presumably impossible for Acantharia which produce reproductive cells (swarmers) that are significantly smaller than Phaeocystis cells (Fig. 2). The Acantharia–Phaeocystis association could nevertheless provide an alternative model to kleptoplastidy for investigating the genomic and physiological mechanisms involved in the early stages of permanent plastid acquisition.
Concluding Remarks
Our study reveals a highly original mode of symbiosis between unicellular planktonic eukaryotes whereby the symbiont is an ecologically prominent component of the ecosystem not only in the symbiotic association, but also in its free-living phase. This association is also noteworthy in that it involves a harmful and bloom-forming microalga. In contrast, the bacterial or fungal symbionts within insects and plants and the microalgal symbionts of corals and other benthic marine organisms are typically very rare in their free-living phase (44), in each case having first been discovered through the association with their host before being found as independent entities in the environment. Establishment of obligate photosymbiotic relationships that rely on horizontal transmission must be challenging in the vast and barren oceanic realm, making this a potentially risky strategy in evolutionary terms for the host. Acantharia an important group of protistan zooplankton, have overcome the problem by developing a flexible association with one of the most abundant eukaryotic phytoplankton taxa in the marine environment. Reports of abundant photosynthetic prokaryotes, including the cyanobacteria Synechococcus and Prochlorococcus, forming symbiotic associations with protists in open oceans (45, 46), indicate that this mode of symbiosis may be well suited for the planktonic realm. This discovery provides further evidence of the remarkably high level of ecological and metabolic interdependency of planktonic organisms and participates in increasing awareness of the significance of symbiosis in structuring a key compartment of the biosphere, the oceanic ecosystem (47).
Materials and Methods
Symbiotic Acantharia were collected at different locations worldwide and individually isolated using a micropipette (Table S1). DNA extraction from single cells was conducted as described previously (23). Partial 18S and 28S rDNA genes from host acantharians were amplified using Radiolaria-specific primers (23). In parallel, 23 strains of Phaeocystis sp. from different culture collections were harvested in exponential growth phase and concentrated by centrifugation. DNA was extracted using the Nucleospin RNA II kit. The 18S and 28S rDNA genes and the plastidial rbcL, and psbA genes from symbiotic and cultured Phaeocystis were PCR amplified. Molecular datasets were aligned with MAFFT v6.818 (48) and the optimal model of evolution was determined with jModelTest (49). Phylogenetic relationships were reconstructed with RAxML (50), and Bayesian inference was conducted using Beast v.1.6.1 and companion software (51). The phylogenetic trees of host (Acantharia and symbiont (Phaeocystis) taxa (each symbiont is associated with one host) were used to perform cophylogenetic analyses. We used an event-based method, Jane 3 (52), and a global fit method, ParaFit (53), implemented in CopyCat (54). Divergence times of Acantharia and Phaeocystis were estimated using Bayesian relaxed-clock methods implemented in BEAST v.1.6.1. BEAUti v.1.6.1 was used to define parameters such as the fossil-based calibrations. To allow some uncertainty, most constraints were assigned a normal prior distribution with means corresponding to the fossil date or the first occurrence of a character and a relatively broad SD encompassing the minimum and maximum age of this calibration. Molecular clock parameters are detailed in SI Materials and Methods.
Aliquots of an exponentially growing P. cordata culture and 50–200 acantharian cells were preserved for DMSP and DMSO measurements. DMSP was measured as the DMS evolved by alkaline hydrolysis, using purge and trap gas chromatography coupled to flame photometric detection (GC-FPD). DMSO was analyzed as DMS, using the cobalt-doped borohydride (NaBH4) reduction method (55). Detailed methods are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank S. Romac, E. Boudoux, Y. Souab, and M. J. Garet-Delmas for technical assistance and N. Suzuki for morphological identification of Acantharia. We thank the institutes and funding agencies that supported the collection of samples: the Laboratoire d'Océanographie de Villefranche-sur-Mer, the InterUniversity Institute for Marine Sciences in Eilat (Association of European Marine Biological Laboratories-EU FP7 I3 project 227799), the Stazione Zoologica Anton Dohrn in Naples, the Akajima Marine Science Laboratory (Centre National de la Recherche Scientifique–Japan Science and Technology Agency program), the Station Biologique de Roscoff, and the TaraOceans expedition. This research was supported by the Region Bretagne (Diversité de la Photosymbiose Pélagique DIPHOPE 044763), the French Agence Nationale de la Recherche 09-BLAN-0348 POSEIDON, and the French “Investissements d’Avenir” projects OCEANOMICS and European Marine Biological Resource Centre-France.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. JQ697697–JQ6977738 and JX660702–JX660995).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1212303109/-/DCSupplemental.
References
- 1.Margulis L, Fester R. Symbiosis as a Source of Evolutionary Innovation: Speciation and Morphogenesis. Cambridge, MA: MIT Press; 1991. [PubMed] [Google Scholar]
- 2.Muscatine L, Falkowski PG, Porter JW, Dubinsky Z. Fate of photosynthetic fixed carbon in light- and shade- adapted colonies of the symbiotic coral Stylophora pistillata. Proc R Soc Lond B Biol Sci. 1984;222:181–202. [Google Scholar]
- 3.Yellowlees D, Rees TAV, Leggat W. Metabolic interactions between algal symbionts and invertebrate hosts. Plant Cell Environ. 2008;31(5):679–694. doi: 10.1111/j.1365-3040.2008.01802.x. [DOI] [PubMed] [Google Scholar]
- 4.Stanley GD., Jr Ecology, photosymbiosis and the evolution of modern coral reefs. Science. 2006;312(5775):857–858. doi: 10.1126/science.1123701. [DOI] [PubMed] [Google Scholar]
- 5.Carlos A, Baillie B, Kawachi M, Maruyama T. Phylogenetic position of Symbiodinium (Dinophyceae) isolates from tridacnids (Bivalvia), cardiids (Bivalvia), a sponge (Porifera), a soft coral (Anthozoa), and a free-living strain. J Phycol. 1999;35:1054–1062. [Google Scholar]
- 6.LaJeunesse T. Investigating the biodiversity, ecology, and phylogeny of endosymbiotic dinoflagellates in the genus Symbiodinium using the ITS region: In search of a ‘‘species’’ level marker. J Phycol. 2001;37:866–880. [Google Scholar]
- 7.Pochon X, Pawlowski J, Zaninetti L, Rowan R. High genetic diversity and relative specificity among Symbiodinium-like endosymbiotic dinoflagellates in soritid foraminiferans. Mar Biol. 2001;139:1069–1078. [Google Scholar]
- 8.Haeckel E. 1887. Report on the Radiolaria collected by H.M.S. Challenger. Zoology 18:1—1803. Available at http://caliban.mpiz-koeln.mpg.de/haeckel/challenger/. Accessed October 4, 2012.
- 9.Coffroth MA, Lewis CF, Santos SR, Weaver JL. Environmental populations of symbiotic dinoflagellates in the genus Symbiodinium can initiate symbioses with reef cnidarians. Curr Biol. 2006;16(23):R985–R987. doi: 10.1016/j.cub.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 10.Littman RA, van Oppen MJH, Willis BL. Methods for sampling free-living Symbiodinium (zooxanthellae) and their distribution and abundance at Lizard Island (Great Barrier Reef) J Exp Mar Biol Ecol. 2008;364:48–53. [Google Scholar]
- 11.Stoecker DK, Johnson MD, de Vargas C, Not F. Acquired phototrophy in aquatic protists. Aquat Microb Ecol. 2009;57:279–310. [Google Scholar]
- 12.Shaked Y, de Vargas C. Pelagic photosymbiosis: rDNA assessment of diversity and evolution of dinoflagellate symbionts and planktonic foraminiferal hosts. Mar Ecol Prog Ser. 2006;325:59–71. [Google Scholar]
- 13.Gast RJ, Caron DA. Molecular phylogeny of symbiotic dinoflagellates from planktonic foraminifera and radiolaria. Mol Biol Evol. 1996;13(9):1192–1197. doi: 10.1093/oxfordjournals.molbev.a025684. [DOI] [PubMed] [Google Scholar]
- 14.Gast RJ, Caron DA. Photosymbiotic associations in planktonic foraminifera and radiolaria. Hydrobiologia. 2001;461:1–7. [Google Scholar]
- 15.Anderson OR. The radiolarian symbiosis. In: Goff L, editor. Algal Symbiosis: A Continuum of Interaction Strategies. Cambridge, UK: Cambridge Univ Press; 1983a. pp. 69–89. [Google Scholar]
- 16.Falkowski PG, et al. The evolution of modern eukaryotic phytoplankton. Science. 2004;305(5682):354–360. doi: 10.1126/science.1095964. [DOI] [PubMed] [Google Scholar]
- 17.Zas’ko DN, Vedernikov VI. A comparative analysis of the vertical distribution of epipelagic radiolaria, chlorophyll, and zooplankton in different areas of the North Atlantic Ocean (from June to September 2001) Oceanology. 2003;43:63–71. [Google Scholar]
- 18.Michaels AF, Caron DA, Swanberg NR, Howse FA, Michaels CM. Planktonic sarcodines (acantharia, radiolaria, foraminifera) in surface waters near Bermuda: Abundance, biomass and vertical flux. J Plankton Res. 1995;17:131–163. [Google Scholar]
- 19.Swanberg NR, Caron DA. Patterns of feeding in epipelagic oceanic plankton. J Plankton Res. 1991;13:287–312. [Google Scholar]
- 20.Michaels AF. Vertical distribution and abundance of Acantharia and their symbionts. Mar Biol. 1988;97:559–569. [Google Scholar]
- 21.Schewiakoff WT. The Acantharia. Fauna e Flora del Golfo di Napoli. 1926;37:1–755. [Google Scholar]
- 22.Taylor FJR. Symbioses in marine microplankton. Ann Inst Oceanogr Paris. 1982;58(S):61–90. [Google Scholar]
- 23.Decelle J, Suzuki N, Mahé F, de Vargas C, Not F. Molecular phylogeny and morphological evolution of the Acantharia (Radiolaria) Protist. 2012;163(3):435–450. doi: 10.1016/j.protis.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Febvre J, Febvre-Chevalier C. Ultrastructural study of zooxanthellae of 3 species of Acantharia (Protozoa: Actinopoda), with details of their taxonomic position in the Prymnesiales (Prymnesiophyceae Hibberd, 1976) J Mar Biol Assoc U K. 1979;59:215–226. [Google Scholar]
- 25.Schoemann V, Becquevort S, Stefels J, Rousseau V, Lancelot C. Phaeocystis blooms in the global ocean and their controlling mechanisms: A review. J Sea Res. 2005;53:43–66. [Google Scholar]
- 26.Verity PG, Smetacek V. Organism life cycles, predation, and the structure of marine pelagic ecosystems. Mar Ecol Prog Ser. 1996;30:277–293. [Google Scholar]
- 27.Arrigo KR, et al. Phytoplankton community structure and the drawdown of nutrients and CO2 in the southern ocean. Science. 1999;283(5400):365–367. doi: 10.1126/science.283.5400.365. [DOI] [PubMed] [Google Scholar]
- 28.Stefels J, Steinke M, Turner S, Malin G, Belviso S. Environmental constraints on the production and removal of the climatically active gas dimethulsulphide (DMS) and implications for ecosystem modelling. Biogeochemistry. 2007;83:245–275. [Google Scholar]
- 29.Medlin L, Zingone A. A taxonomic review of the genus Phaeocystis. Biogeochemistry. 2007;83:3–18. [Google Scholar]
- 30.Sachs JL, Essenberg CJ, Turcotte MMM. New paradigms for the evolution of beneficial infections. Trends Ecol Evol. 2011;26(4):202–209. doi: 10.1016/j.tree.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Heckman DS, et al. Molecular evidence for the early colonization of land by fungi and plants. Science. 2001;293(5532):1129–1133. doi: 10.1126/science.1061457. [DOI] [PubMed] [Google Scholar]
- 32.Cardenas AL, Harries PJ. Effect of nutrient availability on marine origination rates throughout the Phanerozoic eon. Nat Geosci. 2010;3:430–434. [Google Scholar]
- 33.Sunda W, Kieber DJ, Kiene RP, Huntsman S. An antioxidant function for DMSP and DMS in marine algae. Nature. 2002;418(6895):317–320. doi: 10.1038/nature00851. [DOI] [PubMed] [Google Scholar]
- 34.Yost DM, Mitchelmore CL. Dimethylsulfoniopropionate (DMSP) lyase activity in different strains of the symbiotic alga Symbiodinium microadriaticum. Mar Ecol Prog Ser. 2009;386:61–70. [Google Scholar]
- 35.Van Bergeijk SA, Stal LJ. Dimethylsulfoniopropionate and dimethylsulfide in the marine flatworm Convoluta roscoffensis and its algal symbiont. Mar Biol. 2001;138:209–216. [Google Scholar]
- 36.Kodama Y, Fujishima M. 2009. Infection of Paramecium bursaria by symbiotic Chlorella species. Endosymbionts in Paramecium, Microbiology Monographs 12, ed Fujishima M (Springer-Verlag, Berlin and Heidelberg), pp 31–55. [Google Scholar]
- 37.Fishman Y, Zlotkin E, Sher D. Expulsion of symbiotic algae during feeding by the green hydra—A mechanism for regulating symbiont density? PLoS ONE. 2008;3(7):e2603. doi: 10.1371/journal.pone.0002603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gates RD, Baghdasarian G, Muscatine L. Temperature stress causes host cell detachment in symbiotic cnidarians: Implications for coral bleaching. Biol Bull. 1992;182:324–332. doi: 10.2307/1542252. [DOI] [PubMed] [Google Scholar]
- 39.Castro-Sanguino C, Sánchez JA. Dispersal of Symbiodinium by the stoplight parrotfish Sparisoma viride. Biol Lett. 2012;8(2):282–286. doi: 10.1098/rsbl.2011.0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson MD, Oldach D, Delwiche CF, Stoecker DK. Retention of transcriptionally active cryptophyte nuclei by the ciliate Myrionecta rubra. Nature. 2007;445(7126):426–428. doi: 10.1038/nature05496. [DOI] [PubMed] [Google Scholar]
- 41.Gast RJ, Moran DM, Dennett MR, Caron DA. Kleptoplasty in an Antarctic dinoflagellate: Caught in evolutionary transition? Environ Microbiol. 2007;9(1):39–45. doi: 10.1111/j.1462-2920.2006.01109.x. [DOI] [PubMed] [Google Scholar]
- 42.Hansen PJ, Fenchel T. The bloom-forming ciliate Mesodinium rubrum harbours a single permanent endosymbiont. Mar Biol Res. 2006;2:169–177. [Google Scholar]
- 43.Okamoto N, Inouye I. A secondary symbiosis in progress? Science. 2005;310(5746):287. doi: 10.1126/science.1116125. [DOI] [PubMed] [Google Scholar]
- 44.Nyholm SV, McFall-Ngai MJ. The winnowing: Establishing the squid-vibrio symbiosis. Nat Rev Microbiol. 2004;2(8):632–642. doi: 10.1038/nrmicro957. [DOI] [PubMed] [Google Scholar]
- 45.Foster RA, Carpenter EJ, Bergman B. Unicellular cyanobionts in open ocean dinoflagellates, radiolarians, and tintinnids: Ultrastructural characterization and immuno-localization of phycoerythrin and nitrogenase. J Phycol. 2006;42:453–463. [Google Scholar]
- 46.Foster RA, Collier JL, Carpenter EJ. Reverse transcription PCR amplification of cyanobacterial symbiont 16S rRNA sequences from single non-photosynthetic eukaryotic marine planktonic host cells. J Phycol. 2006;42:243–250. [Google Scholar]
- 47.Strom SL. Microbial ecology of ocean biogeochemistry: A community perspective. Science. 2008;320(5879):1043–1045. doi: 10.1126/science.1153527. [DOI] [PubMed] [Google Scholar]
- 48.Katoh K, Toh H. Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform. 2008;9(4):286–298. doi: 10.1093/bib/bbn013. [DOI] [PubMed] [Google Scholar]
- 49.Posada D. jModelTest: Phylogenetic model averaging. Mol Biol Evol. 2008;25(7):1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- 50.Stamatakis A, Hoover P, Rougemont J. A rapid boostrap algorithm for the RAxML Web-Servers. Syst Biol. 2008;75(5):758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- 51.Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conow C, Fielder D, Ovadia Y, Libeskind-Hadas R. Jane: A new tool for the cophylogeny reconstruction problem. Algorithms Mol Biol. 2010;5:16. doi: 10.1186/1748-7188-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Legendre P, Desdevises Y, Bazin E. A statistical test for host-parasite coevolution. Syst Biol. 2002;51(2):217–234. doi: 10.1080/10635150252899734. [DOI] [PubMed] [Google Scholar]
- 54.Meier-Kolthoff JP, Auch AF, Huson DH, Göker M. COPYCAT: Cophylogenetic analysis tool. Bioinformatics. 2007;23(7):898–900. doi: 10.1093/bioinformatics/btm027. [DOI] [PubMed] [Google Scholar]
- 55.Simó R, Vila-Costa M. Ubiquity of algal dimethylsulfoxide in the surface ocean: Geographic and temporal distribution patterns. Mar Chem. 2006;100:136–146. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.