Abstract
The world’s coral reefs are being degraded, and the need to reduce local pressures to offset the effects of increasing global pressures is now widely recognized. This study investigates the spatial and temporal dynamics of coral cover, identifies the main drivers of coral mortality, and quantifies the rates of potential recovery of the Great Barrier Reef. Based on the world’s most extensive time series data on reef condition (2,258 surveys of 214 reefs over 1985–2012), we show a major decline in coral cover from 28.0% to 13.8% (0.53% y−1), a loss of 50.7% of initial coral cover. Tropical cyclones, coral predation by crown-of-thorns starfish (COTS), and coral bleaching accounted for 48%, 42%, and 10% of the respective estimated losses, amounting to 3.38% y−1 mortality rate. Importantly, the relatively pristine northern region showed no overall decline. The estimated rate of increase in coral cover in the absence of cyclones, COTS, and bleaching was 2.85% y−1, demonstrating substantial capacity for recovery of reefs. In the absence of COTS, coral cover would increase at 0.89% y−1, despite ongoing losses due to cyclones and bleaching. Thus, reducing COTS populations, by improving water quality and developing alternative control measures, could prevent further coral decline and improve the outlook for the Great Barrier Reef. Such strategies can, however, only be successful if climatic conditions are stabilized, as losses due to bleaching and cyclones will otherwise increase.
Keywords: climate change, disturbance, anthropogenic risk, world heritage, reef management
There is increasing concern about the progressive degradation of the world’s coral reefs (1–3). Major anthropogenic risk factors include mortality and reduced growth of the reef-building corals due to their high sensitivity to rising seawater temperatures, ocean acidification, water pollution from terrestrial runoff and dredging, destructive fishing, overfishing, and coastal development (4). These anthropogenic risks interact with other large-scale acute disturbances, especially tropical storms and population outbreaks of the coral-eating crown-of-thorns starfish (COTS) Acanthaster planci, which may also increase in frequency and intensity in response to human activities (5, 6).
Regional policies cannot protect coral reefs from global-scale risks due to climate change-associated heat stress and intensifying tropical storms. Efforts are therefore shifting toward management of local and regional anthropogenic pressures to strengthen reef resilience (7–9). However, assessment of the likely effectiveness of reductions of local anthropogenic pressures requires a sound understanding of the processes that determine the ecosystem trajectories.
The Great Barrier Reef (GBR) represents a particularly relevant case study to investigate ecosystem trajectories and potential mitigation, because it is the world’s largest coral reef ecosystem, containing ∼3,000 individual coral reefs within an area of 345,000 km2. Its outstanding universal values were recognized by World Heritage listing in 1981. GBR reefs have been classified as the world’s least threatened coral reefs (4) due to their distance from the relatively small human population centers and strong legal protection (10, 11). Local anthropogenic disturbances (e.g., destructive fishing, industrial and urban pollution, tourism overuse, anchor damage, vessel groundings, oil spills) have had minor adverse effects on the GBR to date. Fishing, although intense near the coast and urban centers, is banned in 33% of the GBR and is regulated elsewhere (11). Nonetheless, the GBR has been subject to severe disturbances, including COTS outbreaks, mass coral bleaching and declining growth rates of coral due to increasing seawater temperatures, terrestrial runoff, tropical cyclones, and coral diseases (2, 3, 12–14). The runoff of soils, fertilizers, and pesticides from agricultural and coastal development has significantly affected inshore coral reefs (12, 15–17), and has likely increased COTS outbreak frequencies (5, 18). Conclusions of scientific studies on the condition of the GBR, based on different datasets and various time periods, have ranged from evidence for fluctuations from localized disturbances (13, 14) to ecosystem-wide declines (1, 2).
The objectives of this study were threefold: (i) to investigate spatial patterns and temporal dynamics of coral cover for the whole GBR; (ii) to identify the main causes of coral mortality by combining field estimates of coral cover with observed and modeled environmental data; and (iii) to assess the capacity of reefs to recover in the absence of various disturbances and to estimate future coral cover, given that levels of disturbance remain similar to those of 1985–2012. The study is based on 2,258 reef surveys from 214 different reefs over 27 y (Fig. 1A) by the Australian Institute of Marine Science (AIMS) Long-Term Monitoring Program using a standardized manta-tow sampling protocol (19). Estimated trends and forecasts of coral cover were made for the whole GBR and separately for three subregions, namely: (i) the remote northern region (11.9–15.4°S), which is sparsely inhabited and only lightly altered by human activities; (ii) the central region (15.4–20.0°S), which has more intense agriculture and grazing, as well as a progressively developed coastline; and (iii) the southern region (20.0–23.9°S), where inshore reefs are under pressure from coastal development and agricultural runoff but offshore reefs receive protection due to their greater distance from the coast (Fig. 1A). This regionalization helped identify different reef trajectories and effects of disturbances along the >2,000-km-long GBR.
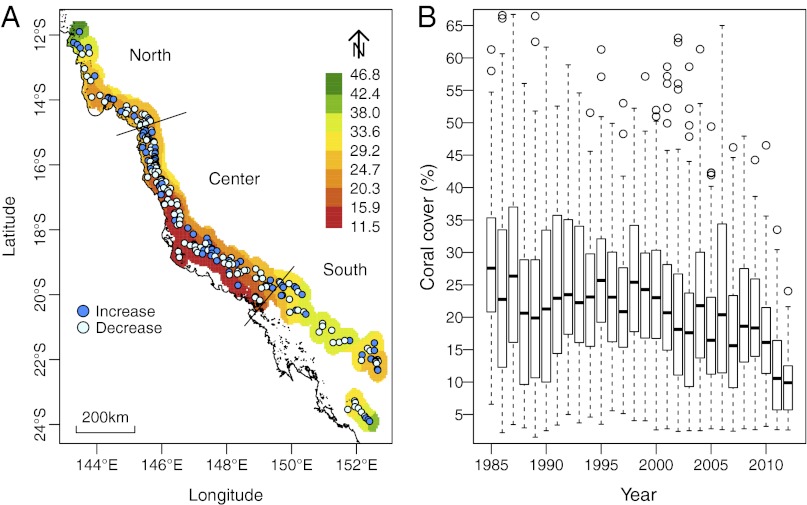
Fig. 1.
Coral cover on the GBR. (A) Map of the GBR with color shading indicating mean coral cover averaged over 1985–2012. Points show the locations of the 214 survey reefs in the northern, central, and southern regions, and their color indicates the direction of change in cover over time. (B) Box plots indicate the percentiles (25%, 50%, and 75%) of the coral cover distributions within each year and suggest a substantial decline in coral cover over the 27 y.
Results
Coral cover averaged 22.9% over the 214 reefs and 27 y, and spatial variation was strong, with the highest values in the far northern (>35%) and southern (>30%) GBR and the lowest values in central inshore reefs (<20%) (Fig. 1A). The cover on individual reefs ranged from 1.50 to 79.7% across space and time (Fig. 1B).
Coral cover data were analyzed using logistic regression models. All models included random effects of reefs and a continuous autoregressive structure over time for each reef. The first analyses consisted of a purely temporal model comprising a smoothed trend for the whole GBR and for each region separately. For the whole GBR, this showed that from 1985 to 2012, mean coral cover declined nonlinearly from 28.0% [95% confidence interval (CI) = (26.6, 29.4)] to 13.8% (95% CI = 12.4, 15.3) (Fig. 2A), a total decline of 14.2% (0.53% y−1). This is equivalent to a loss of 50.7% of the initial cover. Two-thirds of that decline has occurred since 1998, the current rate of decline is 1.51% y−1, and from 2006 to 2012, the rate of decline has consistently been >1.4% y−1 (Fig. 2A). Fitting similar models to the three regions showed that temporal trends varied among them (Fig. 2 B–D), with consistent cover of ∼24% in the north, a nonlinear decline from 26.4 to 14.1% in the center, and a recent severe decline from 37.4 to 8.2% in the south. Overall, cover increased on 32.2% and declined on 67.8% of the 214 reefs (Fig. 1A).
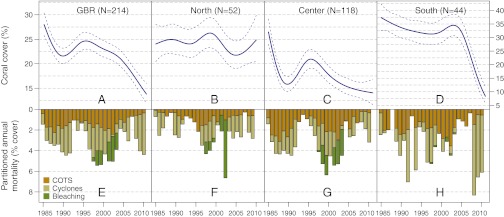
Fig. 2.
Temporal trends in coral cover (A–D) and annual mortality due to COTS, cyclones, and bleaching (E–H) for the whole GBR and the northern, central, and southern regions over the period 1985–2012 (N, number of reefs). (A–D) Trends in coral cover, with blue lines indicating estimated means (±2 SEs) of each trend. (E–H) Composite bars indicate the estimated mean coral mortality for each year, and the sub-bars indicate the relative mortality due to COTS, cyclones, and bleaching. The periods of decline of coral cover in A–D reflect the high losses shown in E–H.
The effects of three main forms of acute disturbances, namely, observed COTS densities, modeled maximum wind speeds of 34 tropical cyclones, and mass coral bleaching in 1998 and 2002, were estimated by adding them to the temporal logistic model. These analyses were conducted for the whole GBR and for each region separately (Fig. 1). Disturbances due to COTS, cyclones, and bleaching occurred frequently from 1985 to 2012, with only 3 of the 214 reefs remaining impact-free. COTS were observed on 31.8% of reef visits, cyclones had affected reefs in the 18-mo window before 46.0% of visits, and the two mass bleaching events had affected reefs in the 2-y window before 9.2% of visits. For the GBR as a whole, there were cyclical effects due to COTS but no evidence of increasing levels of mortality from disturbance across years (Fig. 2E). The presence of COTS at the active outbreak density of one COTS per 200 m of manta tow gave an estimated coral mortality of 5.48% y−1 (SE = 0.66%) for a reef with 20% coral cover. Cyclonic winds of 40 ms−1 resulted in a mean mortality of 7.36% (SE = 0.78%) cover, and bleaching led to a mean mortality of 3.11% (SE = 0.55%) cover at 20% coral cover.
The estimated coral cover profiles strongly reflected the patterns of disturbance over time, both overall and for each region (Fig. 2 A–H). The remote northern region had relatively low mortality from COTS and cyclones, and cover was stable with the exception of a slight decline due to bleaching from 1998 to 2003. In the central region, mortality was high for most years, except for a low-disturbance period in the early 1990s, during which reefs showed strong recovery. The southern region also had substantial mortality due to COTS and experienced the greatest impacts from cyclones, especially in the period 2009–2012. Losses from bleaching were negligible in this region.
The mean annual reef mortality was estimated for each of the three forms of disturbance (Fig. 2 E–H and Table 1) for 1985–2011, because the 2012 disturbance data were incomplete. For the whole GBR, COTS, cyclones, and bleaching accounted for mortality rates of 1.42%, 1.62%, and 0.34% y−1 (42, 48, and 10%), respectively, giving a mean total mortality of 3.38% y−1. Given the estimated rate of decline of 0.53% y−1 for 1985–2012, the estimated net growth of coral cover was 2.85% y−1 for coral cover of 20%, and indicates the potential for recovery, given that disturbances can be reduced. This estimate can be interpreted as a lower bound of the growth of coral cover because this rate of decline does not take into account any losses due to other agents (e.g., reduced calcification due to thermal stress and ocean acidification, diseases).
Table 1.
Estimated rates (% y−1) and SEs of (i) decline, growth, and total mortality of coral cover and (ii) total coral mortality partitioned between COTS, cyclones, and bleaching
| GBR | North | Center | South | ||
| i | Decline | 0.53 (0.08) | 0.11 (0.14) | 0.44 (0.08) | 1.04 (0.16) |
| Growth | 2.85 (0.26) | 2.07 (0.44) | 2.78 (0.26) | 2.34 (0.52) | |
| Total mortality | 3.38 (0.19) | 2.18 (0.35) | 3.22 (0.18) | 3.38 (0.44) | |
| ii | COTS mortality | 1.42 (0.17) | 0.77 (0.25) | 1.54 (0.24) | 1.59 (0.27) |
| Cyclone mortality | 1.62 (0.22) | 1.05 (0.23) | 1.29 (0.14) | 1.75 (0.32) | |
| Bleaching mortality | 0.34 (0.08) | 0.36 (0.13) | 0.39 (0.09) | 0.04 (0.11) |
All rates are based on 20% coral cover and are averaged over 1985–2011. Results are presented for the whole GBR and for the northern, central, and southern regions.
The observed coral cover profiles (Fig. 2 A–D) and estimates of growth and mortality due to the three forms of disturbance (Table 1) enable us to infer future trends in coral cover. For example, if mean coral cover of the GBR continues to decline from the current 13.8% at its mean rate of 0.53% y−1 for 1985–2012, cover will be 10.0% (SE = 1.7%) by 2022. This assumption may be overoptimistic, however, because the rate of decline from 2006 to 2012 has consistently been substantially higher at ∼1.45% y−1 (Fig. 2A); based on that rate, estimated coral cover would be only 5.1% (SE = 1.2%) by 2022. For the northern, central, and southern regions, the mean rates of coral cover decline are −0.19% (i.e., an increase), 0.47%, and 1.12% y−1, respectively, and by 2022, estimated coral cover would be 24.5% (SE = 3.1%), 10.7% (SE = 2.1%), and 0.04% (SE = 0.02%). The last of these estimates is clearly unreliable due to the influence of the unusually extreme cyclone activity in the past 3 y.
The rates of coral growth, mortality, and disturbances (Table 1) can also be used to assess the likely effects of intervention to restore coral cover and changes in coral cover due to changes in patterns of disturbance. For example, in the absence of COTS, the mean coral cover decline of 0.53% y−1 would become an increase of 0.89% y−1, and in the absence of cyclones, it would become an increase of 1.09% y−1. Projecting these recoveries to 2022 gives estimated mean coral cover of 22.8% (SE = 2.4%) and 25.3% (SE = 2.9%), representing increases of >50% relative to current coral cover. However, if coral cover declines at the 2006–2012 rate of 1.45% y−1, in the absence of COTS and cyclones, estimated coral cover in 2022 would be 14.0% (SE = 1.8%) and 15.7% (SE = 2.2%), respectively, representing negligible recoveries of 0.2% and 1.9%.
Discussion
This study has shown a major decline in hard coral cover from 28.0 to 13.8% (0.53% y−1) over 27 y, based on data derived from a single program of methodologically consistent surveys. This loss of over half of initial cover is of great concern, signifying habitat loss for the tens of thousands of species associated with tropical coral reefs. The rate of decline has also increased substantially, and has averaged ∼1.45% y−1 since ∼2006. Both the overall and more recent rates of decline are higher than previous estimates (13, 14), which were either based on time series that ended in 2005 (14) or covered a shorter period (1995–2009) and surveyed far fewer reefs using a different survey method (13). The disturbance data for COTS or cyclones show periodic and random fluctuations but no systematic long-term variation over the 27-y observation period, and given that GBR coral cover was likely higher than 28% before 1985 (2), the decline in coral cover may have started long before then.
This study suggests the GBR is on a trajectory similar to that of reefs in the Caribbean, where coral cover has declined by ∼1.4% y−1 (compare with 1.51% y−1 for the GBR current rate of decline) from ∼55% in 1977 to ∼10% today (20, 21). Importantly, however, the processes leading to decline differ for the two systems. Caribbean reefs do not have COTS or other similarly effective coral predators. In contrast, the rapid decline in coral cover in the Caribbean has been attributed to a combination of coral diseases and storms, together with a phase shift from coral to algal dominance due to the loss of all major groups of herbivores from overexploitation, diseases, and possibly elevated nutrient runoff (20–22). Such a prominent role for coral disease has not been observed on the GBR to date (13); neither are there indications for a phase shift to algal dominance, because macroalgal dominance is restricted to nutrient-enriched inshore areas and herbivorous fishes face insignificant fishing pressure (12, 23).
One commonality between both systems is that disturbances, especially from tropical storms, are a major driver of coral cover, and more acute disturbances affect reefs today compared with 50–100 y ago. Cyclone intensities are increasing with warming ocean temperatures, although projected increases are greater for the Northern Hemisphere than for the Southern Hemisphere (6). The recent frequency and intensity of mass coral bleaching are of major concern, and are directly attributable to rising atmospheric greenhouse gases (3). To date, the GBR has lost fewer corals to bleaching and diseases than many other regions in the world (13, 24), but bleaching mortality will almost certainly increase in the GBR, given the upward trend in temperatures (25).
Water quality is a key environmental driver for the GBR. Central and southern rivers now carry five- to ninefold higher nutrient and sediment loads from cleared, fertilized, and urbanized catchments into the GBR compared with pre-European settlement (16). Global warming is also increasing rainfall variability (26), resulting in more frequent intense drought-breaking floods that carry particularly high nutrient and sediment loads (16, 18). River runoff of nutrients and sediments directly affects about 15% of reefs (12, 16). On these reefs, coral cover does not directly depend on water quality (17); however, reefs exposed to poor water clarity and elevated nutrient concentrations show significant increases in macroalgal cover and reduced coral species richness and recruitment (12, 17). There is also strong evidence that water quality affects the frequency of COTS outbreaks in the central and southern GBR (5, 18). Survival of the plankton-feeding larvae of COTS is high in nutrient-enriched flood waters, whereas few larvae complete their development in seawater with low phytoplankton concentrations. Models have shown that the frequency of COTS outbreaks on the GBR has likely increased from one in 50–80 y before European agricultural nutrient runoff, to the currently observed frequency of one in ∼15 y (5).
Coral cover depends not only on mortality from acute disturbances but on rates of growth. Rates of coral calcification on the GBR and many other reef systems around the world have declined by 15–20% since ∼1990 due to increasing thermal stress (27, 28). With our conservative estimate for coral cover growth of 2.85% y−1, this translates into a decline in cover of 0.44–0.57% y−1, equivalent to 29–38% of the current coral cover decline of 1.51% y−1. Due to other causes of coral losses, such as disease, that are unaccounted for in our model, true coral cover growth will likely be higher than 2.85%; hence, the estimated losses due to reduced calcification are also likely to be higher than 0.44–0.57%.
Without significant changes to the rates of disturbance and coral growth, coral cover in the central and southern regions of the GBR is likely to decline to 5–10% by 2022. The future of the GBR therefore depends on decisive action. Although world governments continue to debate the need to cap greenhouse gas emissions, reducing the local and regional pressures is one way to strengthen the natural resilience of ecosystems (7, 9). Our analyses show that in the absence of cyclones, COTS, and bleaching, the estimated rate of increase in coral cover is 2.85% y−1, demonstrating substantial capacity for recovery of reefs. In the absence of COTS alone, coral cover could increase by 0.89% y−1 despite ongoing losses due to cyclones and bleaching. Reducing COTS populations by improving water quality and developing alternative control measures could prevent further coral decline and improve the outlook for the GBR in the short term. In the longer term, success of this strategy requires stabilization of global temperatures to prevent additional losses due to bleaching and cyclones. Intervention to control COTS populations has been rejected in the past when their effects on coral cover, and the link of COTS outbreaks to water quality, were less understood. In 2003, Australian governments committed to improving water quality in the GBR Lagoon (15). However, this study shows that more decisive measures to improve water quality are needed, which specifically target COTS larval survival in the high-risk central region where population outbreaks originate. The recent reemergence of COTS outbreaks in that region adds to the urgency to evaluate additional scientific solutions to controlling COTS populations.
In conclusion, coral cover on the GBR is consistently declining, and without intervention, it will likely fall to 5–10% within the next 10 y. Mitigation of global warming and ocean acidification is essential for the future of the GBR. Given that such mitigation is unlikely in the short term, there is a strong case for direct action to reduce COTS populations and further loss of corals. Without intervention, the GBR may lose the biodiversity and ecological integrity for which it was listed as a World Heritage Area.
Materials and Methods
Coral cover and densities of COTS were surveyed around the perimeter of entire reefs with the manta-tow technique (19) by the AIMS Long-Term Monitoring Program between 1985 and 2012. The number of tows per reef varied from 3 to 325. Data were reef-averaged, and reefs with fewer than 5 surveys in the 27-y sampling period were excluded. The final data consisted of 2,258 reef surveys from 214 different reefs, comprehensively covering the GBR.
The maximum wind speed and the number of hours with wind speeds at or exceeding gale force (>17 ms−1) were estimated for each 4-km grid cell within the GBR for each of the 34 tropical cyclones during the 27-y observation period. Meteorological data were provided by the Australian Bureau of Meteorology and by Knapp et al. (29). Surface winds were calculated for each cell as 10-min maximum wind speeds for every hour of each storm. Maximum cyclone winds averaged 32.8 ms−1 (range: 17.9–55.7 ms−1), and the mean duration of exposure to gales was 12.6 h (range: 1–95 h).
Estimates of coral bleaching in 1998 and 2002 were based on aerial surveys conducted on ∼650 reefs along >3,000-km flying paths during the height of each of the two coral mass-bleaching events (30). Nearest neighbor analysis was used to predict whether or not survey reefs that were not covered by the aerial surveys did bleach. Other known bleaching events had few or incomplete records and were not included in this work.
Logistic regression models were used for all analyses. The response for all models was reef-averaged proportional coral cover, p, and all analyses were weighted by the number of tows per reef. In addition to the fixed predictors, random effects of reefs and continuous autoregressive errors were included. The latter better captured the relationships of observations across time within reefs compared with other options, such as random smooth or linear temporal effects for each reef. All model estimates are expressed as percentages of coral cover rather than proportions for ease of interpretation. These estimates involve rates of change of coral cover with covariates, such as time or environmental drivers. For the logistic model, these rates vary as dp/dx ∝ p(1 − p), where x denotes the covariate. Thus, on the observed scale, effect sizes are largest when P = 0.5 and shrink as p → 0 or p → 1. In all cases, effect sizes are estimated at 20% coral cover (close to the overall mean observed coral cover) unless otherwise stated.
The first group of analyses modeled temporal change in coral cover and how that change varied in the northern, central, and southern sections of the GBR. The second group of analyses included the effects of the environmental drivers (COTS, cyclones, and bleaching) in addition to the temporal and spatial effects. For all analyses, the smoothness of temporal trends was estimated using natural splines and generalized cross-validation (31). From the latter analyses, we extracted the environmental effects and then reconstructed temporal change under various scenarios, such as absence of COTS or absence of all environmental drivers. The modeling approach used in this work can thus provide forecasts of the likely effects of management practices, such as COTS control, and/or estimates of likely effects of consequences of future climate change, such as more frequent cyclones or bleaching events.
Two issues were considered before the use of the environmental predictors in the analyses. First, the environmental predictors were measured or generated in different ways. COTS were counted in situ at the same time and place that coral cover was observed. Conversely, cyclone and bleaching data were interpolated from GBR-wide spatial-temporal models, and are thus less likely to represent true conditions at the reefs across space and time. It thus follows that for the same given strength of relationship between response and predictor, these spatially modeled data are more likely to underestimate effect sizes than those based on observed in situ data. Second, the effects of the environmental predictors on coral cover are likely to occur either later than the time of observation (e.g., bleaching) or over a window of time. To optimize prediction, it was necessary to find the best temporal window for each predictor and to integrate these effects across the window. For each series of COTS on each reef, we used both the abundance at the time of observed cover and that from the preceding survey. For the two cyclone measures, maximum speed and duration, as well as for bleaching, the optimum time window over which to average values was found by searching through a limited collection of window widths and times of onset relative to the time of survey. For cyclones, only maximum wind speed was found to be an effective predictor, and it predicted best when based on the 1.5 y preceding the observation of coral cover. For the two bleaching events, the optimum window was 2 y before the coral cover observation. Additionally, predictors were transformed to linearize the relationships between the log-odds of proportional coral cover and the predictors; COTS abundances were fourth root-transformed, and cyclone measures were square root-transformed.
Spatial mapping of estimated data values was used to illustrate the distributions of coral cover and the predictors. Relative distance across and along the GBR was used as a spatial coordinate system rather than longitude and latitude, because the former provide more accurate spatial estimates.
The R statistical software package (32) was used for all data analyses.
Acknowledgments
We thank the AIMS Long-Term Monitoring Program for providing the coral and COTS data, and Ray Berkelmans for the bleaching data. This work was supported by the Australian Institute of Marine Science and the National Environmental Research Program of the Australian Government.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 17734.
References
- 1.Bellwood DR, Hughes TP, Folke C, Nyström M. Confronting the coral reef crisis. Nature. 2004;429:827–833. doi: 10.1038/nature02691. [DOI] [PubMed] [Google Scholar]
- 2.Bruno JF, Selig ER. Regional decline of coral cover in the Indo-Pacific: Timing, extent, and subregional comparisons. PLoS ONE. 2007;2:e711. doi: 10.1371/journal.pone.0000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoegh-Guldberg O, et al. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- 4.Burke L, Reytar K, Spalding M, Perry A. Reefs at Risk Revisited. Washington, DC: World Resources Institute; 2011. [Google Scholar]
- 5.Fabricius KE, Okaji K, De’ath G. Three lines of evidence to link outbreaks of the crown-of-thorns seastar Acanthaster planci to the release of larval food limitation. Coral Reefs. 2010;29:593–605. [Google Scholar]
- 6.Knutson T, et al. Tropical cyclones and climate change. Nat Geosci. 2010;3:157–163. [Google Scholar]
- 7.Folke C, Carpenter S, Walker B, Scheffer M, Elmqvist T. Regime shifts, resilience, and biodiversity in ecosystem management. Annu Rev Ecol Evol Syst. 2004;35:557–581. [Google Scholar]
- 8.Hughes TP, Graham NA, Jackson JB, Mumby PJ, Steneck RS. Rising to the challenge of sustaining coral reef resilience. Trends Ecol Evol. 2010;25:633–642. doi: 10.1016/j.tree.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Secretariat of the Convention on Biological Diversity . Strategic Plan for Biodiversity 2011-2020 and the Aichi Targets. Montreal: Secretariat of the Convention on Biological Diversity; 2010. [Google Scholar]
- 10.Commonwealth of Australia . Great Barrier Reef Marine Park Act. Canberra, Australia: Commonwealth Consolidated Acts; 1975. [Google Scholar]
- 11.Commonwealth of Australia . Great Barrier Reef Marine Park Zoning Plan. Townsville, Australia: Great Barrier Reef Marine Park Authority; 2003. [Google Scholar]
- 12.De’ath G, Fabricius K. Water quality as a regional driver of coral biodiversity and macroalgae on the Great Barrier Reef. Ecol Appl. 2010;20:840–850. doi: 10.1890/08-2023.1. [DOI] [PubMed] [Google Scholar]
- 13.Osborne K, Dolman AM, Burgess SC, Johns KA. Disturbance and the dynamics of coral cover on the Great Barrier Reef (1995-2009) PLoS ONE. 2011;6:e17516. doi: 10.1371/journal.pone.0017516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sweatman H, Delean S, Syms C. Assessing loss of coral cover on Australia’s Great Barrier Reef over two decades, with implications for longer term-trends. Coral Reefs. 2011;30:521–531. [Google Scholar]
- 15.The State of Queensland and Commonwealth of Australia . Reef Water Quality Protection Plan. Brisbane, Australia: Queensland Department of Premier and Cabinet; 2009. [Google Scholar]
- 16.Kroon FJ, et al. River loads of suspended solids, nitrogen, phosphorus and herbicides delivered to the Great Barrier Reef lagoon. Mar Pollut Bull. 2012;65:167–181. doi: 10.1016/j.marpolbul.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Fabricius KE, et al. A bioindicator system for water quality on inshore coral reefs of the Great Barrier Reef. Mar Pollut Bull. 2012;65:320–332. doi: 10.1016/j.marpolbul.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Brodie J, Fabricius K, De’ath G, Okaji K. Are increased nutrient inputs responsible for more outbreaks of crown-of-thorns starfish? An appraisal of the evidence. Mar Pollut Bull. 2005;51:266–278. doi: 10.1016/j.marpolbul.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 19.Miller I, Jonker M, Coleman G. 2009 Crown-of-thorns starfish and coral surveys using the manta tow and SCUBA search techniques. Long-Term Monitoring of the Great Barrier Reef Standard Operation Procedure. Technical report (Australian Institute of Marine Science, Townsville, Australia) [Google Scholar]
- 20.Gardner TA, Côté IM, Gill JA, Grant A, Watkinson AR. Long-term region-wide declines in Caribbean corals. Science. 2003;301:958–960. doi: 10.1126/science.1086050. [DOI] [PubMed] [Google Scholar]
- 21.Côté IM, Gill JA, Gardner TA, Watkinson AR. Measuring coral reef decline through meta-analyses. Philos Trans R Soc Lond B Biol Sci. 2005;360:385–395. doi: 10.1098/rstb.2004.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aronson RB, Precht WF. White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia. 2001;460:1–3. [Google Scholar]
- 23.Bruno JF, Sweatman H, Precht WF, Selig ER, Schutte VGW. Assessing evidence of phase shifts from coral to macroalgal dominance on coral reefs. Ecology. 2009;90:1478–1484. doi: 10.1890/08-1781.1. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson C. Status of Coral Reefs of the World: 2008. Townsville, Australia: Global Coral Reef Monitoring Network; 2008. [Google Scholar]
- 25.Fuessel HM. An updated assessment of the risks from climate change based on research published since the IPCC Fourth Assessment Report. Clim Change. 2009;97:469–482. [Google Scholar]
- 26.Lough J. Great Barrier Reef coral luminescence reveals rainfall variability over northeastern Australia since the 17th century. Paleoceanography. 2011 doi: 10.1029/2010PA002050. [DOI] [Google Scholar]
- 27.De’ath G, Lough JM, Fabricius KE. Declining coral calcification on the Great Barrier Reef. Science. 2009;323:116–119. doi: 10.1126/science.1165283. [DOI] [PubMed] [Google Scholar]
- 28.Cantin NE, Cohen AL, Karnauskas KB, Tarrant AM, McCorkle DC. Ocean warming slows coral growth in the central Red Sea. Science. 2010;329:322–325. doi: 10.1126/science.1190182. [DOI] [PubMed] [Google Scholar]
- 29.Knapp KR, Kruk MC, Levinson DH, Diamond HJ, Neumann CJ. The international best track archive for climate stewardship (ibtracs): Unifying tropical cyclone best track data. Bull Am Meteorol Soc. 2010;91:363–376. [Google Scholar]
- 30.Berkelmans R, De’ath G, Kininmonth S, Skirving W. A comparison of the 1998 and 2002 coral bleaching events on the Great Barrier Reef: Spatial correlation, patterns, and predictions. Coral Reefs. 2004;23:74–83. [Google Scholar]
- 31.Wood S. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J Am Stat Assoc. 2004;99:673–686. [Google Scholar]
- 32.R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2012. [Google Scholar]