Abstract
Endothelial cell (EC) Ca2+-activated K channels (SKCa and IKCa channels) generate hyperpolarization that passes to the adjacent smooth muscle cells causing vasodilation. IKCa channels focused within EC projections toward the smooth muscle cells are activated by spontaneous Ca2+ events (Ca2+ puffs/pulsars). We now show that transient receptor potential, vanilloid 4 channels (TRPV4 channels) also cluster within this microdomain and are selectively activated at low intravascular pressure. In arterioles pressurized to 80 mmHg, ECs generated low-frequency (∼2 min−1) inositol 1,4,5-trisphosphate receptor-based Ca2+ events. Decreasing intraluminal pressure below 50 mmHg increased the frequency of EC Ca2+ events twofold to threefold, an effect blocked with the TRPV4 antagonist RN1734. These discrete events represent both TRPV4-sparklet- and nonsparklet-evoked Ca2+ increases, which on occasion led to intracellular Ca2+ waves. The concurrent vasodilation associated with increases in Ca2+ event frequency was inhibited, and basal myogenic tone was increased, by either RN1734 or TRAM-34 (IKCa channel blocker), but not by apamin (SKCa channel blocker). These data show that intraluminal pressure influences an endothelial microdomain inversely to alter Ca2+ event frequency; at low pressures the consequence is activation of EC IKCa channels and vasodilation, reducing the myogenic tone that underpins tissue blood-flow autoregulation.
Keywords: endothelial cell calcium, cremaster arterioles, mesenteric arteries
Ca2+-activated K+ (KCa) channels in arteriolar endothelial cells (ECs) are activated by intrinsic spontaneous or receptor-mediated Ca2+ events, each leading to hyperpolarization of smooth muscle cells (SMCs) and vasodilation independent of nitric oxide or prostacyclin—the endothelium-dependent hyperpolarization (EDH) response. This hyperpolarization spreads both radially and longitudinally through the vascular wall via patent gap junctions to evoke local and conducted dilation, and it is central to cardiovascular function (1, 2).
EDH is the predominant endothelium-dependent mechanism in smaller “resistance” arteries and arterioles. The underlying hyperpolarization is generated by two subtypes of KCa channels found in the EC, but not SMC, membrane, the small (SKCa,KCa2.3) and intermediate (IKCaK, Ca3.1) conductance forms that may be activated independently of each other (3). The physiological importance of independent activation is apparent from studies with KCa3.1-deficient mice in which the mean blood pressure is raised by ∼7 mmHg, but further elevated by disrupting both KCa channels (4). In mesenteric resistance arteries, IKCa channels are focused within EC projections through the internal elastic lamina (IEL) termed myoendothelial junctions (MEJs). MEJs can contain gap junctions (MEGJs) coupling ECs to SMCs, and EDH can spread by direct electrical coupling and/or a diffusible factor (5, 6). The IKCa channels enriched within MEJs can be activated by spontaneous inositol 1,4,5-trisphosphate (IP3)-mediated Ca2+ events, discovered in unpressurized arteries and termed Ca2+ pulsars (7). Block of these intrinsic Ca2+ pulsars, or of IKCa channels, depolarizes the adjacent SMC by ∼8 mV, suggesting that the two are functionally linked and continually suppress SMC membrane potential (7). Although ECs in pressurized resistance arteries/arterioles in vivo and in vitro generate spontaneous EC Ca2+ events, it is not known whether changes in intraluminal pressure influence these events and whether there is a functional consequence against myogenic tone (8–10).
Because the endothelium has a marked inhibitory influence on vascular tone in vivo, particularly by emitting hyperpolarization, it is puzzling that removal of this monolayer does not seem to alter the myogenic response per se (11, 12). Furthermore, changes in intraluminal pressure in arterioles appear not to alter global EC [Ca2+]i (13). However, the spatial and temporal resolution of [Ca2+]i measurements has improved greatly since the early 1990s, revealing, for example, that SMCs and ECs generate elementary and spatially restricted Ca2+ events spontaneously. These rapid, subcellular events are not necessarily reflected by global [Ca2+]i; e.g., localized Ca2+ sparks in cerebral artery SMCs suppress tone by activating BKCa channels in the adjacent sarcolemma (14).
We therefore investigated the possibility that arteriolar intraluminal pressure might influence the generation of spontaneous Ca2+ events in the endothelium and thereby alter myogenic tone. Myogenic tone reflects SMC constriction that is related to the level of intraluminal pressure. Acute increases in pressure evoke vasoconstriction apparent at pressures of 30–40 mmHg and above, reflecting divergence of the active and passive pressure/diameter curves (15). Of direct relevance to the present study, in vivo measurement of intravascular pressure in rat cremaster muscle arterioles at rest revealed pressures of 70–80 mmHg (16). The myogenic response stabilizes local blood flow and capillary filtration pressure as blood pressure changes and provides a basal tone that can be enhanced or attenuated by nerve transmitters, autacoids, and hormones (15). Myogenic contraction relies in large part on SMC depolarization, so hyperpolarization originating from either cell type will exert a significant influence on tone (17).
Our data reveal a unique mechanism intrinsic to ECs, whereby low (<50 mmHg) intraluminal pressure activates TRPV4 channels to increase the frequency of EC Ca2+ events within MEJs. The increase is sufficient to activate EC IKCa channels and, as a consequence, reverse or suppress myogenic tone. These observations extend the concept of MEJs as signaling microdomains and reveal a physiological role for these thin membrane structures.
Results
All experiments were performed using isolated, cannulated, and pressurized rat cremaster arterioles, with a passive internal diameter of 160–200 μm at 80 mmHg, a pressure at which they developed spontaneous myogenic tone.
Effect of Intraluminal Pressure on EC and SMC Ca2+ Events and Myogenic Tone.
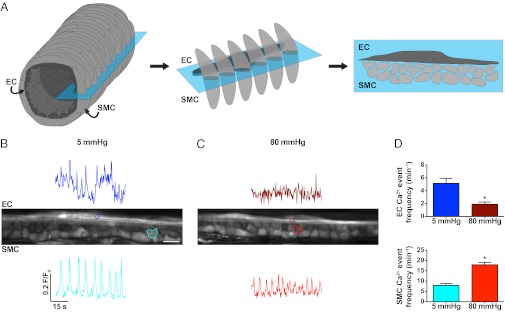
We developed a method to simultaneously record EC and SMC Ca2+ events in adjacent cells using multiphoton microscopy at the arteriole midplane. By changing intraluminal pressure, we observed reciprocal changes in Ca2+ events within the two cell types. Decreasing pressure (80–5 mmHg) reduced the frequency of spontaneous Ca2+ events in the SMCs from 17.9 ± 1.2 to 7.9 ± 1.0 min−1. At the same time, the frequency of Ca2+ events in the adjacent ECs increased from 1.9 ± 0.3 to 5.1 ± 0.8 min−1 (Fig. 1D and Movie S1).
Fig. 1.
Midplane imaging of spontaneous Ca2+ events in pressurized cremaster arteriolar ECs and SMCs. (A) Schematic representation of an arteriole (Left) showing the SMCs (light gray) wrapped circumferentially around the axially aligned ECs (dark gray, Center). Focusing at the midplane of an arteriole (blue box) allows both cell types to be imaged simultaneously (Right). (B and C) Midplane images of an intact arteriole pressurized to either 5 (B) or 80 (C) mmHg (ECs left to right, SMCs in cross section). Regions of interest (ROIs) shown on the image correspond to the temporal fluorescence traces from either ECs (Upper) or SMCs (Lower). (Scale bar, 10 μm; Movie S1). (D) Summary data of events recorded simultaneously in both cell types. The pressure-dependent frequency of EC Ca2+ events (Upper) changed in the opposite direction to SMCs (Lower). Note that changes in fluorescence intensity do not directly relate to amplitudes in [Ca2+]i. *P < 0.05, significantly different from 5 mmHg (n = 5). Images were acquired at ∼3 Hz.
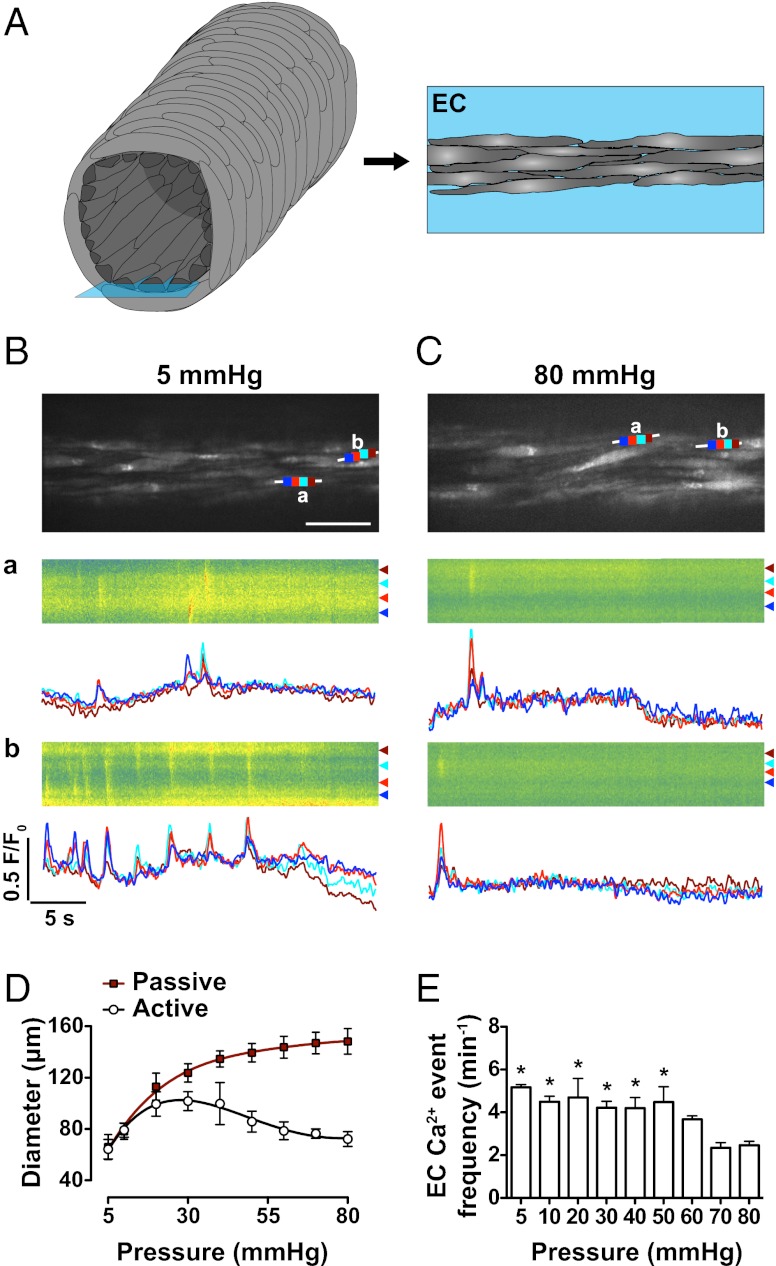
Responses at the midplane were confirmed by lowering the plane of focus to view ECs at the bottom of the arteriole (Fig. 2). The majority of spontaneous EC Ca2+ events were nonpropagating (local) (5 mmHg, 73%; 80 mmHg, 75% of events), whereas the remaining subset propagated along the EC axis (waves). Of note, spontaneous EC Ca2+ events were not reflected by global Ca2+ measurements (Fig. S1 and Movie S2). To establish the pressure at which the activation of EC Ca2+ events changed, full myogenic response curves were performed. Step increases in intraluminal pressure (5–80 mmHg) were associated with passive distension of the vessel, and beyond 30 mmHg, myogenic tone developed in a pressure-dependent manner (Fig. 2D). The diameter of arterioles was similar at 5 mmHg (no tone, 64.2 ± 7.6 μm, n = 3) and 80 mmHg (myogenic tone, 72.2 ± 5.8 μm, n = 3), and localized, spontaneous EC Ca2+ events were present at each pressure. The frequency of ECs events was significantly increased at and below 50 mmHg, as was the percentage of active cells (5 mmHg, 61 ± 7%, compared with 80 mmHg, 42 ± 5%; paired data, n = 14, P < 0.05). A similar profile was observed in mesenteric resistance arteries (not myogenically active; Fig. S2B). The spontaneous EC Ca2+ events in cremaster arterioles did not synchronize between cells, and although Ca2+ events seemed to originate from the same subcellular region of an EC, in some cases they propagated (Fig. 2B).
Fig. 2.
Bottom-plane imaging of EC Ca2+ events in pressurized cremaster arterioles. (A) Imaging ECs at the lowest (Right, blue box) plane in an arteriole enables both spatial and temporal resolution of local and propagating Ca2+ events. (B and C) Confocal fluorescence images of ECs at an intraluminal pressure of 5 mmHg (B) (Movies S1 and S2) and 80 mmHg (C) in the same vessel. (Scale bar, 30 μm.) White lines indicate the site of line-scan analysis with each color (dark blue, light red, light blue, and dark red) corresponding to traces in a and b (low Ca2+, green; high Ca2+, red), raw data, with corresponding Ca2+ events from the defined subcellular regions plotted below as F/F0 over time. (D) Summary data of diameter change in pressurized arterioles, showing myogenic tone developing above 30 mmHg during step increases in intraluminal pressure; active (open circles) arterial diameter associated with an increase in the corresponding frequency of EC Ca2+ events at lower pressures (E). Note that arteriolar diameter at 5 mmHg (no tone) was effectively the same as at 80 mmHg (myogenic tone). Corresponding Ca2+-free passive diameters (red squares) for each dataset are also shown. *P < 0.05; significantly different from 80 mmHg (paired observations; n = 3). Images were acquired at ∼9 Hz.
TRPV4 Channels Underlie Increases in EC Ca2+ Events at Low Pressure.
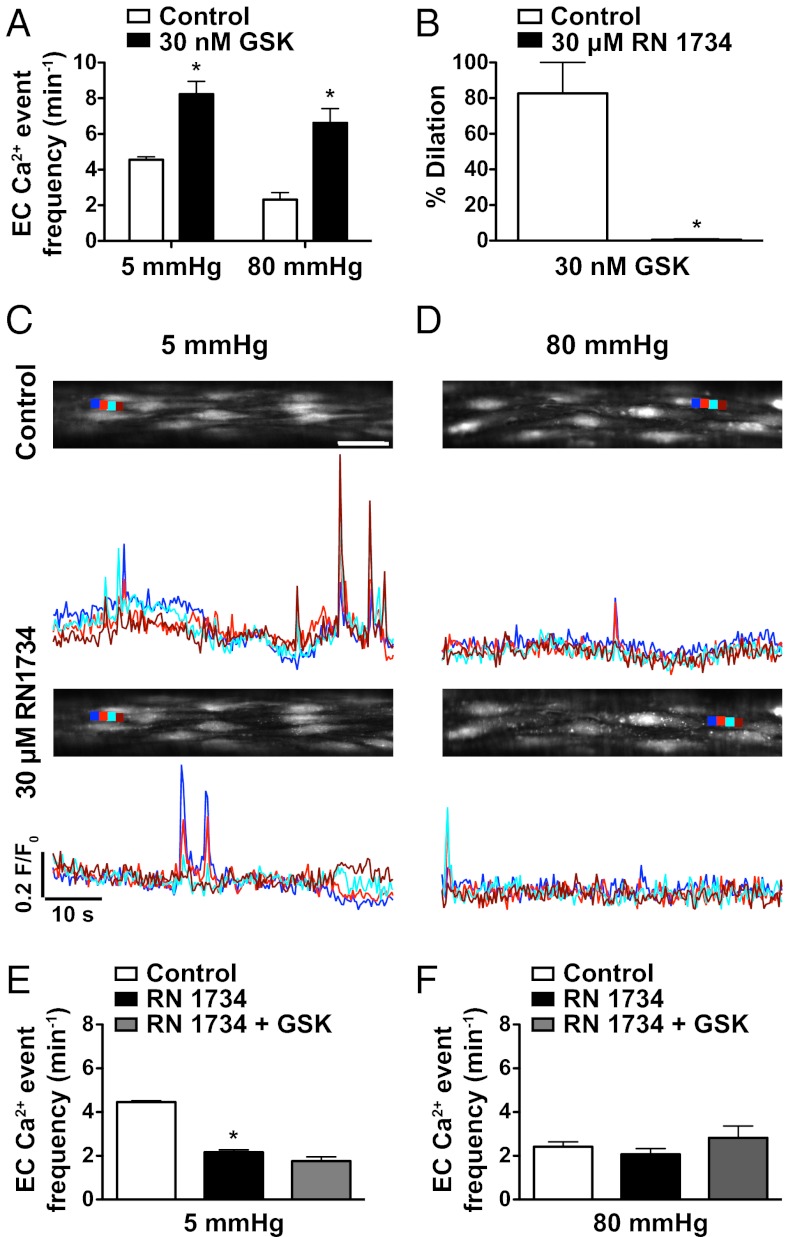
The TRPV4 channel agonist GSK1016790A (GSK, 30 nM) evoked local increases in EC Ca2+ at 5 and 80 mmHg (Fig. 3A). With 100 nM GSK, these events evolved into cell-wide Ca2+ waves, but this concentration visibly damaged the preparation (as also observed by ref. 18). As a consequence, regardless of GSK concentration used, each experiment was terminated after exposure to the agonist. GSK also evoked arteriolar dilation, an effect blocked by the TRPV4 channel antagonist RN1734 (30 μM; Fig. 3B). RN1734 also reduced the frequency of spontaneous EC Ca2+ events at low pressure, but not at 80 mmHg, although it did block increases to 30 nM GSK at both pressures (Fig. 3 C–F) and reduced the EC-dependent vasodilation to ACh (Fig. S3). Similar effects were obtained with another TRPV4 antagonist (HC067047, 10 μM; Fig. S4) and in mesenteric arteries (Fig. S2 C and D). RN1734 also blocked ATP-sensitive K+ channels, although this action did not account for the effects against EC Ca2+ events (Table S1).
Fig. 3.
TRPV4 channels underlie activation of EC Ca2+ events by a low-pressure-dependent mechanism. (A) The TRPV4 agonist GSK1016790A (GSK; 30 nM) increased EC Ca2+ events at both 5 and 80 mmHg. (B) In arterioles with myogenic tone at 80 mmHg, 30 nM GSK evoked 80% dilation, an effect blocked by the TRPV4 antagonist RN1734 (30 μM). (C and D) OGB-1 fluorescence reporting EC Ca2+ events in the bottom confocal plane of pressurized arterioles at an intraluminal pressure of either 5 (C) or 80 (D) mmHg before (Upper) and after (Lower) incubation with 30 μM RN1734. (Scale bar, 30 μm.) Ca2+ events in subcellular regions (color coded, dark blue, light red, light blue, and dark red) are plotted below as F/F0 changes with time. (E and F) Summary data show the effect of RN1734 against basal EC Ca2+ events at 5 (E) and 80 (F) mmHg. *P < 0.05; significantly different from control (paired observations, n = 4). In the same arterioles, RN1734 also blocked any increase in EC Ca2+ event frequency to 30 nM GSK. Images were acquired at ∼3 Hz.
Spatial Correlation Between EC Ca2+ Events and TRPV4 Channels.
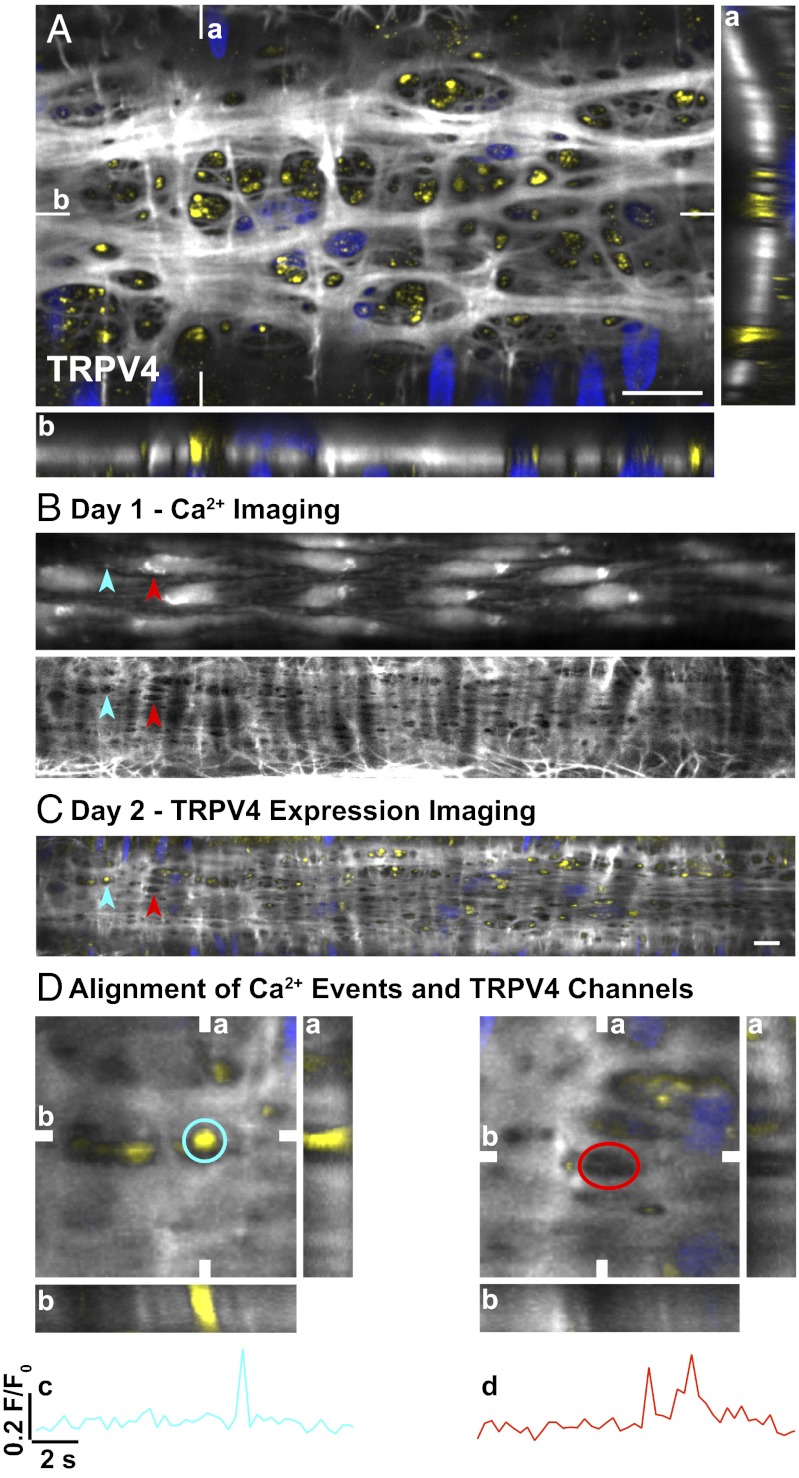
Spontaneous EC Ca2+ events were mainly focused within holes through the IEL (71 ± 1% of cases, 213/300 events, n = 3). In the same arterioles, 58 ± 6% of these events correlated with signal for TRPV4 channels (Fig. 4). In general, the expression of TRPV4 channels also corresponded to holes through the IEL in cremaster (Fig. 4A) and mesentery (Fig. S2A). Punctate, membrane-localized expression of TRPV4 channels was also evident in SMCs (Movie S3).
Fig. 4.
Correlation between EC spontaneous Ca2+ events, holes in the IEL, and expression of TRPV4 channels in pressurized cremaster arterioles. (A) Confocal merged image of three (0.2-μm) z-axis planes of a pressurized arteriole at the level of the IEL. TRPV4 expression (yellow) localized primarily to holes through the IEL (white with nuclear staining blue). Data correlating TRPV4 expression and EC Ca2+ events were accumulated over 2 d in the same pressurized arteriole. Spontaneous EC Ca2+ events were measured, and the elastin was stained (5 mmHg; day 1). Sites of spontaneous Ca2+ activity were identified and analyzed post hoc to determine the correlation with holes in the IEL (overlay in Lower). (B) Spontaneous Ca2+ events were restricted to holes in the IEL in ∼71% of cases (n = 3 arterioles; blue and red arrowheads). (C) The arteriole was then fixed in situ, and TRPV4 expression was determined the next day (day 2). (D) EC spontaneous Ca2+ events aligned with TRPV4 expression in 58 ± 6% (n = 3) of cases (C, blue arrowhead; D, blue ROI; c, time-series plot). Spontaneous Ca2+ activity indicated by the red arrowhead in B and C aligned with a hole in the IEL but not with TRPV4 expression (D, red ROI; d, time-series plot). (A and D) Reconstructed z-stacks are shown in both the vertical (a) and horizontal (b) planes indicated on the merged image. (Scale bars, 10 μm.) Field of view in D, 23 μm2. Images were acquired at ∼3 Hz.
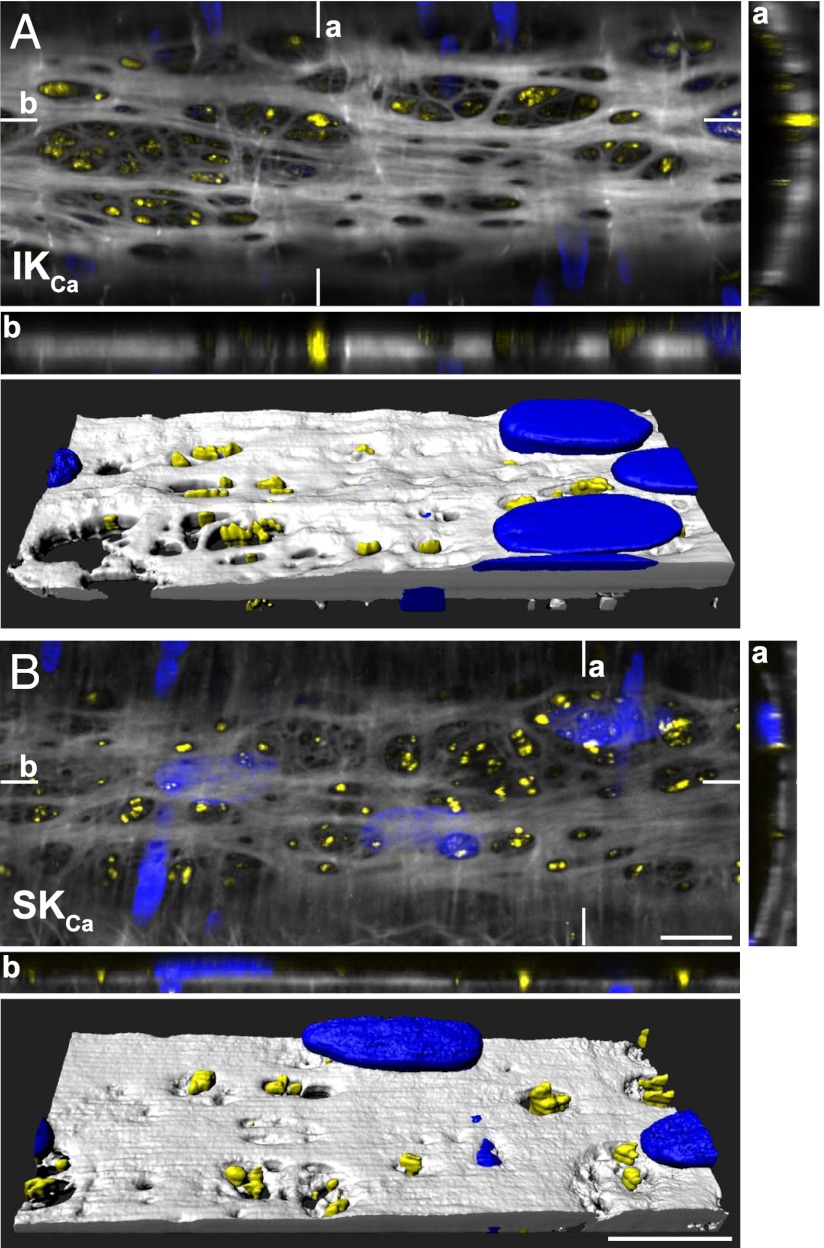
TRPV4, IKCa and SKCa Channels Cluster in Holes Through the IEL.
The 3D reconstruction of z-stack confocal sections through the wall of pressurized arterioles revealed dense clusters of both KCa3.1 and KCa2.3 channels within holes in the IEL (Fig. 5 and Movie S3).
Fig. 5.
Association between KCa3.1 and KCa2.3 channel expression and holes through the IEL in pressurized cremaster arterioles. Confocal image from a pressurized arteriole (merged image of three 0.2-μm z-axis planes at the level of the IEL). KCa3.1 (IKCa channel; A) and KCa2.3 (SKCa channel; B) expression is indicated in yellow, IEL in white, and nuclear staining in blue. Reconstructed z-stacks are shown for the corresponding vertical (a) and horizontal (b) planes indicated on the main, merged image. Note the localization of IKCa and SKCa channels primarily within holes through the IEL, highlighted in the 3D volume reconstruction (A and B, Lower). (Scale bars, 10 μm.) Data are representative of images from three arterioles (Movie S3).
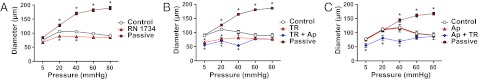
Blocking TRPV4 and IKCa Channels Increases Myogenic Tone.
Nitric oxide did not modulate myogenic tone at any pressure (Fig. S5A). In the presence of N-nitro-l-arginine methyl ester (l-NAME) to block NO synthase, inhibition of TRPV4 channels with 30 μM RN1734 significantly increased myogenic tone at low pressure (20 and 40 mmHg, n = 4), although this effect was intermediate to the increase in myogenic tone that developed after selective block of IKCa channels with 1 μM TRAM-34 (n = 3; Fig. 6). The ability of TRAM-34 to increase myogenic tone at pressures <40 mmHg was not mimicked by block of SKCa channels with apamin (n = 3), although the combined presence of TRAM-34 and apamin did increase myogenic tone slightly more than TRAM-34 alone (n = 6). When the endothelium was physically disrupted, myogenic tone at low pressures was unaffected by TRAM-34, apamin, and l-NAME (Fig. S5B).
Fig. 6.
EC TRPV4 and IKCa channels suppress the development of myogenic tone in cremaster arterioles. (A and B) At low intraluminal pressure, block of either TRPV4 channels with RN1734 (30 μM, n = 4; A) or IKCa channels with TRAM-34 (TR, 1 μM, n = 3; B) significantly increased the level of myogenic tone. (C) In contrast, block of SKCa channels with apamin (Ap) did not alter the pressure/diameter profile (100 nM, n = 3). The passive diameters obtained in Ca2+-free buffer for each dataset are also shown. *P < 0.05, significantly different from control (100 μM l-NAME) at the same pressure (paired observations).
Signaling Pathways Underlying EC Ca2+ Events.
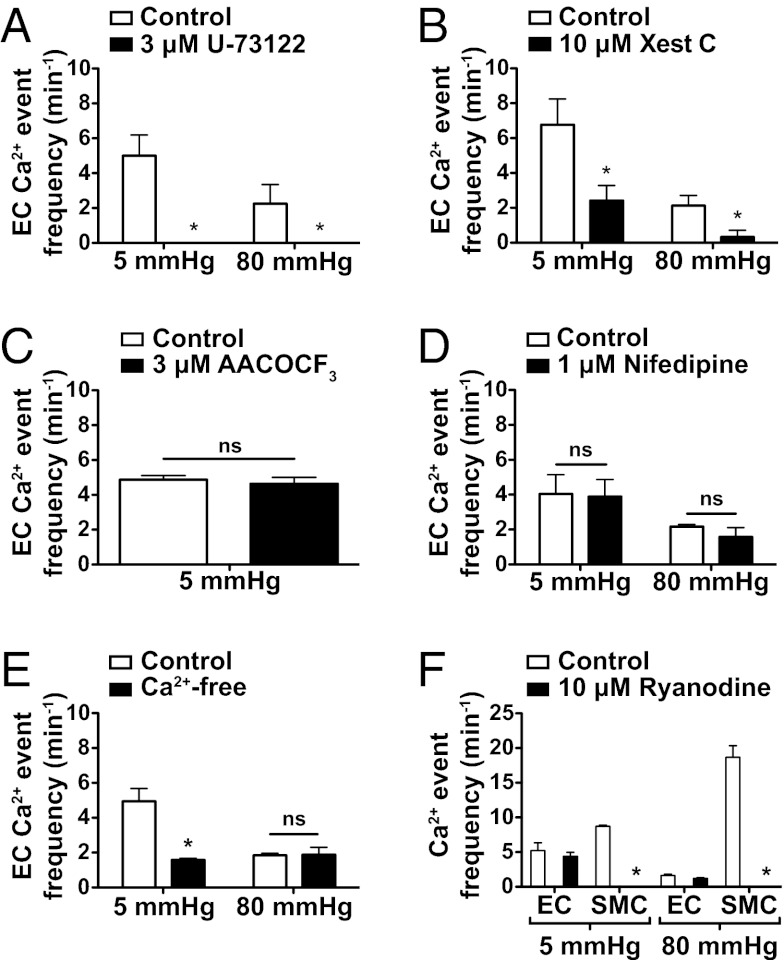
The phospholipase C (PLC) inhibitor U-73122 (3 μM) abolished spontaneous Ca2+ events in ECs at both 5 and 80 mmHg after intraluminal incubation (Fig. 7A) and fully blocked increases stimulated with 1 μM ACh. Intraluminal perfusion of 10 μM xestospongin C significantly reduced the frequency of EC Ca2+ events at 5 mmHg and almost abolished events at 80 mmHg (Fig. 7B). In contrast, neither inhibition of phospholipase A2 (PLA2) with 3μM AACOCF3 (Fig. 7C), voltage-gated Ca2+ channels (VGCC) with 1 μM nifedipine (Fig. 7D), nor block of ryanodine receptors with 10 μM ryanodine (Fig. 7F) affected the frequency of EC spontaneous Ca2+ events. Removal of extracellular Ca2+ did not alter the EC spontaneous Ca2+ events at 80 mmHg but reduced the event frequency at 5 mmHg by ∼50% (Fig. 7E), a similar profile seen with the TRPV4 channel antagonist RN1734 (Fig. 3 E and F).
Fig. 7.
EC Ca2+ events involve IP3R signaling and Ca2+ influx but not arachidonic acid derivatives, VGCC, or ryanodine receptors. (A) EC spontaneous Ca2+ events in pressurized cremaster arterioles were completely abolished by the PLC antagonist U-73122 (3 μM) at 5 mmHg (n = 3) and 80 mmHg (n = 3). (B) IP3R blockade with xestospongin C (Xest C, 10 μM) attenuated spontaneous EC Ca2+ event frequency at both 5 mmHg (n = 3) and 80 mmHg (n = 4). (C) Block of PLA2, a liberator of arachidonic acid, with AACOCF3 (3 μM) did not alter (ns, not significant) EC Ca2+ event frequency at 5 mmHg. (D) Block of VGCC with nifedipine (1 μM) had no effect on Ca2+ event frequency at either 5 or 80 mmHg (n = 4) but prevented the development of myogenic tone. (E) Removal of extracellular Ca2+ (Ca2+-free) reduced EC activity at 5 mmHg but not at 80 mmHg (n = 3). (F) Ryanodine (10 μM) abolished asynchronous propagated SMC Ca2+ events (waves) at both 5 and 80 mmHg intraluminal pressure in cremaster arterioles (n = 3) but did not affect EC spontaneous Ca2+ activity in the same arterioles (n = 3). *P < 0.05, significantly different from control at each pressure.
Discussion
Little is known about the functional importance of spontaneous Ca2+ events generated by vascular ECs in situ. We now show an intrinsic mechanism involving EC Ca2+ events in pressurized arteries and arterioles that activates hyperpolarization and dilation in response to low intraluminal pressure. The mechanism involves TRPV4 channels focused within MEJs that are activated by low pressure to increase local EC Ca2+ event frequency. Direct activation of TRPV4 channels leads to Ca2+ sparklets (18), and, indirectly, sparklets will stimulate other [Ca2+]i-sensitive Ca2+ signaling components [e.g., PLC, IP3 receptors (IP3Rs)]. Whether direct or indirect, the increase in local [Ca2+] activates adjacent EC IKCa channels, causing EC-dependent vasodilation at low pressures, reducing myogenic tone (Fig. S6). The fact that similar changes in frequency in response to pressure occur in mesenteric resistance arteries in the absence of myogenic tone suggests that the relationship is a general property of arterial ECs, providing that TRPV4 and KCa channels are present. As such, these direct and indirect contributions should now be considered when measuring the frequency and spatial location of spontaneous EC Ca2+ events in arteries and arterioles.
ECs in intact arteries and arterioles can generate spontaneous, localized, and asynchronous Ca2+ events that are universally ryanodine-insensitive, and, at least in part IP3R-based, i.e., Ca2+ puffs/pulsars. The spontaneous events can propagate to generate Ca2+ waves across ECs (7–10, 19, 20). However, it is not clear whether there is any functional correlate for these spontaneous EC Ca2+ events, particularly in the absence of agonist stimulation. By studying isolated, intact arteries and arterioles under physiological intraluminal pressures, we observed spontaneous EC Ca2+ events predominantly in the vicinity of holes through the IEL and at a similar frequency to the recently defined Ca2+ pulsars in mouse mesenteric resistance artery ECs (4–6 min−1 in unpressurized en face arteries and ∼2 min−1 in arteries pressurized to 80 mmHg) (7). Our data now extend these seminal observations, showing a subpopulation of EC Ca2+ events activated specifically by intraluminal pressures <50 mmHg. Before the recent identification of Ca2+ sparklets by Sonkusare et al. (18), most of the events we observe would have been considered pulsars.
We also show that the EC Ca2+ events induced by low intraluminal pressure involve TRPV4 channels. ECs contain a range of different TRP channels (21), and in resistance arteries, TRPV4 channels are intimately involved in vasodilation to EC-dependent agonists (22, 23) and shear stress-induced dilation (24). Sustained activation to low pressure is a unique addition to this list. The Ca2+ events stimulated by low pressure can be identified by removing extracellular Ca2+ or by pharmacologically blocking TRPV4 channels (schematically depicted in Fig. S6). Interestingly, both mesenteric resistance arteries and cremaster arterioles displayed an increase in frequency of EC Ca2+ events at 5 mmHg. This increase required Ca2+ influx through TRPV4 channels, because TRPV4 antagonists suppressed the frequency at 5 mmHg to a rate similar to 80 mmHg, i.e., ∼2 min−1. This activity profile correlated with dense immunohistochemical signal for TRPV4 channels in the regions generating the EC Ca2+ events. TRPV4 expression was also apparent in some SMCs, but GSK evoked dilation, so any potential role is not clear.
Although the low-pressure-induced EC Ca2+ events appear primarily due to activation of TRPV4 channels, it was not possible to separate the contribution due to influx through TRPV4 channels from indirect activation of other [Ca2+]i-sensitive pathways resulting in Ca2+ influx and/or release. Together, all these routes can potentially generate cell-wide waves in the endothelium of pressurized cremaster arterioles. Importantly, because TRPV4 channels are themselves [Ca2+]i-sensitive (25), sparklets may also propagate into waves via activation of adjacent TRPV4 channels.
How low intraluminal pressure might activate EC TRPV4 channels is not clear. Although TRPV4 channels can be activated by arachidonic acid derivatives (epoxyeicosatrienoic acids; ref. 26), this possibility could be ruled out because a PLA2 inhibitor had no effect on the EC Ca2+ events. The most likely explanation is that activation somehow follows a reduction in the luminally generated radial compressive force experienced by the EC monolayer. TRPV4 channels are sensitive to changes in cell shape, as demonstrated by activation in response to increasing shear stress or as a result of hypotonic cell swelling (27, 28). In our experiments, EC Ca2+ events were decreased to a similar extent at 80 mmHg (as in vivo; ref. 16) in cremaster arterioles, whether or not the arterioles developed myogenic tone (with or without nifedipine present). These observations were consistent with our experiments using myogenically inactive mesenteric arteries and suggest that it is not tone per se that turns off EC Ca2+ events. In each case, the ECs would experience different tension, but the compressive effect of 80-mmHg intraluminal pressure would be relatively constant. If the cell shape assumed when radial compression is low does activate TRPV4 channels, the subcellular mechanism remains to be defined.
A link between low intraluminal pressure, EC Ca2+ events, and myogenic tone involving TRPV4 channels suggests that Ca2+ sparklets are of fundamental importance for vascular function. These elementary events are revealed by block of IP3-mediated Ca2+-release and appear to operate cooperatively within a four-channel metastructure (18). As few as three TRPV4 channels per cell have been suggested as sufficient to activate EC IKCa channels, leading to hyperpolarization and vasodilation (7, 18). Our data now show that low pressure is sufficient to increase EC Ca2+ event frequency by twofold to threefold and drive significant vasodilation. This mechanism is intrinsic to the arteriole, operating independently of the surrounding tissue. What is not clear is how Ca2+ sparklets might enhance puff/pulsar frequency, but this may simply reflect the known ability of Ca2+ to stimulate IP3Rs (29). Consistent with this suggestion, IP3Rs are densely clustered within MEJs (7), and vasodilation to the TRPV4 agonist, GSK1016790A, was greatly reduced after block of IP3R-signaling (18).
The fact that ECs in arteries/arterioles are responsive to intraluminal pressure will have important physiological consequences. The inverse relationship between EC Ca2+ events and intraluminal pressure, and the fact this change persists at steady-state, suggests that reducing intraluminal pressure will cause vasodilation in myogenically active arteries/arterioles and sustain tissue blood flow. For example, when systemic blood pressure decreases or if local ischemia or an upstream stenosis drops arteriolar pressure, EC Ca2+ events will generate hyperpolarization and give rise to conducted dilation (1, 2). This vascular coordination involves electrical coupling via homo- and heterocellular gap junctions to sustain the spread of hyperpolarization through the endothelium. Conducted dilation occurs in arteries, whether they are myogenically active or not, and serves to increase total tissue blood flow and coordinate vascular networks (1, 2). The resulting increase in tissue blood flow will then raise intra-arteriolar pressure, remove the dilation signal, and as a result increase vascular tone.
The uniqueness of the present study lies in the discovery that changes in intraluminal pressure can influence myogenic tone by modulating spontaneous EC Ca2+ events (TRPV4-associated sparklets/events) and thereby influence arteriolar diameter. Further, this control mechanism is part of a membrane-signaling microdomain located within MEJs. These EC projections are a focus for IP3R-mediated Ca2+ events, and this Ca2+ will generate SMC hyperpolarization predominantly by activation of the EC IKCa channels densely expressed within this microdomain (1, 5, 7).
Materials and Methods
Arterial Preparation.
Rat cremaster arterioles were isolated and cannulated onto glass pipettes. After warming to 34 °C, intraluminal pressure was raised to 80 mmHg, and arterioles developed myogenic tone and fully dilated to the EC-dependent dilator ACh. See SI Materials and Methods.
Measurement of EC and/or SMC [Ca2+]i Changes in Pressurized Arterioles.
Oregon Green 488 BAPTA-1 AM was loaded into ECs, SMCs, or EC and SMCs. To simultaneously image EC and SMC Ca2+ events, a multiphoton laser (790 nm) was used to image the arterial wall at the midplane. Visible (488 nm) lasers were used to image either EC or SMC Ca2+ events at the bottom-plane of the arteries. Results are presented as frequency (events⋅cell⋅min−1) or F/Fo, calculated by dividing the fluorescence (F) by an average baseline fluorescence (Fo). See SI Materials and Methods.
Experimental Protocols and Drugs.
Data Analysis.
In all cases results are summarized as the mean ± SEM of n arterioles, one per animal.
Supplementary Material
Acknowledgments
This work was supported by a Wellcome Trust, UK, Programme grant. K.A.D. is a British Heart Foundation Senior Basic Science Research Fellow. C.J.G. is a Royal Society–Wolfson Merit Award Holder.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1211946109/-/DCSupplemental.
References
- 1.Garland CJ, Hiley CR, Dora KA. EDHF: Spreading the influence of the endothelium. Br J Pharmacol. 2011;164(3):839–852. doi: 10.1111/j.1476-5381.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagher P, Segal SS. Regulation of blood flow in the microcirculation: role of conducted vasodilation. Acta Physiol (Oxf) 2011;202(3):271–284. doi: 10.1111/j.1748-1716.2010.02244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crane GJ, Gallagher N, Dora KA, Garland CJ. Small- and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery. J Physiol. 2003;553(Pt 1):183–189. doi: 10.1113/jphysiol.2003.051896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brähler S, et al. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation. 2009;119(17):2323–2332. doi: 10.1161/CIRCULATIONAHA.108.846634. [DOI] [PubMed] [Google Scholar]
- 5.Dora KA, Gallagher NT, McNeish A, Garland CJ. Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ Res. 2008;102(10):1247–1255. doi: 10.1161/CIRCRESAHA.108.172379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396(6708):269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 7.Ledoux J, et al. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci USA. 2008;105(28):9627–9632. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duza T, Sarelius IH. Increase in endothelial cell Ca(2+) in response to mouse cremaster muscle contraction. J Physiol. 2004;555(Pt 2):459–469. doi: 10.1113/jphysiol.2003.051029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McSherry IN, Spitaler MM, Takano H, Dora KA. Endothelial cell Ca2+ increases are independent of membrane potential in pressurized rat mesenteric arteries. Cell Calcium. 2005;38(1):23–33. doi: 10.1016/j.ceca.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Kansui Y, Garland CJ, Dora KA. Enhanced spontaneous Ca2+ events in endothelial cells reflect signalling through myoendothelial gap junctions in pressurized mesenteric arteries. Cell Calcium. 2008;44(2):135–146. doi: 10.1016/j.ceca.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang A, Sun D, Koller A. Endothelial dysfunction augments myogenic arteriolar constriction in hypertension. Hypertension. 1993;22(6):913–921. doi: 10.1161/01.hyp.22.6.913. [DOI] [PubMed] [Google Scholar]
- 12.Falcone JC, Davis MJ, Meininger GA. Endothelial independence of myogenic response in isolated skeletal muscle arterioles. Am J Physiol. 1991;260(1 Pt 2):H130–H135. doi: 10.1152/ajpheart.1991.260.1.H130. [DOI] [PubMed] [Google Scholar]
- 13.Falcone JC. Endothelial cell calcium and vascular control. Med Sci Sports Exerc. 1995;27(8):1165–1169. [PubMed] [Google Scholar]
- 14.Nelson MT, et al. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270(5236):633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 15.Davis MJ. Perspective: Physiological role(s) of the vascular myogenic response. Microcirculation. 2012;19(2):99–114. doi: 10.1111/j.1549-8719.2011.00131.x. [DOI] [PubMed] [Google Scholar]
- 16.Hill MA, Trippe KM, Li QX, Meininger GA. Arteriolar arcades and pressure distribution in cremaster muscle microcirculation. Microvasc Res. 1992;44(1):117–124. doi: 10.1016/0026-2862(92)90106-y. [DOI] [PubMed] [Google Scholar]
- 17.Kotecha N, Hill MA. Myogenic contraction in rat skeletal muscle arterioles: smooth muscle membrane potential and Ca(2+) signaling. Am J Physiol Heart Circ Physiol. 2005;289(4):H1326–H1334. doi: 10.1152/ajpheart.00323.2005. [DOI] [PubMed] [Google Scholar]
- 18.Sonkusare SK, et al. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336(6081):597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burdyga T, Shmygol A, Eisner DA, Wray S. A new technique for simultaneous and in situ measurements of Ca2+ signals in arteriolar smooth muscle and endothelial cells. Cell Calcium. 2003;34(1):27–33. doi: 10.1016/s0143-4160(03)00019-8. [DOI] [PubMed] [Google Scholar]
- 20.Ying X, Minamiya Y, Fu C, Bhattacharya J. Ca2+ waves in lung capillary endothelium. Circ Res. 1996;79(4):898–908. doi: 10.1161/01.res.79.4.898. [DOI] [PubMed] [Google Scholar]
- 21.Yao X, Garland CJ. Recent developments in vascular endothelial cell transient receptor potential channels. Circ Res. 2005;97(9):853–863. doi: 10.1161/01.RES.0000187473.85419.3e. [DOI] [PubMed] [Google Scholar]
- 22.Saliez J, et al. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation. 2008;117(8):1065–1074. doi: 10.1161/CIRCULATIONAHA.107.731679. [DOI] [PubMed] [Google Scholar]
- 23.Earley S, et al. TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am J Physiol Heart Circ Physiol. 2009;297(3):H1096–H1102. doi: 10.1152/ajpheart.00241.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendoza SA, et al. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol. 2010;298(2):H466–H476. doi: 10.1152/ajpheart.00854.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strotmann R, Schultz G, Plant TD. Ca2+-dependent potentiation of the nonselective cation channel TRPV4 is mediated by a C-terminal calmodulin binding site. J Biol Chem. 2003;278(29):26541–26549. doi: 10.1074/jbc.M302590200. [DOI] [PubMed] [Google Scholar]
- 26.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res. 2005;97(12):1270–1279. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe H, et al. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002;277(16):13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- 28.Liedtke W, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103(3):525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol. 1990;95(6):1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.