Abstract
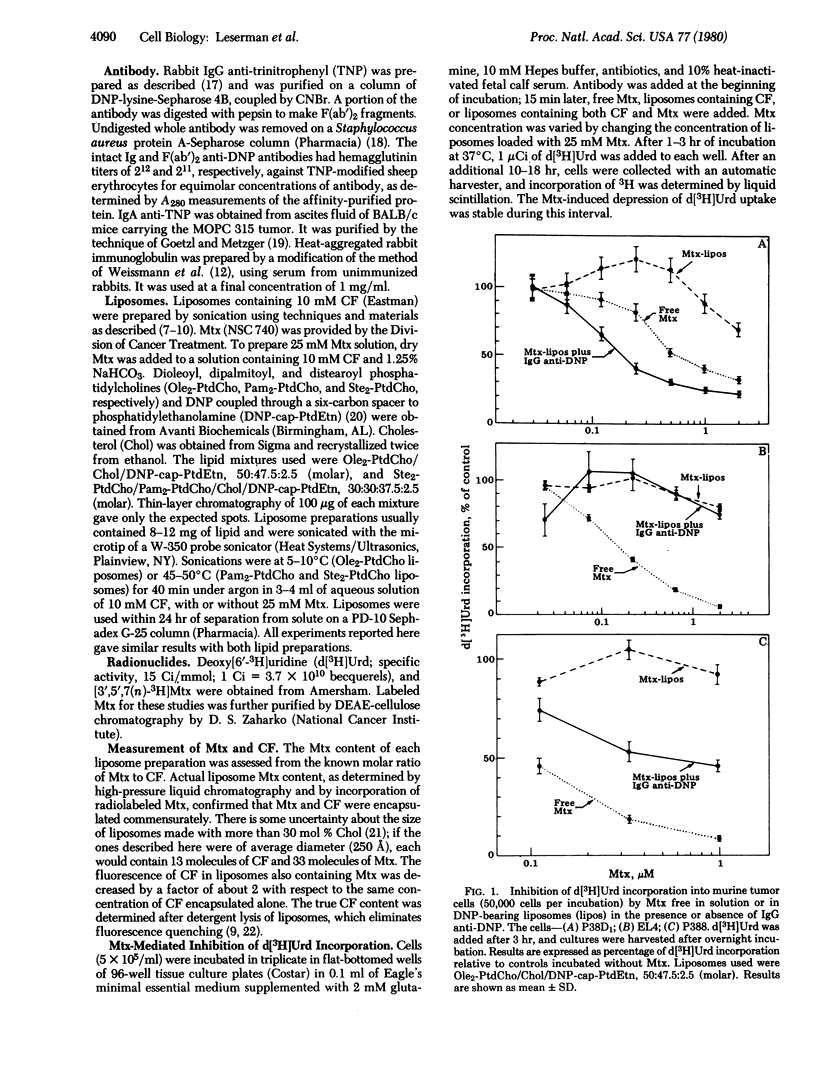
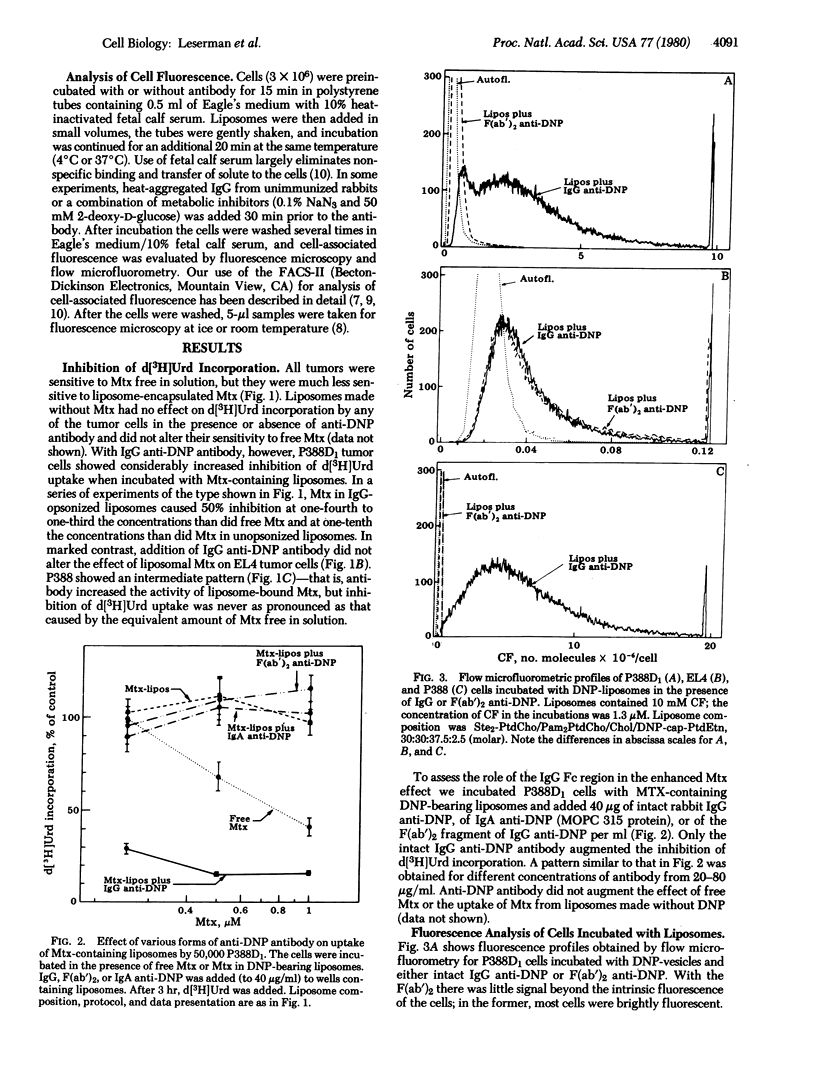
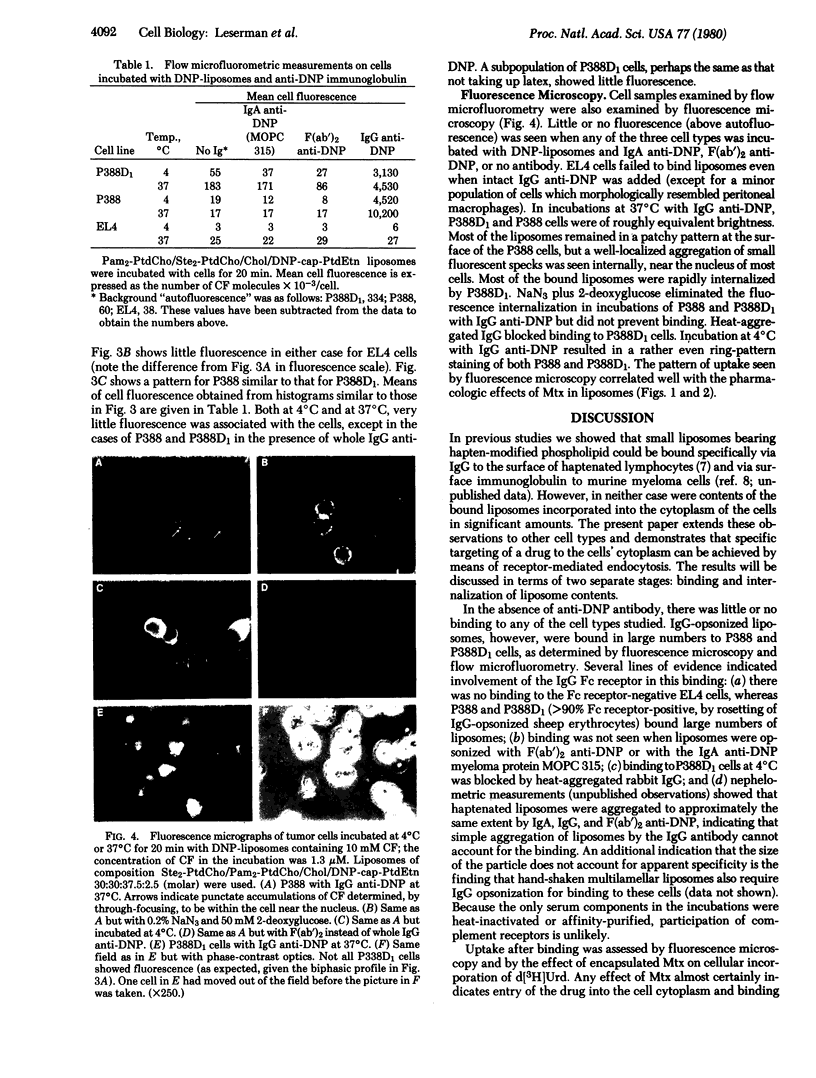
Specific receptor-mediated delivery of the contents of small, sonicated liposomes was studied with three murine tumor cell types: an IgG Fc receptor-negative nonphagocytic line (EL4); an Fc receptor-positive phagocytic line (P388D1); and an Fc receptor-positive nonphagocytic line (P388). The liposomes (formed from phosphatidylcholines, cholesterol, and dinitrophenyl-substituted phosphatidylethanolamine) contained carboxyfluorescein as a fluorescent marker and methotrexate as a pharmacologic agent. Binding and internalization of the liposomes were observed by fluorescence microscopy and measured by flow microfluorometry. The hapten-derivatized lipid was used as a binding point on the liposome for the antibody-combining site of the immunoglobulin. In the presence of IgG anti-dinitrophenyl, but not F(ab')2 or IgA anti-dinitrophenyl, liposomes bound to the Fc receptor-bearing cells. The liposomes underwent endocytosis by the P388D1 cells and, to a lesser extent, by the P388 cells. As measured by depression of [3H]deoxyuridine incorporation, methotrexate in IgG-opsonized liposomes had a much greater pharmacologic effect on the P388D1 cells than did the same amount in unopsonized liposomes or in free solution. This observation indicates that an appropriately chosen drug, incorporated in liposomes, can exert its effect on a cytoplasmic target after endocytosis. P388 cells showed a moderate effect of the drug in liposomes. Neither P388 nor P388D1 cells bound or ingested unopsonized liposomes, and the Fc receptor-negative EL4 line neither bound nor ingested opsonized liposomes. The data demonstrate specific interaction of opsonized liposomes with the cells' IgG Fc receptor.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenthal R., Weinstein J. N., Sharrow S. O., Henkart P. Liposome--lymphocyte interaction: saturable sites for transfer and intracellular release of liposome contents. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5603–5607. doi: 10.1073/pnas.74.12.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. M., Weissmann G., Hoffstein S., Awasthi Y. C., Srivastava S. K. Introduction of purified hexosaminidase A into Tay-Sachs leukocytes by means of immunoglobulin-coated liposomes. Biochemistry. 1976 Jan 27;15(2):452–460. doi: 10.1021/bi00647a034. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Use of staphylococcal protein A as an immunological reagent. J Immunol Methods. 1978;20:241–253. doi: 10.1016/0022-1759(78)90259-4. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Metzger H. Affinity labeling of a mouse myeloma protein which binds nitrophenyl ligands. Kinetics of labeling and isolation of a labeled peptide. Biochemistry. 1970 Mar 3;9(5):1267–1278. doi: 10.1021/bi00807a031. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Neerunjun D. E., Hunt R. Fate of a liposome-associated agent injected into normal and tumour-bearing rodents. Attempts to improve localization in tumour tissues. Life Sci. 1977 Aug 1;21(3):357–369. doi: 10.1016/0024-3205(77)90516-1. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Neerunjun E. D. Homing of liposomes to target cells. Biochem Biophys Res Commun. 1975 Jul 22;65(2):537–544. doi: 10.1016/s0006-291x(75)80180-x. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G. The carrier potential of liposomes in biology and medicine (first of two parts). N Engl J Med. 1976 Sep 23;295(13):704–710. doi: 10.1056/NEJM197609232951305. [DOI] [PubMed] [Google Scholar]
- Handwerger B. S., Koren H. S. The nature of the effector cell in antibody-dependent, cell-mediated cytolysis (ADCC): the cytotoxic activity of murine tumor cells and peritoneal macrophages. Clin Immunol Immunopathol. 1976 Mar;5(2):272–281. doi: 10.1016/0090-1229(76)90032-5. [DOI] [PubMed] [Google Scholar]
- Koren H. S., Handwerger B. S., Wunderlich J. R. Identification of macrophage-like characteristics in a cultured murine tumor line. J Immunol. 1975 Feb;114(2 Pt 2):894–897. [PubMed] [Google Scholar]
- Leserman L. D., Weinstein J. N., Blumenthal R., Sharrow S. O., Terry W. D. Binding of antigen-bearing fluorescent liposomes to the murine myeloma tumor MOPC 315. J Immunol. 1979 Feb;122(2):585–591. [PubMed] [Google Scholar]
- Lewis J. T., McConnell H. M. Model lipid bilayer membranes as tragets for antibody-dependent, cellular- and complement-mediated immune attack. Ann N Y Acad Sci. 1978;308:124–138. doi: 10.1111/j.1749-6632.1978.tb22018.x. [DOI] [PubMed] [Google Scholar]
- Newman G. C., Huang C. Structural studies on phophatidylcholine-cholesterol mixed vesicles. Biochemistry. 1975 Jul 29;14(15):3363–3370. doi: 10.1021/bi00686a012. [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Weinstein J. N. Interactions of liposomes with mammalian cells. Annu Rev Biophys Bioeng. 1978;7:435–468. doi: 10.1146/annurev.bb.07.060178.002251. [DOI] [PubMed] [Google Scholar]
- Segal D. M., Hurwitz E. Dimers and trimers of immunoglobulin G covalently cross-linked with a bivalent affinity label. Biochemistry. 1976 Nov 30;15(24):5253–5258. doi: 10.1021/bi00669a009. [DOI] [PubMed] [Google Scholar]
- Six H. R., Uemura K. I., Kinsky S. C. Effect of immunoglobulin class and affinity on the initiation of complement-dependent damage to liposomal model membranes sensitized with dinitrophenylated phospholipids. Biochemistry. 1973 Sep 25;12(20):4003–4011. doi: 10.1021/bi00744a034. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Pike M. C., Fischer D. G., Koren H. S. Biologic and biochemical activities of continuous macrophage cell lines P388D1 and J774.1. J Immunol. 1977 Dec;119(6):2060–2066. [PubMed] [Google Scholar]
- Uhr J. W. Passive sensitization of lymphocytes and macrophages by antigen-antibody complexes. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1599–1606. doi: 10.1073/pnas.54.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. N., Blumenthal R., Sharrow S. O., Henkart P. A. Antibody-mediated targeting of liposomes. Binding to lymphocytes does not ensure incorporation of vesicle contents into the cells. Biochim Biophys Acta. 1978 May 18;509(2):272–288. doi: 10.1016/0005-2736(78)90047-0. [DOI] [PubMed] [Google Scholar]
- Weinstein J. N., Yoshikami S., Henkart P., Blumenthal R., Hagins W. A. Liposome-cell interaction: transfer and intracellular release of a trapped fluorescent marker. Science. 1977 Feb 4;195(4277):489–492. doi: 10.1126/science.835007. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Bloomgarden D., Kaplan R., Cohen C., Hoffstein S., Collins T., Gotlieb A., Nagle D. A general method for the introduction of enzymes, by means of immunoglobulin-coated liposomes, into lysosomes of deficient cells. Proc Natl Acad Sci U S A. 1975 Jan;72(1):88–92. doi: 10.1073/pnas.72.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]