Abstract
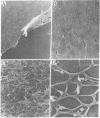
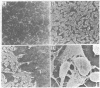
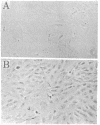
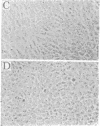
Corneal endothelial cells maintained in tissue culture retain the ability to synthesize and secrete an extracellular matrix (ECM) along their basal cell surface. Treatment of confluent cultures with 0.5% Triton X-100 results in the removal of the cell monolayer, thereby exposing the ECM, which adheres strongly to the tissue culture dish. Dishes coated with ECM were used to study the permissive effect of such a substrate on cell proliferation. The proliferation of bovine granulosa and adrenal cortex cells maintained on plastic tissue culture dishes was compared to that on dishes coated with ECM. Neither cell type, even when exposed to optimal serum concentration, replicated when seeded at low cell density on plastic. In contrast, when seeded on ECM they proliferated actively. None of the cultures maintained on ECM required fibroblast growth factor in order to reach confluence, although when maintained on plastic they were totally dependent on fibroblast growth factor for proliferation. Because cells maintained on plastic do not respond to factors present in serum or plasma, although they do so respond when maintained on ECM, it is likely that the close contact of the cells with the ECM restores their sensitivity to agents present in serum and plasma.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EHRMANN R. L., GEY G. O. The growth of cells on a transparent gel of reconstituted rat-tail collagen. J Natl Cancer Inst. 1956 Jun;16(6):1375–1403. [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- GROBSTEIN C. Morphogenetic interaction between embryonic mouse tissues separated by a membrane filter. Nature. 1953 Nov 7;172(4384):869–870. doi: 10.1038/172869a0. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Birdwell C. R. Determination of cellular shape by the extracellular matrix and its correlation with the control of cellular growth. Cancer Res. 1978 Nov;38(11 Pt 2):4155–4171. [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Birdwell C. R. Determination of cellular shape by the extracellular matrix and its correlation with the control of cellular growth. Cancer Res. 1978 Nov;38(11 Pt 2):4155–4171. [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G. The coating of bovine and rabbit corneas denuded of their endothelium with bovine corneal endothelial cells. Exp Eye Res. 1979 Mar;28(3):249–265. doi: 10.1016/0014-4835(79)90087-3. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Vlodavsky I., Alvarado J., Johnson L. K. The identification and localization of fibronectin in cultured corneal endothelial cells: cell surface polarity and physiological implications. Exp Eye Res. 1979 Nov;29(5):485–509. doi: 10.1016/0014-4835(79)90151-9. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Ill C. R., Birdwell C. R. Effects of fibroblast and epidermal growth factors on ovarian cell proliferation in vitro. I. Characterization of the response of granulosa cells to FGF and EGF. Endocrinology. 1977 Apr;100(4):1108–1120. doi: 10.1210/endo-100-4-1108. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Ill C. R. Do plasma and serum have different abilities to promote cell growth? Proc Natl Acad Sci U S A. 1980 May;77(5):2726–2730. doi: 10.1073/pnas.77.5.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Ill C. R., Hornsby P. J., Gill G. N. Control of bovine adrenal cortical cell proliferation by fibroblast growth factor. Lack of effect of epidermal growth factor. Endocrinology. 1977 Apr;100(4):1080–1089. doi: 10.1210/endo-100-4-1080. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Ill C. Extracellular matrix and control of proliferation of vascular endothelial cells. J Clin Invest. 1980 Jun;65(6):1351–1364. doi: 10.1172/JCI109799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Mescher A. L., Birdwell C. R. Stimulation of corneal endothelial cell proliferations in vitro by fibroblast and epidermal growth factors. Exp Eye Res. 1977 Jul;25(1):75–89. doi: 10.1016/0014-4835(77)90248-2. [DOI] [PubMed] [Google Scholar]
- Greenburg G., Vlodavsky I., Foidart J. M., Gospodarowicz D. Conditioned medium from endothelial cell cultures can restore the normal phenotypic expression of vascular endothelium maintained in vitro in the absence of fibroblast growth factor. J Cell Physiol. 1980 May;103(2):333–347. doi: 10.1002/jcp.1041030219. [DOI] [PubMed] [Google Scholar]
- Grobstein C. Mechanisms of organogenetic tissue interaction. Natl Cancer Inst Monogr. 1967 Sep;26:279–299. [PubMed] [Google Scholar]
- Orly J., Sato G. Fibronectin mediates cytokinesis and growth of rat follicular cells in serum-free medium. Cell. 1979 Jun;17(2):295–305. doi: 10.1016/0092-8674(79)90155-7. [DOI] [PubMed] [Google Scholar]
- Rath N. C., Reddi A. H. Collagenous bone matrix is a local mitogen. Nature. 1979 Apr 26;278(5707):855–857. doi: 10.1038/278855a0. [DOI] [PubMed] [Google Scholar]
- Reid L. M., Rojkind M. New techniques for culturing differentiated cells: reconstituted basement membrane rafts. Methods Enzymol. 1979;58:263–278. doi: 10.1016/s0076-6879(79)58142-7. [DOI] [PubMed] [Google Scholar]
- Saxen L., Lehtonen E., Karkinen-Jäskeläinen M., Nordling S., Wartiovaara J. Are morphogenetic tissue interactions mediated by transmissible signal substances or through cell contacts? Nature. 1976 Feb 26;259(5545):662–663. doi: 10.1038/259662a0. [DOI] [PubMed] [Google Scholar]
- Stenman S., Vaheri A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J Exp Med. 1978 Apr 1;147(4):1054–1064. doi: 10.1084/jem.147.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Johnson L. K., Greenburg G., Gospodarowicz D. Vascular endothelial cells maintained in the absence of fibroblast growth factor undergo structural and functional alterations that are incompatible with their in vivo differentiated properties. J Cell Biol. 1979 Nov;83(2 Pt 1):468–486. doi: 10.1083/jcb.83.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Lui G. M., Gospodarowicz D. Morphological appearance, growth behavior and migratory activity of human tumor cells maintained on extracellular matrix versus plastic. Cell. 1980 Mar;19(3):607–616. doi: 10.1016/s0092-8674(80)80037-7. [DOI] [PubMed] [Google Scholar]
- WESSELLS N. K. SUBSTRATE AND NUTRIENT EFFECTS UPON EPIDERMAL BASAL CELL ORIENTATION AND PROLIFERATION. Proc Natl Acad Sci U S A. 1964 Aug;52:252–259. doi: 10.1073/pnas.52.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Richards J., Bowman P., Guzman R., Enami J., McCormick K., Hamamoto S., Pitelka D., Nandi S. Sustained growth and three-dimensional organization of primary mammary tumor epithelial cells embedded in collagen gels. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3401–3405. doi: 10.1073/pnas.76.7.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]