Abstract
The neuropeptide oxytocin (OXT) can enhance the impact of positive social cues but may reduce that of negative ones by inhibiting amygdala activation, although it is unclear whether the latter causes blunted emotional and mnemonic responses. In two independent double-blind placebo-controlled experiments, each involving over 70 healthy male subjects, we investigated whether OXT affects modulation of startle reactivity by aversive social stimuli as well as subsequent memory for them. Intranasal OXT potentiated acoustic startle responses to negative stimuli, without affecting behavioral valence or arousal judgments, and biased subsequent memory toward negative rather than neutral items. A functional MRI analysis of this mnemonic effect revealed that, whereas OXT inhibited amygdala responses to negative stimuli, it facilitated left insula responses for subsequently remembered items and increased functional coupling between the left amygdala, left anterior insula, and left inferior frontal gyrus. Our results therefore show that OXT can potentiate the protective and mnemonic impact of aversive social information despite reducing amygdala activity, and suggest that the insula may play a role in emotional modulation of memory.
Keywords: emotion, functional imaging, psychophysiology, cognition
Current concepts of the neuromodulatory role of the peptide hormone oxytocin (OXT) in human cognition and behavior emphasize its prosocial effects. Ample evidence for this view comes from a plethora of behavioral experiments in healthy subjects (1), which have demonstrated beneficial effects of a single intranasal dose (24–48 IU) of OXT across a wide range of cognitive tasks, probing, for example, interpersonal trust and cooperation (2, 3; see also ref. 4), generosity (5), social recognition (6–8) and related memory (9–11), social reinforcement learning and emotional empathy (12), and social judgments (13–15).
This prosocial perspective on OXT is challenged, however, by evidence that OXT also enhances envy and schadenfreude (gloating) (16), ethno-centrism (including prejudice, xenophobia, and racial bias) (4), and outgroup derogation (17). Moreover, OXT hinders trust and cooperation when social information about interaction partners is lacking (18). Furthermore, OXT appears to negatively bias recollections of maternal care and closeness and to diminish trust and cooperation in insecurely or anxiously attached individuals (19, 20).
In an attempt to reconcile this controversial evidence, it has been proposed that the social effects of OXT could be mediated by reduced anxiety or by an increased perceptual salience of social cues (21). The anxiolytic action of OXT has been confirmed by showing reduced amygdala responses to aversive social stimuli in healthy people (22–25; but see also refs. 26 and 27), and subjects with social phobia (28). It is compatible with decreased endocrine and subjective responses to social stress (29), as well as reduced negative cognitive self-appraisal in individuals scoring high in trait-anxiety (30). In contrast, the social salience hypothesis has gained substantial support from studies demonstrating increased eye contact (31) and improved mind-reading from facial expressions (32) as a result of OXT treatment. Whether these mechanisms quintessentially yield positive or negative social outcomes may vary depending on contextual or person-specific characteristics (21). An alternative view holds that emotional valence may be the key in guiding the social effects of OXT, with it facilitating social approach to positive cues and inhibiting social withdrawal from negative ones (33).
Against this empirical background we performed two experiments, using paradigms in the social-protective domain, to disentangle the valence-specific effects of OXT and to determine if anxiolytic, salience, or approach-enhancing mechanisms are most influential. Specifically, Exp. 1 examined the emotional modulation of the acoustic startle reflex (ASR) and we predicted that anxiolytic effects should lead to overall diminished ASR magnitudes and less pronounced potentiation of the ASR in the context of negative stimuli. On the other hand, the social-salience hypothesis would be compatible with facilitated emotional modulation both with negative and positive stimuli, whereas the social-approach/withdrawal hypothesis would predict differential modulation, with startle responses being reduced to negative (withdrawal) and increased to positive (approach) stimuli. Exp. 2 addressed the question of how OXT shapes the neural correlates of emotion perception and subsequent memory, thus enabling us to characterize the neuromodulatory influence of OXT on information of both immediate and future relevance. Decreased amygdala responses to negative stimuli and reduced memory for them should be a consequence of anxiolytic effects, whereas an increased salience of such stimuli could improve subsequent memory performance. On the other hand, if OXT enhances social approach behavior then one might predict either no effect—because negative stimuli would not normally promote approach behavior—or possibly make them less memorable because of weakening of the normal withdrawal response.
Results
Sample Characteristics.
From 80 subjects enrolled in Exp. 1, 11 subjects [OXT, n = 5; PLC (placebo: sodium chloride solution), n = 6] were excluded from further analysis because of technical failures during data acquisition (n = 4), mood alterations (n = 1), or lack of distinct startle responses (n = 6). Consequently, subsequent analyses included data from 69 subjects (OXT, n = 36; PLC, n = 33). From the 73 subjects enrolled in Exp. 2, three were excluded from further analysis because of technical failures during data acquisition. Consequently, subsequent analyses were performed on the data obtained from 70 subjects (OXT, n = 35; PLC, n = 35). In both experiments, treatment groups showed no differences regarding demographics, pretreatment neuropsychological performance, attention, or anxiety (Tables S1 and S2).
Experiment 1.
Emotion-modulated startle.
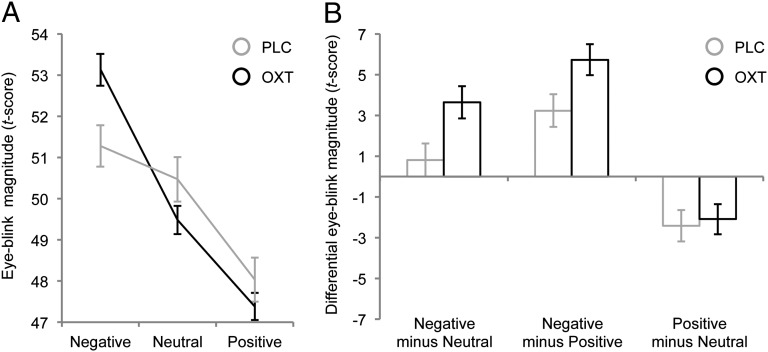
A repeated-measures ANOVA with “group” (PLC, OXT) as between-subjects factor, “valence” (negative, neutral, or positive) as within-subjects factor, and “ASR magnitude” as dependent variable revealed a main effect of valence [F(2, 134) = 34.49, P < 0.01, η2 = 0.34] and an interaction effect of valence and group [F(2, 134) = 4.09, P = 0.02, η2 = 0.06]. Both groups showed a significant linear trend for valence (i.e., negative > neutral > positive), but a comparison of the effect sizes indicated that the emotional modulation in the OXT group [F(1, 35) = 84.10, P < 0.01, η2 = 0.71] was more than twice as large as in the PLC group [F(1, 32) = 13.78, P < 0.01, η2 = 0.30] (Fig. 1A). Post hoc Bonferroni-corrected two-sample t tests showed that the ASR magnitude in the OXT group was significantly larger than in the PLC group during the viewing of negative [t(67) = 2.92, P = 0.01, η2 = 0.11], but not neutral [t(54.94) = −1.54, P = 0.37, η2 = 0.04] or positive [t(53.91) = −1.04, P = 0.91, η2 = 0.02] pictures. The contrasts “negative minus neutral” [t(57.77) = 2.56, P = 0.01, η2 = 0.09] and “negative minus positive” [t(59.07) = 2.33, P = 0.02, η2 = 0.08] yielded significantly larger difference scores in the OXT group, but there was no OXT effect for the contrast “positive minus neutral” [t(67) = 0.31, P = 0.76, η2 < 0.01] (Fig. 1B). In summary, administration of OXT profoundly potentiated the emotional modulation of the ASR specifically for negative stimuli.
Fig. 1.
Exp. 1 examined the effects of OXT relative to PLC on emotion-modulated startle reactivity. (A) Both groups showed a significant linear trend for valence (i.e., negative > neutral > positive), but the emotional modulation of eye-blink magnitudes in the OXT group was more than twice as large as in the PLC group. (B) The difference in eye-blink magnitude between negative and neutral as well as negative and positive conditions (but not between positive and neutral) was significantly larger in the OXT group than in the PLC group. Taken together, these results show that OXT profoundly potentiated the emotional modulation of the startle response specifically for negative stimuli. Error bars indicate the SEM.
Emotion ratings.
A repeated-measures ANOVA with “group” (PLC, OXT) as between-subjects factor, “valence” (negative, neutral, positive) as within-subjects factor, and “valence ratings” as dependent variable yielded a main effect of valence [F(1.38, 92.46) = 270.68, P < 0.01, η2 = 0.80], but no interaction effect [F(1.38, 92.46) = 0.22, P = 0.72, η2 < 0.01]. As expected, negative pictures were rated as more aversive than neutral ones and positive pictures as more pleasant than neutral ones (Table S3). For the arousal ratings, there was a main effect of valence [F(2, 134) = 302.18, P < 0.01, η2 = 0.82] but no interaction effect [F(2, 134) = 0.16, P = 0.85, η2 < 0.01]. Negative and positive pictures were therefore rated as equally more arousing than neutral ones in both groups (Table S3).
Baseline startle response.
Raw startle response magnitudes from the interstimulus intervals were individually examined. At visual inspection, the overall startle magnitude seemed to be smaller in the OXT group (mean ± SD, 31.0 ± 46.2 μV) than in the PLC group (47.1 ± 40.4 μV); however, an independent-samples t test revealed no significant difference [t(67) = −1.54, P = 0.13, η2 = 0.03]. Similarly, the raw startle response magnitude in the neutral condition for the OXT group (25.9 ± 42.7 μV) was not significantly different from that of the PLC group (40.8 ± 34.6 μV) [t(67) = −1.6, P = 0.12, η2 = 0.04].
Experiment 2.
Behavioral data.
Two-sample t tests revealed no between-group differences in the overall number of subsequently remembered items nor in the numbers of subsequently remembered negative or neutral items (all P values > 0.20). Thus, OXT had no influence on memory capacity in general nor on a valence-specific effect. However, we observed a shift in the proportion of subsequently remembered negative to neutral items. Specifically, the OXT group showed a subsequent memory bias toward negative information at the cost of neutral information [remembered negative items minus remembered neutral items: OXT, 4.9 ± 2.8; PLC, −2.8 ± 3.0; t(68) = −2.98, P < 0.01, η2 = 0.12] (Fig. 2A and Table S4).
Fig. 2.
Exp. 2 examined the effects of OXT relative to PLC on subsequent memory for emotional stimuli. (A) Both groups did not differ in their total amount of later remembered items (absolute numbers are given in the bars) in the surprise free-recall test 24 h postscan. However, OXT induced a shift in the relative proportion of later remembered items, evident in a recall bias toward aversive social information. (B) Successful encoding of negative stimuli was associated with larger left anterior insula responses (MNI: x = −38, y = 22, z = 4) in the OXT group, an effect that was reversed in the PLC group. Extraction of individual percent signal changes confirmed this pattern. For illustration purposes, results are displayed at uncorrected significance (P < 0.001) thresholds. Error bars indicate the SEM.
Whole-brain analysis of functional MRI data.
The pooled sample showed greater activity in response to negative stimuli in left inferior occipital gyrus [t(34) = 8.87, PFWE < 0.05, Montreal Neurological Institute (MNI): x = −44, y = −70, z = −6] and right middle occipital gyrus [t(34) = 8.58, PFWE < 0.05, MNI: x = 48, y = −80, z = 4] (main effect of “valence”). In general, successful encoding was associated with greater responses in left fusiform gyrus [t(34) = 6.08, PFWE < 0.05, MNI: x = −30, y = −48, z = −14] (main effect of “subsequent memory”). Separate analyses for the PLC and OXT groups yielded no significant clusters of activation for these contrasts. Although there was no main effect of OXT treatment, we found a group × subsequent memory interaction effect in the left anterior insula for negative but not for neutral stimuli [t(68) = 6.08, PFWE < 0.05, MNI: x = −38, y = 22, z = 4) (Fig. 2B and Table S5). Extraction of individual percent signal changes revealed that successful encoding of negative stimuli was associated with greater left anterior insula responses in the OXT group, an effect that was reversed in the PLC group (Fig. 2B). Combined masking confirmed that this interaction effect was independent of simple main effects.
Regions of interest analysis of functional MRI data.
In line with previous findings (22–25; but see also refs. 26 and 27), amygdala activity was globally reduced as a result of OXT treatment (Table S5). This reduction was histoprobabilistically mapped to the superficial amygdala, reflecting the demonstrated importance of this amygdala subregion in social stimulus processing (34, 35).
Functional connectivity analysis of functional MRI data.
Given that the amygdala has been identified as a key target of OXT effects in the brain (22–27), we focused, in a first analysis, on potential effects of OXT treatment on functional connectivity of the amygdala using a generalized form of context-dependent psychophysiological interactions (gPPIs) analysis (36). Our results showed that OXT reduced functional coupling between the left amygdala and left anterior cingulate cortex (ACC) [t(68) = 4.35, PFWE < 0.05, MNI: x = −4, y = 26, z = 14] for all stimuli regardless of valence. For negative stimuli OXT decreased, whereas for neutral stimuli it increased functional coupling between the right amygdala and left insula [t(68) = 4.23, PFWE < 0.05, MNI: x = −42, y = −2, z = 16]. These profiles were confirmed by extraction of individual parameter estimates (Fig. 3A). In a second analysis, we addressed the question of whether the observed OXT effects in the left anterior insula also influenced functional coupling with interconnected brain regions. Our analysis showed that OXT increased functional coupling between left anterior insula and both left inferior frontal gyrus (IFG) [t(68) = 4.53, PFWE < 0.05, MNI: x = −46, y = 0, z = 24] and left basolateral amygdala (BLA) [t(68) = 3.46, PFWE < 0.05, MNI: x = −30, y = −14, z = −11] during successful encoding of negative stimuli. Extraction of individual parameter estimates confirmed these profiles in the OXT group, whereas the PLC group exhibited the reverse pattern (Fig. 3B). Given a signal distribution of 25% in the hippocampus and 75% in the BLA, histoprobabilistic mapping identified the BLA as the major projection area of OXT-related enhanced cross-talk between the left anterior insula and left amygdala during successful encoding of negative stimuli.
Fig. 3.
Results of the functional connectivity analysis of functional MRI data acquired in Exp. 2. (A) Effects of OXT on functional connectivity of the left and right amygdala (seed regions). OXT reduced functional coupling between the left amygdala and left ACC (Upper) and between the right amygdala and left anterior insula (Lower). (B) Effects of OXT on functional connectivity of the left anterior insula (seed region). OXT increased functional coupling with the left IFG (Upper) and left BLA (Lower). Error bars indicate the SEM. For illustration purposes, results are displayed at uncorrected significance (P < 0.001) thresholds. a.u., arbitrary units.
Discussion
Despite the reported anxiolytic effects of OXT, and extensive evidence from our study and others for its suppression of amygdala reactivity to fear signals (22–25; but see also refs. 26 and 27), our results show that the peptide enhances the impact of aversive social information. Specifically, OXT facilitated startle responses to negative stimuli and increased the number of subsequently remembered negative relative to neutral items. Furthermore, our findings suggest that OXT may be acting to promote this potentiated impact of aversive social stimuli by facilitating anterior insula responses to them and by modifying its functional connectivity with the IFG and amygdala. Importantly, despite OXT reducing superficial amygdala responses to negative stimuli, the latter still produced startle and subsequent memory-modulating effects. Histoprobabilistic mapping indeed identified the BLA as the major projection area of OXT-related enhanced cross-talk between the left anterior insula and left amygdala during successful encoding of negative stimuli, suggesting that OXT puts the BLA under neural control of the insula. By hijacking the modulatory functions of the BLA, the insula may become more influential in biasing emotional and cognitive processing toward information gaining crucial behavioral relevance under conditions of enhanced OXT signaling.
Our original aim was to distinguish between three current hypotheses concerning the role of OXT in the human brain, namely as a facilitator of (i) prosocial behavior, (ii) social salience, and (iii) social approach/withdrawal. Our results show that OXT does not simply facilitate responses to positive social cues and reduce them to negative ones, because in both experiments we found evidence for enhanced emotional modulation by, and subsequent memory for, aversive social information. Instead, our data seem to support specific aspects of the social salience and social-approach/withdrawal hypotheses, although before considering which is most relevant, we need to first discuss the implications of our findings in greater detail.
Our result of an enhanced ASR modulation by aversive information following OXT treatment shows that the peptide is not producing an overall anxiolytic effect. Although we found a trend to significant decrease in ASR baseline after OXT application, which is in line with previous findings (22, 37), these anxiolytic actions are clearly not the most influential effects of OXT in our study. Indeed, we found no evidence that OXT affected either arousal or valence ratings for any of the stimuli used. OXT also had no effect on skin conductance (38) or facial electromyographic (EMG) responses during the actual presentation of emotional stimuli (see SI Text). Thus, OXT facilitation of ASR modulation by aversive pictures appeared to be independent of any physiological arousal or conscious arousal/valence ratings (39, 40). Although we cannot completely rule out a contribution of physiological arousal changes to the observed facilitating effects of OXT, it seems unlikely that altered conscious feelings of arousal were playing a substantial role.
In view of our findings from Exp. 1, those in Exp. 2 showing an OXT-induced bias toward remembering aversive rather than neutral social information are also unlikely to be a result of altered arousal or valence ratings. Although a number of studies demonstrating anxiolytic effects of OXT (29, 41) and suppression of amygdala reactivity to fear signals (22–25; but see also refs. 26 and 27) have suggested a potential amnestic effect for aversive stimuli, direct evidence for this has been limited. In humans, OXT reduced emotional ratings to aversively conditioned (25) and angry faces (14). Although it has been hypothesized that OXT promotes amnesia for pain, there is in fact no direct evidence for this (1). Thus, despite assumptions that OXT release might aid forgetting of negative emotional experiences, actual evidence in humans is lacking. Indeed, results from our study suggest rather that OXT can bias learning in favor of aversive compared with neutral social information in at least some contexts.
Taking these data together, a simple arousal-based explanation of our findings is unlikely. Instead, it seems likely that OXT can enhance some aspect of salience for aversive stimuli independent of arousal. We have shown previously that OXT elevates emotional empathy responses toward pictures of individuals expressing either strong negative or positive emotions (12). In our present study increased activation of the anterior insula for subsequently remembered negative items following OXT treatment may also reflect enhanced empathy and compassion (42), although in this case, empathy and compassion for the pain being suffered by individuals depicted in most of the stimuli. In this context we note that the insula responds both to the actual feeling of pain and also to pain being experienced by someone else (42–44). Although a previous study failed to find any effect of OXT on pain empathy in a paradigm where subjects watched their partner receiving a painful procedure (42), this was arguably a much more salient stimulus than for the subjects in our current study viewing aversive pictures. Indeed, two studies reporting OXT effects on empathy using the “reading the mind in the eyes” (32) or a test of empathic accuracy (45) found that these effects were influenced by task difficulty or initial empathy level. Furthermore, Riem et al. (46) demonstrated OXT-induced decreases of amygdala and increases of insular cortex and IFG responses to crying babies in women without children of their own. The fact that we also found that OXT increased functional coupling between the anterior insula and the IFG provides further evidence of an enhanced empathic response because both regions constitute core areas of the empathy network (42, 47–50). The observed pattern of valence-specific increased anterior insula-IFG coupling thus may reflect enhanced emotional empathic responses (12).
In addition to empathy and compassion, the anterior insula has been implicated in interoceptive awareness and estimation of uncertainty and risk (44, 51–55). In the context of our experiments the effects of OXT in increasing the impact of aversive social information may reflect enhanced perception of visceral reactions and feelings of uncertainty and risk arising from elevated empathic responses, promoting approach and protective behavior toward individuals exposed to social threat.
Our results also reveal an important interplay between the amygdala and the insula after OXT treatment. Not only did OXT reduce superficial amygdala activity but also the functional connectivity between the right amygdala and left insula. Conversely, connectivity between the left insula and left amygdala was strengthened under OXT. Previous research has established amygdala–hippocampal interactions as being key to emotional modulation of memory (56), and the amygdala may also be an important site for OXT modulation of emotional empathy (1, 12). However, our results herein suggest that although OXT suppresses superficial amygdala responses to aversive social stimuli, it may still enhance empathy and modulation of memory in response to these stimuli. This result may be because of the insula taking on a more controlling functional role in mediating these latter behaviors. What would be the advantage of such a dual mechanism of action? One possibility is that it could first promote increased approach behavior toward other individuals exhibiting negative emotional signals but requiring social support (such as crying babies or victims of violence). This result could be achieved by reducing amygdala responses. However, increased caring behavior toward such individuals through enhanced empathy, memory for the emotional event concerned, and heightened preparedness for defense is also required. This result could be achieved by an enhanced insula response. In summary, OXT could alter the balance of interplay between amygdala and insula such that social support and protection is more likely to be offered to individuals who need it, even if they exhibit aversive social signals that would normally be avoided.
Several limitations of our study should be acknowledged. First, our study included only men and, against the background that previous studies reported sex-specific effects of OXT (26, 27), we cannot extrapolate our findings to women. Second, we observed an increased protective/defensive response to social-emotional pictures after OXT administration, but we do not know to what extent these effects are restricted to the social domain.
In summary, OXT clearly does not act simply as an anxiolytic and facilitates recognition of and responses to positive social cues. In terms of the remaining two hypotheses relating to enhancing the salience of social stimuli and increasing approach behavior toward them, our results tend to favor the latter. The absence of altered valence ratings for aversive social stimuli following OXT treatment suggests that increased salience of these stimuli is unlikely because this would also predict altered valence. On the other hand, an increased empathic response toward such stimuli would also predict enhanced approach and potentially protective behavior. However, at the same time our findings of facilitated ASR modulation suggest that in parallel with increased approach behavior, there is also a heightened preparedness for defense or flight. So perhaps a more accurate description of what OXT is promoting in these circumstances is “approach and protective behavior, but with heightened caution.” The empathogenic actions of OXT would clearly facilitate approach behavior but the peptide may also increase preparedness for defense as opposed to flight. The attenuated responsivity of the superficial amygdala to aversive social stimuli may act to reduce the likelihood of flight behavior, whereas the increased insula response may reflect both increased empathy and approach behavior toward threatened and suffering individuals together with a heightened visceral reaction and increased feeling of uncertainty and risk.
Methods
A detailed synopsis of all experimental procedures is provided in the SI Text.
Subjects.
Detailed information on study participants is provided in the SI Text. The present study was approved by the institutional review board of the Medical Faculty of the University of Bonn, registered as a controlled clinical trial (ClinicalTrials.gov Identifiers: NCT01606462 and NCT01607970), and carried out in compliance with the latest revision of the Declaration of Helsinki.
Drug Application.
The two experiments underlying the present study followed a randomized, placebo-controlled, double-blind, between-group design; that is, subjects were randomly assigned to either intranasal administration of OXT (24 IU; Syntocinon-Spray, Novartis; three puffs per nostril, each with 4 IU OXT) or PLC (sodium chloride solution), 45 min after which the experimental tasks were carried out.
Exp. 1.
Participants were exposed to acoustic startle probes presented either alone or paired with a color picture. The paradigm featured 20 negative, 20 neutral, and 20 positive pictures, presented for 5 s each, which were mostly selected from the International Affective Picture System (57). Similar to the procedure reported by Becker et al. (58), the startle stimulus consisted of a single 50-ms burst of white noise (100 dB) with nearly instantaneous rise and was delivered binaurally via headphones during 60% of the pictures (i.e., 12 from each category) at 2–4 s after picture onset. Facial EMG activity was recorded from two Ag/AgCl electrodes placed over the orbicularis oculi muscle below the left eye (59). Furthermore, electrodermal activity was measured, and facial EMG activity was also analyzed in trials without startle probe. After task completion, participants were administered the self-assessment manikin (57) to obtain behavioral pleasance (valence) and arousal ratings for each picture on a scale ranging from 1 (minimum) to 9 (maximum).
Exp. 2.
This experiment consisted of two phases, a functional MRI (fMRI)-scanned encoding phase under drug challenge conditions and a surprise free-recall test 24 h postscan. Critically, for analyzing the subsequent memory (difference due to memory) effect, behavioral responses during free-recall were used to backsort encoding trials as either successful (i.e., subsequently remembered) or not. The fMRI paradigm incorporated 24 aversive and 24 neutral picture stimuli selected from the International Affective Picture System (57) and a verbal description (a noun semantically identical to the picture) (60). Each stimulus was presented four times. Stimuli were shown in a random order for 5 s each, followed by a fixation cross that was presented for 2.5–4.5 s and served as a low-level baseline. MRI data were acquired on a 1.5 Tesla MRI scanner and preprocessed using standard procedures. MRI data were analyzed using SPM8 (Wellcome Trust Centre for Neuroimaging, London, United Kingdom). In the first-level analysis the following conditions were modeled: “neutral: later remembered,” “neutral: later forgotten,” “aversive: later remembered,” and “aversive: later forgotten,” using a stick function convolved with a hemodynamic response function (61). To examine if OXT affected the differential neural responses for later-remembered versus forgotten items separately for the aversive and neutral condition, we contrasted the OXT group with the PLC group (OXTaversive: later remembered > aversive: later forgotten > PLCaversive: later remembered > aversive: later forgotten and OXTneutral: later remembered > neutral: later forgotten > PLCneutral: later remembered > neutral: later forgotten). Furthermore, groups were compared using the contrasts “aversive items > neutral items” (main effect of “valence”); “all subsequently remembered items > all forgotten items” (main effect of “subsequent memory”); and “all items > low-level baseline” (main effect of “group”). Emotion-specific effects of OXT treatment were examined using the contrasts “negative items > low-level baseline” and “neutral items > low-level baseline.” To address OXT’s effects on functional connectivity a PPI analysis was carried out using a gPPI (36). Compared with the standard PPI implementation in SPM, gPPI analysis allows to model more than two task conditions in the same model by spanning the entire experimental space to improve model fit, specificity to true negative findings, and sensitivity to true positive findings (36). First, we examined potential modulatory effects of OXT on functional connectivity of the amygdala. For this aim, we extracted the mean time series for each subject from the left and right amygdala that were defined by using the Wake Forest University Pickatlas (61–63). Second, we examined whether the OXT effect on functional activation in the left anterior insula affected functional coupling of this region with interconnected areas. For this aim, mean time series were extracted from a 6-mm sphere centered at the maximum interaction effect found in the functional activation analysis (MNI: x = −38, y = 22, z = 4). In all analyses, groups were compared using two-sample t tests, and group means were tested using one sample t tests with thresholds of P < 0.05 family-wise error-corrected for multiple comparisons. Details on image acquisition, preprocessing and statistical analysis are reported in the SI Text.
Supplementary Material
Acknowledgments
R.H. was supported by Deutsche Forschungsgemeinschaft Grant HU1302/2-2 and by a Starting Independent Researcher Grant (Neuromodulation of Emotion), jointly provided by the Ministry of Innovation, Science, Research, and Technology of the German State of North Rhine-Westphalia and the University of Bonn.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1208852109/-/DCSupplemental.
References
- 1.Striepens N, Kendrick KM, Maier W, Hurlemann R. Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Front Neuroendocrinol. 2011;32(4):426–450. doi: 10.1016/j.yfrne.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 3.Mikolajczak M, et al. Oxytocin makes people trusting, not gullible. Psychol Sci. 2010;21(8):1072–1074. doi: 10.1177/0956797610377343. [DOI] [PubMed] [Google Scholar]
- 4.De Dreu CK, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJ. Oxytocin promotes human ethnocentrism. Proc Natl Acad Sci USA. 2011;108(4):1262–1266. doi: 10.1073/pnas.1015316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zak PJ, Stanton AA, Ahmadi S. Oxytocin increases generosity in humans. PLoS ONE. 2007;2(11):e1128. doi: 10.1371/journal.pone.0001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer-Shofty M, Shamay-Tsoory SG, Harari H, Levkovitz Y. The effect of intranasal administration of oxytocin on fear recognition. Neuropsychologia. 2010;48(1):179–184. doi: 10.1016/j.neuropsychologia.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Marsh AA, Yu HH, Pine DS, Blair RJ. Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology (Berl) 2010;209(3):225–232. doi: 10.1007/s00213-010-1780-4. [DOI] [PubMed] [Google Scholar]
- 8.Schulze L, et al. Oxytocin increases recognition of masked emotional faces. Psychoneuroendocrinology. 2011;36(9):1378–1382. doi: 10.1016/j.psyneuen.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Guastella AJ, Mitchell PB, Mathews F. Oxytocin enhances the encoding of positive social memories in humans. Biol Psychiatry. 2008;64(3):256–258. doi: 10.1016/j.biopsych.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Savaskan E, Ehrhardt R, Schulz A, Walter M, Schächinger H. Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology. 2008;33(3):368–374. doi: 10.1016/j.psyneuen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Rimmele U, Hediger K, Heinrichs M, Klaver P. Oxytocin makes a face in memory familiar. J Neurosci. 2009;29(1):38–42. doi: 10.1523/JNEUROSCI.4260-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurlemann R, et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci. 2010;30(14):4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theodoridou A, Rowe AC, Penton-Voak IS, Rogers PJ. Oxytocin and social perception: Oxytocin increases perceived facial trustworthiness and attractiveness. Horm Behav. 2009;56(1):128–132. doi: 10.1016/j.yhbeh.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Evans S, Shergill SS, Averbeck BB. Oxytocin decreases aversion to angry faces in an associative learning task. Neuropsychopharmacology. 2010;35(13):2502–2509. doi: 10.1038/npp.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norman GJ, et al. Selective influences of oxytocin on the evaluative processing of social stimuli. J Psychopharmacol. 2011;25(10):1313–1319. doi: 10.1177/0269881110367452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shamay-Tsoory SG, et al. Intranasal administration of oxytocin increases envy and schadenfreude (gloating) Biol Psychiatry. 2009;66(9):864–870. doi: 10.1016/j.biopsych.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 17.De Dreu CK, et al. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328(5984):1408–1411. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- 18.Declerck CH, Boone C, Kiyonari T. Oxytocin and cooperation under conditions of uncertainty: The modulating role of incentives and social information. Horm Behav. 2010;57(3):368–374. doi: 10.1016/j.yhbeh.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Bartz JA, et al. Effects of oxytocin on recollections of maternal care and closeness. Proc Natl Acad Sci USA. 2010;107(50):21371–21375. doi: 10.1073/pnas.1012669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartz J, et al. Oxytocin can hinder trust and cooperation in borderline personality disorder. Soc Cogn Affect Neurosci. 2011;6(5):556–563. doi: 10.1093/scan/nsq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: Context and person matter. Trends Cogn Sci. 2011;15(7):301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Kirsch P, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25(49):11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Domes G, et al. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62(10):1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 24.Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58(4):639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci. 2008;28(26):6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Domes G, et al. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35(1):83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Lischke A, et al. Oxytocin increases amygdala reactivity to threatening scenes in females. Psychoneuroendocrinology. 2012;37(9):1431–1438. doi: 10.1016/j.psyneuen.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Labuschagne I, et al. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35(12):2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54(12):1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 30.Alvares GA, Chen NT, Balleine BW, Hickie IB, Guastella AJ. Oxytocin selectively moderates negative cognitive appraisals in high trait anxious males. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 31.Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry. 2008;63(1):3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 32.Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007;61(6):731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 33.Kemp AH, Guastella AJ. The role of oxytocin in human affect: A novel hypothesis. Curr Dir Psychol Sci. 2012;20(4):222–231. [Google Scholar]
- 34.Bzdok D, Laird AR, Zilles K, Fox PT, Eickhoff SB. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goossens L, et al. Selective processing of social stimuli in the superficial amygdala. Hum Brain Mapp. 2009;30(10):3332–3338. doi: 10.1002/hbm.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. Neuroimage. 2012;61(4):1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ayers LW, Missig G, Schulkin J, Rosen JB. Oxytocin reduces background anxiety in a fear-potentiated startle paradigm: Peripheral vs central administration. Neuropsychopharmacology. 2011;36(12):2488–2497. doi: 10.1038/npp.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gamer M, Büchel C. Oxytocin specifically enhances valence-dependent parasympathetic responses. Psychoneuroendocrinology. 2012;37(1):87–93. doi: 10.1016/j.psyneuen.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Conzelmann A, et al. Methylphenidate normalizes emotional processing in adult patients with attention-deficit/hyperactivity disorder: Preliminary findings. Brain Res. 2011;1381:159–166. doi: 10.1016/j.brainres.2010.12.085. [DOI] [PubMed] [Google Scholar]
- 40.Thibodeau R. Approach and withdrawal actions modulate the startle reflex independent of affective valence and muscular effort. Psychophysiology. 2011;48(7):1011–1014. doi: 10.1111/j.1469-8986.2010.01159.x. [DOI] [PubMed] [Google Scholar]
- 41.Quirin M, Kuhl J, Düsing R. Oxytocin buffers cortisol responses to stress in individuals with impaired emotion regulation abilities. Psychoneuroendocrinology. 2011;36(6):898–904. doi: 10.1016/j.psyneuen.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Singer T, et al. Effects of oxytocin and prosocial behavior on brain responses to direct and vicariously experienced pain. Emotion. 2008;8(6):781–791. doi: 10.1037/a0014195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54(3):2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 44.Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13(8):334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Bartz JA, et al. Oxytocin selectively improves empathic accuracy. Psychol Sci. 2010;21(10):1426–1428. doi: 10.1177/0956797610383439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riem MM, et al. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: A randomized controlled trial. Biol Psychiatry. 2011;70(3):291–297. doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 48.Iacoboni M. Imitation, empathy, and mirror neurons. Annu Rev Psychol. 2009;60:653–670. doi: 10.1146/annurev.psych.60.110707.163604. [DOI] [PubMed] [Google Scholar]
- 49.Schulte-Rüther M, Markowitsch HJ, Fink GR, Piefke M. Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: A functional magnetic resonance imaging approach to empathy. J Cogn Neurosci. 2007;19(8):1354–1372. doi: 10.1162/jocn.2007.19.8.1354. [DOI] [PubMed] [Google Scholar]
- 50.Nummenmaa L, Hirvonen J, Parkkola R, Hietanen JK. Is emotional contagion special? An fMRI study on neural systems for affective and cognitive empathy. Neuroimage. 2008;43(3):571–580. doi: 10.1016/j.neuroimage.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 51.Rilling JK, et al. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology. 2012;37(4):447–461. doi: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ernst J, Northoff G, Böker H, Seifritz E, Grimm S. Interoceptive awareness enhances neural activity during empathy. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clark L, et al. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131(Pt 5):1311–1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huettel SA. Behavioral, but not reward, risk modulates activation of prefrontal, parietal, and insular cortices. Cogn Affect Behav Neurosci. 2006;6(2):141–151. doi: 10.3758/cabn.6.2.141. [DOI] [PubMed] [Google Scholar]
- 55.Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19(4):1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- 56.LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 2006;7(1):54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- 57.Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. Technical Report A-6. Gainesville, FL: Univ of Florida; 2005. [Google Scholar]
- 58.Becker B, et al. Fear processing and social networking in the absence of a functional amygdala. Biol Psychiatry. 2012;72(1):70–77. doi: 10.1016/j.biopsych.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 59.Fridlund AJ, Cacioppo JT. Guidelines for human electromyographic research. Psychophysiology. 1986;23(5):567–589. doi: 10.1111/j.1469-8986.1986.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 60.Hurlemann R, et al. Noradrenergic modulation of emotion-induced forgetting and remembering. J Neurosci. 2005;25(27):6343–6349. doi: 10.1523/JNEUROSCI.0228-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 62.Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21(1):450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 63.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.