Abstract
Nitric oxide (NO) derived from the activity of neuronal nitric oxide synthase (NOS1) is involved in S-nitrosylation of key sarcoplasmic reticulum (SR) Ca2+ handling proteins. Deficient S-nitrosylation of the cardiac ryanodine receptor (RyR2) has a variable effect on SR Ca2+ leak/sparks in isolated myocytes, likely dependent on the underlying physiological state. It remains unknown, however, whether such molecular aberrancies are causally related to arrhythmogenesis in the intact heart. Here we show in the intact heart, reduced NOS1 activity increased Ca2+-mediated ventricular arrhythmias only in the setting of elevated myocardial [Ca2+]i. These arrhythmias arose from increased spontaneous SR Ca2+ release, resulting from a combination of decreased RyR2 S-nitrosylation (RyR2-SNO) and increased RyR2 oxidation (RyR-SOx) (i.e., increased reactive oxygen species (ROS) from xanthine oxidoreductase activity) and could be suppressed with xanthine oxidoreductase (XOR) inhibition (i.e., allopurinol) or nitric oxide donors (i.e., S-nitrosoglutathione, GSNO). Surprisingly, we found evidence of NOS1 down-regulation of RyR2 phosphorylation at the Ca2+/calmodulin-dependent protein kinase (CaMKII) site (S2814), suggesting molecular cross-talk between nitrosylation and phosphorylation of RyR2. Finally, we show that nitroso–redox imbalance due to decreased NOS1 activity sensitizes RyR2 to a severe arrhythmic phenotype by oxidative stress. Our findings suggest that nitroso–redox imbalance is an important mechanism of ventricular arrhythmias in the intact heart under disease conditions (i.e., elevated [Ca2+]i and oxidative stress), and that therapies restoring nitroso–redox balance in the heart could prevent sudden arrhythmic death.
Nitric oxide (NO) is an important regulator of cardiac function via both the activation of cyclic guanosine monophosphate-dependent signaling pathways and direct posttranslational modification of protein thiols (S-nitrosylation) (1). NO derived from the activity of neuronal nitric oxide synthase (NOS1) is involved in S-nitrosylation of key sarcoplasmic reticulum (SR) Ca2+ handling proteins (2). In particular, nitrosylation of both skeletal and cardiac muscle ryanodine receptors (RyR1 and RyR2, respectively) alters their release properties, favoring activation (3, 4). Notably, an increase in RyR2 open probability can cause spontaneous SR Ca2+ release, which may cause arrhythmias. Recently, it was shown that decreased RyR2 S-nitrosylation (RyR2-SNO) through loss of NOS1, was associated with increased spontaneous SR Ca2+ release events in isolated cardiomyocytes, following rapid pacing (5). In a separate study, NOS1 deficiency was shown to decrease spontaneous SR Ca2+ sparks and the open probability of RyR2 under resting conditions in cardiomyocytes and lipid bilayers, respectively (6). These studies suggest that NOS1 deficiency has a variable effect on RyR2 function, likely dependent on the underlying physiological state (i.e., rapid heart rate versus quiescence). It remains unknown, however, whether these changes create a substrate for arrhythmogenesis in the intact heart.
It is increasingly evident that activities of nitric oxide and reactive oxygen species (ROS) are tightly coupled in cardiomyocytes producing nitroso–redox balance. Elevated ROS production (oxidative stress) is a hallmark of several cardiovascular diseases associated with increased risk of fatal ventricular arrhythmias [e.g., myocardial infarction (MI) and heart failure]. Burger et al. (7) recently demonstrated an increased incidence of ventricular arrhythmias following MI in NOS1-deficient mice. These data suggest that a nitroso–redox imbalance may be arrhythmogenic in the setting of MI. However, the molecular basis of the increased arrhythmogenesis is not known.
In the current study, we found that decreased NOS1 activity increased Ca2+-mediated ventricular arrhythmias only in the setting of elevated myocardial [Ca2+]i. These arrhythmias arose from increased spontaneous SR Ca2+ release resulting from a combination of decreased RyR2-SNO and increased RyR2 oxidation (RyR2-SOx) [i.e., increased ROS from xanthine oxidoreductase (XOR) activity] and could be suppressed with xanthine oxidoreductase inhibition (i.e., allopurinol) or nitric oxide donors (i.e., GSNO). Notably, we found evidence of NOS1 regulation of RyR2 phosphorylation at the Ca2+/calmodulin-dependent protein kinase (CaMKII) site (S2814), suggesting molecular cross-talk between the nitrosylation and phosphorylation states of RyR2. Finally, we show that nitroso–redox imbalance due to decreased NOS1 activity sensitizes RyR2 to a severe arrhythmic phenotype under oxidative stress.
Results
Effect of NOS1 Inhibition in the Intact Heart on Susceptibility to Ventricular Arrhythmia.
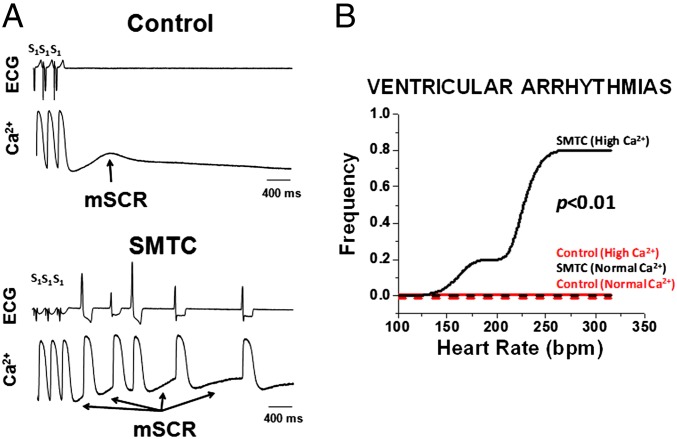
In the intact heart, ventricular arrhythmias were seen following NOS1 inhibition only in the presence of elevated [Ca2+]i. Fig. 1A shows an example of ECG and intracellular Ca2+ transient recordings from the intact heart under elevated [Ca2+]i conditions in the absence (Fig. 1A Upper) and presence (Fig. 1A Lower) of NOS1 inhibition with S-methyl-l-thiocitrulline (SMTC). Following a halt in pacing (S1 − S1 = 190 ms) under control conditions (without NOS1 inhibition) there is a spontaneous (nonelectrically driven) multicellular calcium release (mSCR) event that was not sufficient to produce an ectopic (unstimulated) ventricular beat or arrhythmia (more than three ectopic beats). In contrast, in a heart treated with NOS1 inhibitor, multiple mSCRs (see arrows) developed that were associated with multiple ectopic beats (i.e., ventricular arrhythmia). Summary data confirm that NOS1 inhibition increased susceptibility to pacing-induced ventricular arrhythmias over a broad range of pacing rates, P < 0.01 (Fig. 1B). However, this increased arrhythmia susceptibility following NOS1 inhibition was only evident under elevated (n = 5) [Ca2+]i conditions. In fact, no ventricular arrhythmias were seen with NOS1 inhibition under normal [Ca2+]i conditions (n = 5) nor in control hearts under both normal (n = 4) and elevated (n = 4) [Ca2+]i conditions. Additionally, single ectopic beats were seen under all conditions, yet these were more likely to occur with NOS1 inhibition under elevated [Ca2+]i conditions, P < 0.05. These data show that in well-coupled myocardium, NOS1 inhibition and elevated myocardial Ca2+ produce an arrhythmic substrate for triggered activity.
Fig. 1.
NOS1 inhibition (SMTC) during elevated [Ca2+]i increases susceptibility to triggered ventricular arrhythmias in the whole heart. (A) Example of intracellular calcium recordings and ECG from the intact heart under elevated [Ca2+]i conditions in the absence (Upper) and presence (Lower) of NOS1 inhibition. Following a halt in pacing (S1 − S1 = 190 ms) without NOS1 inhibition (control) there is an mSCR that was not sufficient to produce an ectopic (unstimulated) ventricular beat or arrhythmia (more than three ectopic beats). In contrast, in a heart treated with NOS1 inhibition, multiple mSCRs (see arrows) developed that were associated with multiple ectopic beats (i.e., ventricular arrhythmia). (B) NOS1 inhibition only increased ventricular arrhythmias over a broad range of pacing rates under conditions of elevated [Ca2+]i, (n = 5) compared with NOS1 inhibition with normal [Ca2+]i, (n = 5) and control hearts with normal [Ca2+]i, (n = 4), and elevated [Ca2+]i, (n = 4).
NOS1 Inhibition Combined with Ca2+ Overload Triggers Ca2+-Mediated Arrhythmias.
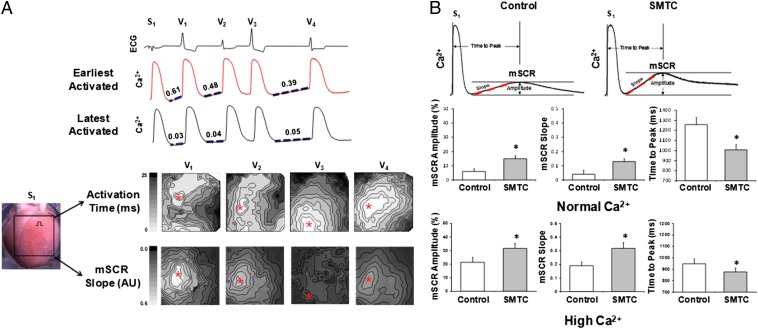
Previously, we have shown that the rate of mSCR rise is an important determinant of Ca2+-mediated triggered activity in intact heart preparations (8, 9). Therefore, to determine whether the arrhythmias we observed are due to mSCR activity following NOS1 inhibition under elevated [Ca2+]i conditions, we compared the origin of consecutive ectopic beats with the location of maximum mSCR slope. Fig. 2A, upper shows an example of a ventricular arrhythmia following NOS1 inhibition under elevated [Ca2+]i conditions. Shown are ECG and Ca2+ recordings from sites of earliest (red trace) and latest (black trace) activation. After pacing was terminated, the mSCR slope (dashed line) preceding the ectopic ventricular beats V1, V2, and V4 is greater at the earliest activated site compared with the latest activated site. Also, for the first beat (V1) the mSCR slope is greater and the preceding diastolic interval (DI) is shorter compared with that for subsequent beats. Contour maps of activation and mSCR slopes for V1–V4 are shown (Fig. 2A, lower). In every beat except V2, the region of earliest activation (red asterisk) corresponds exactly to the region where the mSCR slope is greatest. V2 originated outside the mapping field and may have been a reentrant beat. Importantly, because the endocardium was ablated, the site of earliest activity could not have occurred below the mapping field or from Purkinje fibers. Thus, the earliest site of activation shown is likely the site where the ectopic beat originated.
Fig. 2.
Increased multicellular spontaneous Ca2+ release (mSCR) as cellular mechanism of ventricular arrhythmias with NOS1 inhibition (SMTC). (A) Example of a triggered arrhythmia following NOS1 inhibition. Shown are optical Ca2+ recordings from sites of earliest (red tracing) and latest (black tracing) activation. Notice that the slopes (dashed line) of the mSCRs preceding beats V1, V2, and V4 are greater in the earliest activated site compared with the latest activated site. Activation and mSCR slope maps confirm that the region of earliest activation (red marker) corresponds exactly to the region where mSCR slope is the greatest for each triggered beat. (B) Example of mSCR measured in the whole heart under elevated [Ca2+]i conditions with and without NOS1 inhibition. Summary data show that following inhibition of nNOS under both normal (control, n = 4 and NOS1 inhibition, n = 5) and elevated (control, n = 4 and NOS1 inhibition, n = 5) [Ca2+]i, mSCR amplitude increased and mSCR onset was faster (i.e., increased slope; decreased time to peak) compared with control hearts. *P < 0.05 compared with control.
These findings were consistent across all preparations that developed ventricular arrhythmias. Additionally, NOS1 inhibition did not prolong baseline QT interval compared with control (217 ± 6 ms and 218 ± 6 ms, respectively; P = 0.9), suggesting that ectopy was not the result of early afterdepolarization. Thus, these data suggest that ventricular arrhythmias measured in the intact heart during NOS1 inhibition are Ca2+-mediated triggered arrhythmias.
NOS1 Inhibition Increases Spontaneous Sarcoplasmic Reticulum Ca2+ Release and Peak L-type Ca2+.
Fig. 2B shows a representative example of mSCR activity measured in the intact heart following a period of constant pacing, under elevated [Ca2+]i conditions with and without NOS1 inhibition. As shown, NOS1 inhibition increased mSCR amplitude and slope and decreased the time to mSCR peak compared with control (i.e., without NOS1 inhibition). Summary data (Fig. 2B) show that following NOS1 inhibition mSCR amplitude increased (P < 0.05) and mSCR onset was faster (i.e., increased slope and decreased time to peak, P < 0.05) compared with control hearts (n = 5) under both normal (n = 4) and elevated (n = 5) [Ca2+]i conditions. The combination of NOS1 inhibition and elevated [Ca2+]i were additive in their effect on mSCR activity compared with either in isolation. The addition of ryanodine in a subset of control experiments eradicated the development of mSCRs, confirming that mSCRs are Ca2+ release events from the SR, as shown previously (9). Moreover, these data show that NOS1 inhibition with or without elevated [Ca2+]i increase mSCR activity. However, the combination of NOS1 inhibition and elevated [Ca2+]i appears to be necessary for the development of ventricular arrhythmias in the intact heart (Fig. 1B).
Consistent with previous reports in isolated myocytes from the mouse (5), we found that inhibition of NOS1 in isolated guinea pig cardiomyocytes resulted in frequent spontaneous Ca2+ transients and aftercontractions (100% of myocytes). In contrast, spontaneous Ca2+ transients and aftercontractions were seen significantly less in control myocytes (22% of myocytes, P < 0.01). Moreover, inhibition of NOS1 increased peak L-type Ca2+ current (ICaL) in isolated myocytes compared with control (−9.13 pA/pF vs. −5.92 pA/pF, respectively; P < 0.05). In total, these data are consistent with previous findings in the mouse, demonstrating that loss of NOS1 produces spontaneous RyR Ca2+ release and increased ICaL in isolated cardiomyocytes under normal [Ca2+]i conditions (7).
Altered RyR2 Nitroso–Redox Balance Increases Arrhythmogenesis During NOS1 Inhibition.
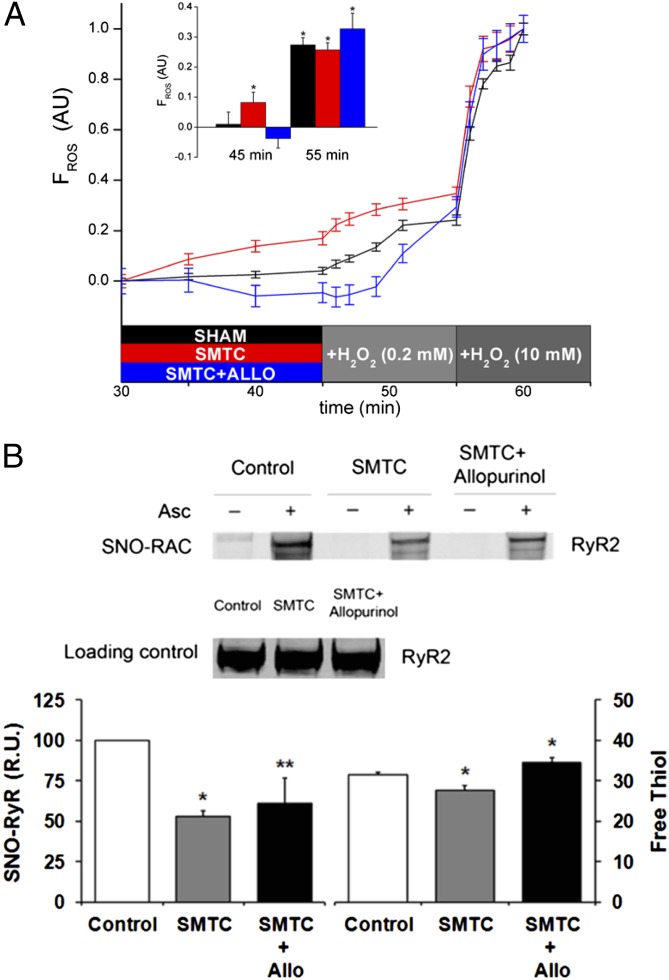
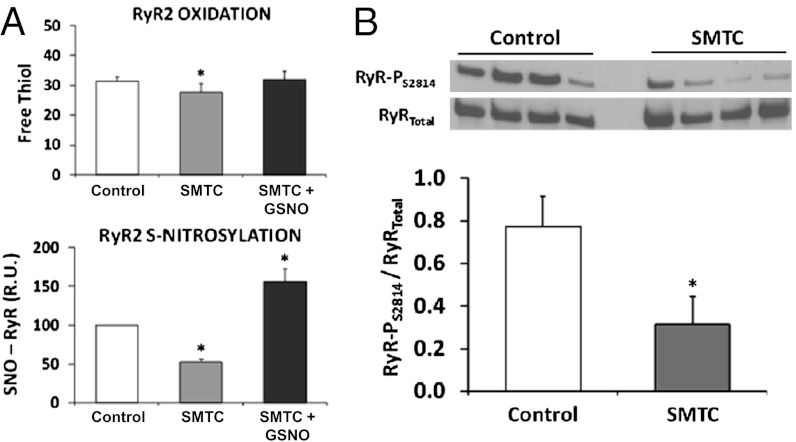
Inhibition of NOS1 can increase reactive oxygen species (ROS), which may significantly disrupt nitroso–redox balance of key SR Ca2+ cycling proteins (e.g., RyR2). Whole heart ROS was assayed as a marker of oxidative stress and shown to increase during NOS1 inhibition compared with control (Fig. 3A, P < 0.05). Importantly, treatment with the xanthine oxidoreductase (XOR) inhibitor allopurinol reversed the ROS increase during NOS1 inhibition (Fig. 3A, P < 0.05) suggesting that NOS1 inhibition increases XOR-derived superoxide and hydrogen peroxide. At the RyR2 level, NOS1 inhibition resulted in significant nitroso–redox imbalance, as evidenced by decreased RyR2-SNO [SNO-resin-assisted capture (RAC) method] as well as total RyR2 thiol content. Collectively these data indicate that increased RyR2-SOx (i.e., decreased RyR2 thiol content) is secondary to increased XOR-derived ROS and loss of NOS1-derived NO (Fig. 3B, P < 0.05).
Fig. 3.
Combination of decreased S-nitrosylation and increased oxidation of RyR2 as molecular mechanisms of ventricular arrhythmias with NOS1 inhibition (SMTC). (A) Whole heart optical imaging of reactive oxygen species (ROS) showed an increase in ROS during NOS1 inhibition (red line; n = 4) compared with control (black line; n = 4). Moreover, treatment with the xanthine oxidoreductase inhibitor, allopurinol during NOS1 inhibition (n = 4) decreased ROS production. (B) Mean data showed that NOS1 inhibition (n = 6) decreased RyR2-SNO and the number of RyR2-free thiols (indicating increased RyR2 oxidation) compared with control hearts (n = 6). Addition of allopurinol during NOS1 inhibition (n = 6) prevented RyR2 oxidation (RyR2-SOx) but did not significantly increase RyR2-SNO. *P < 0.05 compared with control. **P = 0.09 compared with control.
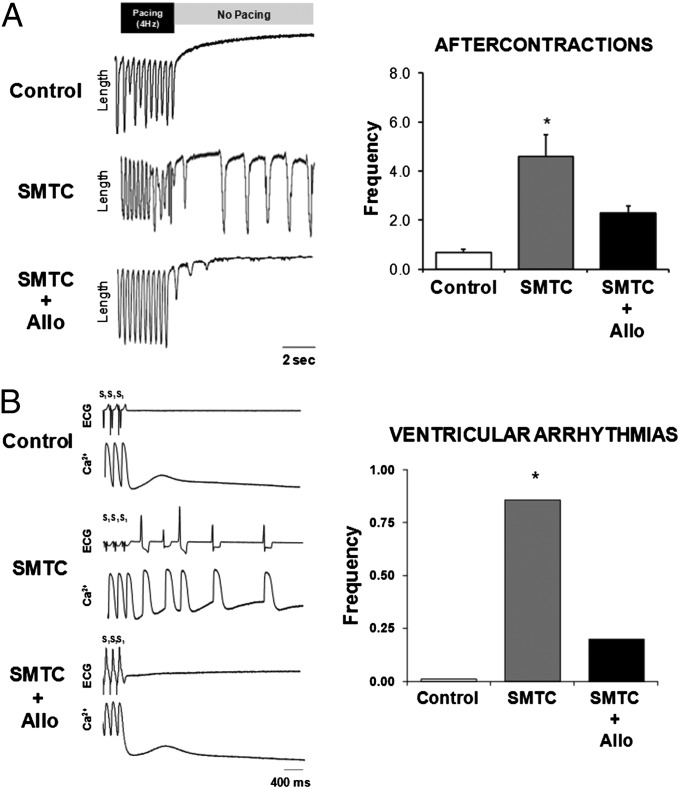
The mechanistic role of decreased RyR2-SNO and increased RyR2-SOx in arrhythmias was further assessed in both isolated cardiomyocytes and the intact heart. Treatment with either allopurinol (XOR inhibition) or GSNO during NOS1 inhibition suppressed spontaneous aftercontractions in isolated myocytes compared with NOS1 inhibition alone (Fig. 4A and Fig. S1, P < 0.05). Consistent with this finding, treatment with allopurinol during NOS1 inhibition in the intact heart decreased both mSCR activity and the incidence of ventricular arrhythmias compared with NOS1 inhibition alone (Fig. 4B, P < 0.01). Notably, treatment with either allopurinol (Fig. 3B) or GSNO (Fig. 5A) during NOS1 inhibition increased the number of RyR2-free thiols, an indication of diminished RyR2-SOx. Also, allopurinol treatment in the absence of NOS1 inhibition increased the number of RyR2-free thiols compared with control (35.8 ± 1.7 vs. 31.5 ± 1.4, respectively; P < 0.01). Moreover, RyR2-SNO was increased by GSNO (Fig. 5A) treatment during NOS1 inhibition. In contrast, RyR2-SNO was not significantly increased by allopurinol treatment during NOS1 inhibition compared with NOS1 inhibition alone (Fig. 3B). Taken together, these data indicate that oxidation and nitrosylation are antipathetic, but the effects of NO to prevent oxidation cannot be explained solely by direct competition for RyR thiols. One interpretation of these data are that the effects of NO are mediated partly through XOR inhibition (10). Thus, rather than nitrosylation and oxidation directly antagonizing each other (e.g., competing for the same sites), the decrease in nitrosylation and concomitant increase in oxidation is mediated by XOR, which increases ROS production when local NO is decreased (via NOS1 inhibition).
Fig. 4.
Allopurinol reverses the effects of NOS1 inhibition (SMTC) on arrhythmogenic aftercontractions in isolated myocytes and ventricular arrhythmias in the intact heart. (A) Tracings of myocyte length showing an increase in aftercontractions with NOS1 inhibition (n = 31) compared with control (n = 27). Treatment with allopurinol (n = 14) during NOS1 inhibition suppressed aftercontractions. (B) Tracings of allopurinol treatment during NOS1 inhibition (n = 4) reversed the effects of NOS1 inhibition (n = 5) on triggered arrhythmias in the intact heart to near control levels (n = 5). *P < 0.05 compared with control.
Fig. 5.
Treatment with GSNO and CaMKII mediated RyR2 phosphorylation. (A) Treatment with GSNO during NOS1 inhibition decreased RyR2-SOx and increased RyR2-SNO compared with NOS1 inhibition alone. (B) Representative Western blots of RyR2-PS2814/RyR2Total during NOS1 inhibition compared with control. As shown, NOS1 inhibition decreased RyR2-PS2814 compared with control. *P < 0.05 compared with control.
Notably, treatment with NOS1 inhibitor + allopurinol (which reduced mSCR activity and arrhythmogenesis) did not reduce ICaL elevation caused by NOS1 inhibition. This suggests that the increased ICaL is not a major mechanism for increasing arrhythmias during NOS1 inhibition. Previously, it has been shown that NOS1 deficiency does not alter PKA-mediated RyR phosphorylation (5). However, in this study, we found that CaMKII-mediated RyR2 phosphorylation was decreased by NOS1 inhibition compared with control (P < 0.05, Fig. 5B).
Collectively these data support the idea that both decreased RyR2-SNO and (resultant) increased RyR2-SOx contribute to create nitroso–redox imbalance, which is sufficient to increase mSCR activity and ventricular arrhythmias during NOS1 inhibition.
Severe Ventricular Arrhythmias Following Combined NOS1 Inhibition and Oxidative Stress.
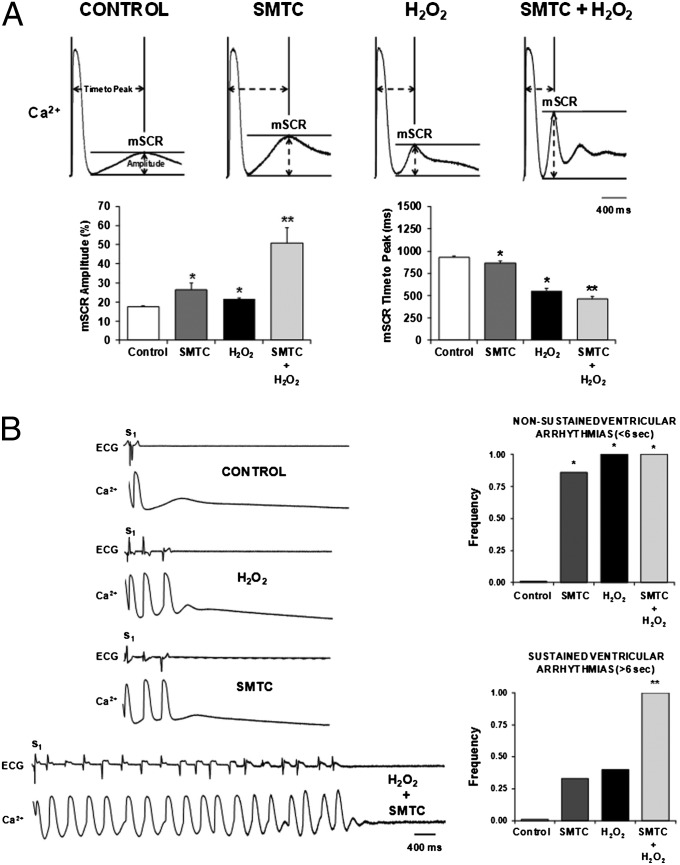
To further illustrate the importance of nitroso–redox imbalance in cardiac arrhythmogenesis and particularly, the synergistic effect of SNO-deficiency and increased oxidation, we investigated the effects of exogenous H2O2 in the intact heart with and without NOS1 inhibition. Fig. 6A shows representative examples of mSCR activity following NOS1 inhibition, H2O2 and combined NOS1 inhibition + H2O2. Both NOS1 inhibition and H2O2 increased mSCR amplitude, and decreased mSCR time to peak compared with control. Notably, the combination of NOS1 inhibition + H2O2 produced an additive increase in mSCR amplitude and decrease in mSCR time to peak compared with NOS1 inhibition and H2O2 alone. Importantly, this increased mSCR activity resulted in a more severe, sustained, arrhythmia phenotype. Specifically, NOS1 inhibition + H2O2 produced ∼150% increase in the frequency of sustained (>5 s) ventricular arrhythmias compared with NOS1 inhibition or H2O2 alone (Fig. 6B, P < 0.01).
Fig. 6.
Combined NOS1 inhibition (SMTC) and exogenous H2O2 produce an additive increase in mSCR and sustained (>5 s) triggered ventricular arrhythmias in the intact heart. (A) Example of spontaneous (nonelectrically driven) calcium release occurring in multiple, neighboring cells under all experimental conditions. Both H2O2 (n = 4) and NOS1 inhibition (n = 7) increased mSCR amplitude, and time to mSCR peak decreased compared with control. Moreover, combined NOS1 inhibition and H2O2 (n = 6) produced an additive increase in mSCR activity. (B) H2O2, NOS1 inhibition, and combined NOS1 inhibition and H2O2 all increased the frequency of nonsustained (≤5 s) triggered arrhythmias compared with control. In contrast, only combined NOS1 inhibition and H2O2 increased the frequency of sustained (>5 s) triggered arrhythmias. *P < 0.05 compared with control. **P < 0.05 compared with control, NOS1 inhibition and H2O2.
RyR2 Nitroso–Redox Imbalance Is Not Responsible for Increase in Ventricular Arrhythmias Following NOS1 Inhibition + Oxidative Stress.
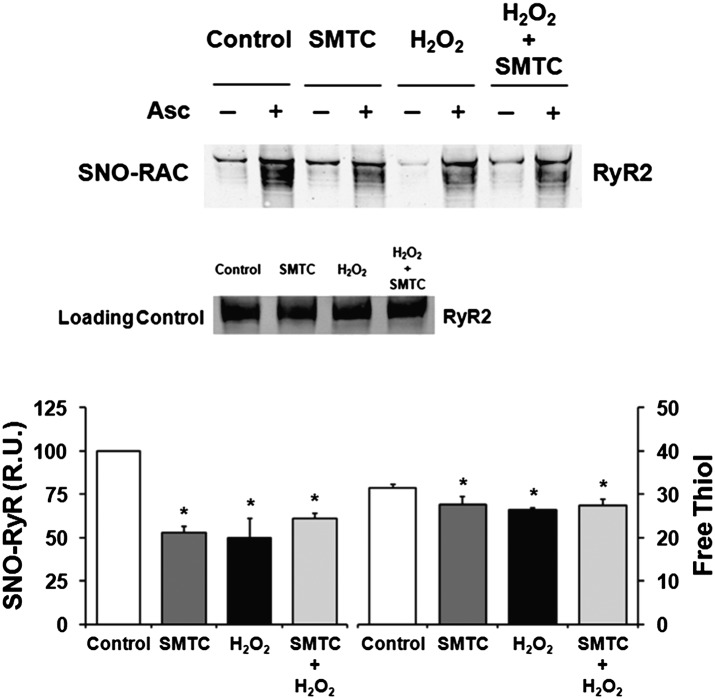
We postulated that combined NOS1 inhibition + H2O2 would produce a greater RyR2 nitroso–redox imbalance (i.e., less free thiols) than either NOS1 inhibition or H2O2 alone (Fig. 7). Both NOS1 inhibition and H2O2 alone decreased RyR2-SNO and increased RyR2-SOx. However, there was no difference in RyR2-SNO and RyR2-SOx following combined NOS1 inhibition + H2O2 compared with NOS1 inhibition or H2O2 alone (Fig. 7). Thus, the additive increase in ventricular arrhythmias following combined NOS1 inhibition + H2O2 may not be solely due to further changes in RyR2 nitroso–redox status. For example, both NOS1 inhibition and H2O2 increased ICaL, and their combination resulted in a near additive increase in ICaL (P = 0.09) compared with either alone. These data suggest that in the presence of increased RyR2 Po (secondary to altered RyR2 nitroso–redox), a further increase in ICaL caused by exogenous H2O2 creates a potent substrate for arrhythmias.
Fig. 7.
Altered RyR2 nitroso–redox balance not responsible for additive increase in ventricular arrhythmias following combined NOS1 inhibition (SMTC) + exogenous H2O2. (Upper) SNO-RAC showing decreased RyR2-SNO with NOS1 inhibition, H2O2, and NOS1 inhibition + H2O2 compared with control. (Lower) Mean data showed that both NOS1 inhibition (n = 6) and H2O2 (n = 6) alone decreased RyR2-SNO and decreased RyR2-free thiols (i.e., increased RyR2-SOx). Interestingly, there was no difference in RyR2-SNO and RyR2-SOx following combined NOS1 inhibition + H2O2 (n = 6) when compared with NOS1 inhibition or oxidative stress alone. *P < 0.05 compared with control. **P = 0.06 compared with control. ^P = 0.08.
Discussion
Our studies establish that diminished NOS1 activity may cause arrhythmia in the intact heart. In particular, we demonstrate that NOS1 inhibition in the intact heart increases susceptibility to Ca2+-mediated triggered arrhythmias only in presence of elevated myocardial [Ca2+]i. These arrhythmias arise from increased mSCR activity (i.e., multicellular spontaneous SR Ca2+ release events) due to a combination of decreased RyR2 S-nitrosylation and increased RyR2 S-oxidation. Restoration of nitroso–redox balance by inhibition of xanthine oxidoreductase (allopurinol) or increasing NO bioavailability (GSNO) reversed the arrhythmogenic phenotype. We also provide evidence for NOS1 regulation of RyR2 phosphorylation at the CaMKII site (S2814), suggesting molecular cross-talk between the nitrosylation, oxidation, and phosphorylation states of RyR2. Finally, oxidative stress (as in ischemic heart disease) may predispose to a strikingly severe arrhythmic phenotype in the absence of NOS1-derived NO.
Effect of NOS1 Inhibition on Ca2+-Mediated Arrhythmias.
In the present study we demonstrate that NOS1 inhibition alone increases triggered arrhythmias but only in the presence of elevated [Ca2+]i. The cellular mechanisms underlying these ventricular arrhythmias are primarily from spontaneous calcium release (i.e., mSCR) that appears to be caused by a primary alteration in RyR2 function. Also, we found that the site of earliest activation of an ectopic beat occurred in the region with the steepest slope of mSCR activity, further suggesting a Ca2+-mediated triggered arrhythmia. This is consistent with previous research from our laboratory, demonstrating that the rate of mSCR rise (i.e., slope) is an important determinant of Ca2+-mediated triggered activity in heart preparations (8). Moreover, we found that increased ICaL is not a required mechanism for spontaneous calcium release during NOS1 inhibition, further supporting a primary role of altered RyR2 function.
These findings extend the observations of Gonzalez et al. (5). in isolated myocytes to the realm of system physiology and disease. Gonzalez showed that deletion of NOS1 increased SR Ca2+ leak and diastolic Ca2+ waves. Our data establish in the intact heart that NOS1-mediated alterations in RyR2 function are not sufficient to produce arrhythmias. For arrhythmias to develop with NOS1 inhibition, elevated myocardial [Ca2+] (a characteristic of disease) is necessary. These data highlight that the mere presence of spontaneous SR Ca2+ release events in a cardiomyocyte does not necessarily translate into a triggered beat or arrhythmia in coupled myocardium and provide unique insight into the basis of arrhythmias in disease.
Molecular Mechanisms Driving Ca2+-Mediated Arrhythmogenesis with NOS1 Inhibition.
The molecular mechanisms regulating spontaneous calcium release in the intact heart are complex. Posttranslational modifications of RyR2 by S-nitrosylation and/or S-oxidation have been shown to affect SR Ca2+ leak and sparks in cardiomyocytes (5, 11). In the current study we demonstrate that the combination of reduced RyR2-SNO and increased RyR2-SOx increase spontaneous calcium release and ventricular arrhythmias in the intact heart. Importantly, these arrhythmias could be reversed with the xanthine oxidoreductase inhibitor allopurinol or the NO donor GSNO. These data are consistent with a model in which RyR2-SNO regulates RyR2-SOx, and where SR nitroso–redox imbalance underlies arrhythmogenesis. However, it is possible that other mechanisms are involved in NO-deficiency–mediated arrhythmogenesis.
Notably, we demonstrated that NOS1 inhibition also increased ICaL and decreased RyR2 phosphorylation at the CaMKII binding site (S2814). ICaL is not primarily responsible for increasing arrhythmia because allopurinol suppressed arrhythmia without decreasing ICaL, and despite the importance of CaMKII signaling in abnormal Ca2+ cycling (12), the significance of decreased phosphorylation is unclear. Recently, Respress et al. (13) showed a reduction in RyR2-P2814 in mice with ischemic cardiomyopathy, a condition known to have increased RyR2 Ca2+ leak. However, S2814 mutant mice did not show increased RyR2 Ca2+ leak. Our findings provide unique evidence that NOS1 is involved in the regulation of RyR2-P2814 and suggest cross-talk between the nitrosylation and phosphorylation states of proteins (i.e., RyR2). This suggests an additional level of signaling complexity in posttranslational regulation of RyR2.
The mechanisms responsible for reduced RyR2-P2814 during NOS1 inhibition are likely complex. It could be postulated that either alone or in combination decreased RyR2-SNO or increased RyR2-SOx produces a conformational change in RyR2, such that the S2814 phosphorylation site is less available for phosphorylation. Alternatively, it has been shown that S-nitrosylation of phosphatases can inhibit their activity (14). Thus, it is possible that NOS1 inhibition decreases S-nitrosylation of a key protein phosphatase (e.g., PP2A); in turn reducing RyR2 phosphorylation at the CaMKII site (S2814).
Importantly, our findings may explain differences between Gonzalez et al. (5) and Wang et al. (6) regarding the effect NOS1 deficiency on RyR Ca2+ leak and Ca2+ sparks/waves. Gonzalez et al. (5) reported increased Ca2+ wave frequency in cardiomyocytes from NOS1-KO mice whereas Wang et al. (6) reported a decrease in Ca2+ spark frequency. In studies by us and Gonzalez et al. (5) rapid pacing was performed to induce Ca2+ waves and arrhythmias, whereas very slow pacing was used by Wang et al. (6). Pacing can alter the redox state of the cardiomyocyte or increase SR [Ca2+], thereby altering the probability of Ca2+ sparks/waves.
Combination of Decreased NOS1 Activity and Oxidative Stress Markedly Increase Arrhythmogenesis.
Oxidative stress associated with disease has been shown to increase the frequency of Ca2+ sparks, afterdepolarizations, and ventricular arrhythmias. Saraiva et al. (15) reported an increased mortality in NOS1 knockout (KO) mice following MI and Burger et al. (7) reported both an increased mortality and incidence of ventricular arrhythmias post-MI (left anterior descending coronary artery ligation) in NOS1-KO mice. Importantly, many of these arrhythmias occurred within 1 h of spontaneous death. Burger et al. (7) postulated that this increase in arrhythmogenesis was related to increased ICaL as both [Ca2+]I elevation and ventricular arrhythmias were decreased with verapamil. However, they measured ICaL in isolated cardiomyocytes from hearts without MI and did not assess RyR2-SOx or other posttranslational modifications.
To simulate disease we imposed oxidative stress with H2O2 and found that NO deficiency produced a striking increase in mSCRs and ventricular arrhythmias. Interestingly, the present study shows little qualitative difference in RyR2 oxidation state (net RyR2 nitroso–redox state) with the combination of NOS1 inhibition and H2O2 versus either alone. This implies that nitroso–redox imbalance within RyR2 is not solely responsible for the severe arrhythmias we report. Our methods do not exclude the possibility, however, that the populations of thiols oxidized under various conditions may be different. Additionally, NOS1 inhibition and H2O2 showed synergistic effects on ICaL, and it is possible, that this synergistic increase in ICaL may in part explain the increased probability and severity of spontaneous calcium release and arrhythmias.
Clinical Significance.
Our findings have important clinical implications as they highlight the role NO derived from NOS1 in the genesis of triggered arrhythmias and identify a potential therapeutic approach (i.e., xanthine oxidoreductase inhibition in combination with NO-based therapy). There is evidence to suggest that obesity, diabetes, and heart failure are all associated with decreased NOS1 activity (16–20). Junttila et al. (21) recently reported a markedly increased incidence of sudden arrhythmic death in diabetic patients after MI compared with nondiabetic controls. Diabetic patients with left ventricle ejection fractions (LVEF) >35% had a sudden death rate equal to nondiabetic patients with LVEF <35%. It could be postulated that this increased arrhythmic risk in diabetic patients is secondary to the combined effects of oxidative stress (i.e., ischemic cardiomyopathy) and impaired NOS1 activity (i.e., diabetes). Similarly, there is preliminary evidence that xanthine oxidoreductase inhibition in heart failure patients with elevated uric acid improved clinical outcomes (composite of heart failure morbidity, mortality, and quality of life) (22). Our findings suggest that therapies targeting the restoration of nitroso–redox balance in the heart could prevent sudden arrhythmic death.
Materials and Methods
Whole Heart Studies.
Experiments were performed in accordance with the Institutional Animal Care and Use Committee of Case Western Reserve University. Hearts from guinea pigs were Langendorff perfused with oxygenated (100% O2) Tyrode’s solution (pH 7.45, 34 °C). Endocardial cryoablations were performed to eliminate the Purkinje fibers and deeper layers of myocardium. Indo-1 AM (10 μM; Invitrogen) was used to measure intracellular Ca2+ transients from the anterior surface of the heart in the following groups: (i) control, normal [Ca2+]I; (ii) NOS1 inhibition (S-methyl-l-thiocitrulline), normal [Ca2+]I; (iii) control, elevated [Ca2+]i (5.5 mM); (iv) NOS1 inhibition, elevated [Ca2+]I; (v) NOS1 inhibition + allopurinol (elevated [Ca2+]i); (vi) H2O2 (elevated [Ca2+]i) and NOS1 inhibition + H2O2 (elevated [Ca2+]i). Constant pacing for 1 min followed by a halt in pacing was used to elicit mSCR activity and arrhythmias as previously described (9). In separate hearts, the concentration of ROS in the intact heart was measured by perfusing for 15 min with the ROS-sensitive dye dihydrorhodamine 6G (Invitrogen). For further information, see SI Materials and Methods.
Isolated Myocytes.
Myocytes were isolated from guinea pig hearts and sarcomere shortening, intracellular Ca2+ transients, and the L-type Ca2+ current were measured. For further information, see SI Materials and Methods.
Biochemistry.
To measure RyR2 S-nitrosylation, SNO-RAC was performed in SR vesicles isolated from guinea pig whole heart homogenate as described previously (23). Quantification of RyR2-free thiols was also assessed in SR vesicles that were incubated with the fluorescent indicator monobromobimane (MBB) (24). Finally, RyR2 phosphorylation (RyR2-PS2814) was measured in tissue obtained from the left ventricular free wall in control and NOS1-inhibited hearts. For further information, see SI Materials and Methods.
Statistical Analysis.
Statistical differences were assessed with ANOVA, Student t test, Kruskal–Wallis test, and χ2 test as appropriate. When unequal variance was detected, the Wilcoxon rank sum test was used. Results were expressed as mean ± SEM.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health (NIH) Grant HL-054807 (to D.S.R.), NIH Grants HL-084142 and HL-100105 (to K.R.L.), NIH Grants HL-075443, HL-095463, AI-080633, and DARPA N66001-10-C-2015 (to J.S.S.), and fellowship awards from the Heart Rhythm Society, NIH National Research Service Award, and the Department of Medicine Physician Scientist Pathway at the MetroHealth Campus of Case Western Reserve University (to M.J.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1210565109/-/DCSupplemental.
References
- 1.Tamargo J, Caballero R, Gómez R, Delpón E. Cardiac electrophysiological effects of nitric oxide. Cardiovasc Res. 2010;87(4):593–600. doi: 10.1093/cvr/cvq214. [DOI] [PubMed] [Google Scholar]
- 2.Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res. 2010;106(4):633–646. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun J, et al. Regulation of the cardiac muscle ryanodine receptor by O(2) tension and S-nitrosoglutathione. Biochemistry. 2008;47(52):13985–13990. doi: 10.1021/bi8012627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stamler JS, Sun QA, Hess DT. A SNO storm in skeletal muscle. Cell. 2008;133(1):33–35. doi: 10.1016/j.cell.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez DR, Beigi F, Treuer AV, Hare JM. Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc Natl Acad Sci USA. 2007;104(51):20612–20617. doi: 10.1073/pnas.0706796104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H, et al. Regulation of myocyte contraction via neuronal nitric oxide synthase: Role of ryanodine receptor S-nitrosylation. J Physiol. 2010;588(Pt 15):2905–2917. doi: 10.1113/jphysiol.2010.192617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burger DE, et al. Neuronal nitric oxide synthase protects against myocardial infarction-induced ventricular arrhythmia and mortality in mice. Circulation. 2009;120(14):1345–1354. doi: 10.1161/CIRCULATIONAHA.108.846402. [DOI] [PubMed] [Google Scholar]
- 8.Hoeker GS, Katra RP, Wilson LD, Plummer BN, Laurita KR. Spontaneous calcium release in tissue from the failing canine heart. Am J Physiol Heart Circ Physiol. 2009;297(4):H1235–H1242. doi: 10.1152/ajpheart.01320.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plummer BN, Cutler MJ, Wan X, Laurita KR. Spontaneous calcium oscillations during diastole in the whole heart: The influence of ryanodine reception function and gap junction coupling. Am J Physiol Heart Circ Physiol. 2011;300(5):H1822–H1828. doi: 10.1152/ajpheart.00766.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan SA, et al. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc Natl Acad Sci USA. 2004;101(45):15944–15948. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez DR, Treuer AV, Castellanos J, Dulce RA, Hare JM. Impaired S-nitrosylation of the ryanodine receptor caused by xanthine oxidase activity contributes to calcium leak in heart failure. J Biol Chem. 2010;285(37):28938–28945. doi: 10.1074/jbc.M110.154948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bers DM. Ryanodine receptor S2808 phosphorylation in heart failure: Smoking gun or red herring. Circ Res. 2012;110(6):796–799. doi: 10.1161/CIRCRESAHA.112.265579. [DOI] [PubMed] [Google Scholar]
- 13.Respress JL, et al. Role of RyR2 phosphorylation at S2814 during heart failure progression. Circ Res. 2012;110(11):1474–1483. doi: 10.1161/CIRCRESAHA.112.268094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Numajiri N, et al. On-off system for PI3-kinase-Akt signaling through S-nitrosylation of phosphatase with sequence homology to tensin (PTEN) Proc Natl Acad Sci USA. 2011;108(25):10349–10354. doi: 10.1073/pnas.1103503108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saraiva RM, et al. Deficiency of neuronal nitric oxide synthase increases mortality and cardiac remodeling after myocardial infarction: Role of nitroso-redox equilibrium. Circulation. 2005;112(22):3415–3422. doi: 10.1161/CIRCULATIONAHA.105.557892. [DOI] [PubMed] [Google Scholar]
- 16.Umar S, van der Laarse A. Nitric oxide and nitric oxide synthase isoforms in the normal, hypertrophic, and failing heart. Mol Cell Biochem. 2010;333(1–2):191–201. doi: 10.1007/s11010-009-0219-x. [DOI] [PubMed] [Google Scholar]
- 17.Koo JR, Vaziri ND. Effects of diabetes, insulin and antioxidants on NO synthase abundance and NO interaction with reactive oxygen species. Kidney Int. 2003;63(1):195–201. doi: 10.1046/j.1523-1755.2003.00728.x. [DOI] [PubMed] [Google Scholar]
- 18.Yu WJ, et al. Insulin restores neuronal nitric oxide synthase expression in streptozotocin-induced diabetic rats. Life Sci. 2000;68(6):625–634. doi: 10.1016/s0024-3205(00)00967-x. [DOI] [PubMed] [Google Scholar]
- 19.Silberman GA, et al. Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation. 2010;121(4):519–528. doi: 10.1161/CIRCULATIONAHA.109.883777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saraiva RM, et al. Reduced neuronal nitric oxide synthase expression contributes to cardiac oxidative stress and nitroso-redox imbalance in ob/ob mice. Nitric Oxide. 2007;16(3):331–338. doi: 10.1016/j.niox.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Junttila MJ, et al. Sudden cardiac death after myocardial infarction in patients with type 2 diabetes. Heart Rhythm. 2010;7(10):1396–1403. doi: 10.1016/j.hrthm.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 22.Hare JM, et al. OPT-CHF Investigators Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT-CHF study. J Am Coll Cardiol. 2008;51(24):2301–2309. doi: 10.1016/j.jacc.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 23.Forrester MT, et al. Proteomic analysis of S-nitrosylation and denitrosylation by resin-assisted capture. Nat Biotechnol. 2009;27(6):557–559. doi: 10.1038/nbt.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kosower NS, Kosower EM. Thiol labeling with bromobimanes. Methods Enzymol. 1987;143:76–84. doi: 10.1016/0076-6879(87)43015-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.