Abstract
Calcifying echinoid larvae respond to changes in seawater carbonate chemistry with reduced growth and developmental delay. To date, no information exists on how ocean acidification acts on pH homeostasis in echinoderm larvae. Understanding acid–base regulatory capacities is important because intracellular formation and maintenance of the calcium carbonate skeleton is dependent on pH homeostasis. Using H+-selective microelectrodes and the pH-sensitive fluorescent dye BCECF, we conducted in vivo measurements of extracellular and intracellular pH (pHe and pHi) in echinoderm larvae. We exposed pluteus larvae to a range of seawater CO2 conditions and demonstrated that the extracellular compartment surrounding the calcifying primary mesenchyme cells (PMCs) conforms to the surrounding seawater with respect to pH during exposure to elevated seawater pCO2. Using FITC dextran conjugates, we demonstrate that sea urchin larvae have a leaky integument. PMCs and spicules are therefore directly exposed to strong changes in pHe whenever seawater pH changes. However, measurements of pHi demonstrated that PMCs are able to fully compensate an induced intracellular acidosis. This was highly dependent on Na+ and HCO3−, suggesting a bicarbonate buffer mechanism involving secondary active Na+-dependent membrane transport proteins. We suggest that, under ocean acidification, maintained pHi enables calcification to proceed despite decreased pHe. However, this probably causes enhanced costs. Increased costs for calcification or cellular homeostasis can be one of the main factors leading to modifications in energy partitioning, which then impacts growth and, ultimately, results in increased mortality of echinoid larvae during the pelagic life stage.
Keywords: pH microelectrode, Strongylocentrotus droebachiensis, acid–base regulation, Na+-HCO3− transport, epithelial transport
Sea urchin larvae have been shown to react with particular sensitivity to CO2-induced reductions in seawater pH (1–4). When larvae are chronically exposed to elevated seawater pCO2 of >0.1 kPa, e.g., as is predicted to occur during the next century in response to anthropogenic CO2 emissions or through upwelling of low-pH deep water, this sensitivity is reflected in reduced growth and developmental rates (5, 6). Echinoderm larvae are considered to be especially vulnerable to seawater pH reduction and to the associated changes in calcium carbonate saturation state of seawater (ΩCal) because their internal skeleton is composed of high magnesium calcite, a highly soluble form of CaCO3 (7, 8). However, long-term reductions in growth and development might just as well be evoked by other physiological mechanisms that are also sensitive to hypercapnia and the related acid–base disturbances. Recent studies conducted on several marine taxa including mollusks (9) and echinoderms (10) demonstrated increased metabolic rates in response to elevated seawater pCO2. It was concluded that reductions in somatic growth and rate of development were caused by a shift of energy partitioning toward vital functions such as cellular acid–base homeostasis (9, 10). For example, sea urchin larvae exposed to acidified seawater (0.35 kPa CO2, pH 7.25) maintained a fully calcified larval phenotype, albeit at a reduced rate of body growth (4). This indicates that biomineralization mechanisms remain functional despite CO2-induced changes in the seawater carbonate system.
The calcification process in sea urchin larvae has been well investigated (e.g., refs. 7, 11–14). Primary mesenchyme cells (PMCs), located within the extracellular matrix of the primary body cavity, form a syncytium around the growing spicules of the pluteus larvae. This syncytial sheath covers the entire surface of the spicules and communicates with the extracellular environment of the primary body cavity (8, 15). The high magnesium calcitic spicules are formed through the production of a transient amorphous calcium carbonate (ACC) phase within vesicles in PMCs. ACC is subsequently released into the spicular cavity (7, 8, 12, 16). To fuel the calcification process, bicarbonate (HCO3−) is absorbed from the seawater (40%) as well as generated from metabolic CO2 (60%) (11). On the other hand, Ca2+ is exclusively obtained directly from the seawater (13). Although the general principle of calcification is well understood, mechanistic information concerning the nature of the transporters that facilitate Ca2+ or HCO3− uptake in PMCs is limited. Several pharmacological studies suggested that Ca2+ channels and transporters are key players in the provision of Ca2+ for spicule formation (17, 18). To remove protons generated during CaCO3 precipitation, PMCs must possess efficient acid–base regulatory properties (19, 20). However, the mechanistic basis of acid–base regulation in PMCs has not been explored so far. Typically, a cell is required to secrete acid equivalents (e.g., H+) or to increase intracellular buffering capacity via the import of buffers (e.g., HCO3−) to compensate for an intracellular acidosis. Acid–base equivalent transport is facilitated through ion transporters such as Na+/H+ exchangers (NHEs), Na+-dependent HCO3− transporters of the SLC4 transporter family, or primary active transporters (e.g., V-type H+-ATPases) (21, 22).
Here, we investigate how changes in seawater pCO2 affect the direct environment of the calcifying PMCs and whether sea urchin larvae are able to regulate extracellular pH in response to both acute and chronic seawater acidification. Furthermore, we study acid–base regulatory features of the PMCs to provide information on the extent and mechanisms of intracellular pH regulatory abilities. This knowledge is crucial to understand the mechanisms underlying the control of pH homeostasis in PMCs and, thus, calcification during environmental hypercapnia. We hypothesize that a higher fraction of energy spent on acid–base compensatory processes of PMCs in response to CO2-induced seawater acidification may be a critical reason for the reported reductions in growth and development of echinoid larvae.
Results and Discussion
Lack of pH Regulation Within the Primary Body Cavity.
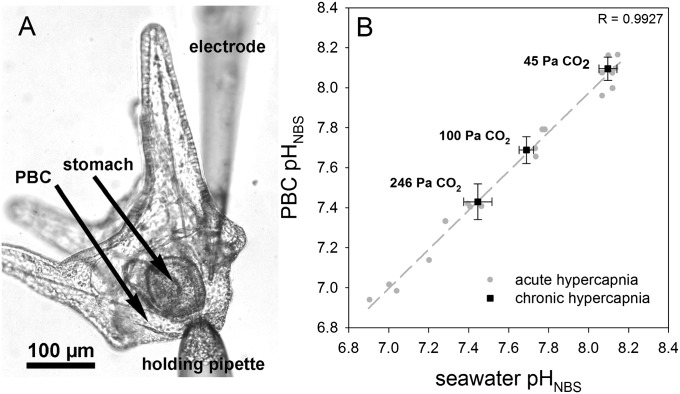
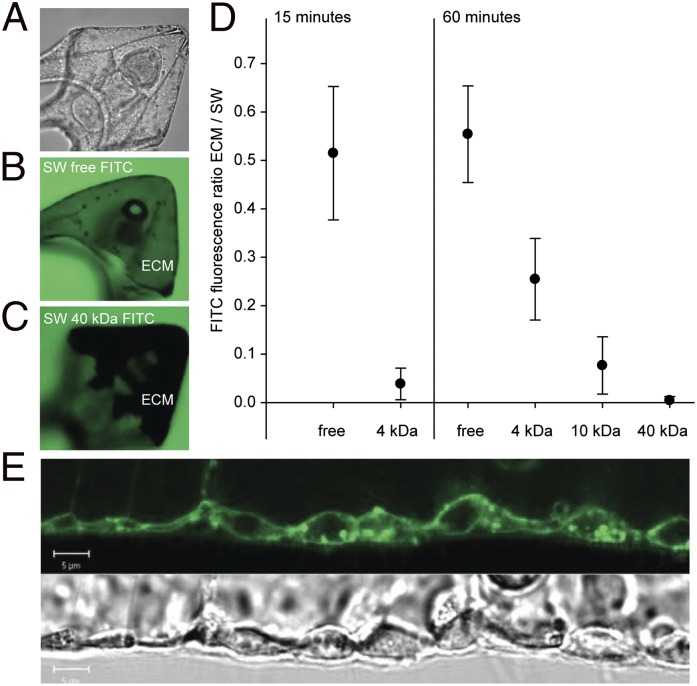
H+-selective microelectrodes were adapted for extracellular pH (pHe) measurements in marine invertebrate larvae. Surprisingly, pHe in the body cavity followed seawater pH (pHsw) without any measurable signs of active pHe compensation. This observation was independent of acute or chronic exposure to elevated seawater pCO2 (Fig. 1). Additionally, pHe was also monitored noninvasively in larvae loaded with the pH-sensitive dye 2′,7′-bis-(carboxyethyl)-5(6)-carboxyfluorescein (BCECF) in an experiment to validate our electrode measurements. These measurements confirmed the lack of significant pHe regulatory ability (Fig. S1). A bias on pH electrode measurements was excluded by performing nonselective voltage electrode measurements. No transepithelial potential difference was recorded between the bath solution and extracellular matrix (Fig. S2). The lack of any difference in pHsw vs. pHe is surprising because it would indicate very shallow pCO2 diffusion gradients from the extracellular compartment to the seawater. Typically, pCO2 values in extracellular fluids of heterotrophic marine metazoans are at least 0.1 kPa higher than those of the surrounding seawater, thereby enabling diffusive CO2 excretion across a delimitating barrier, thus rendering pHe more acidic than seawater (23). The pluteus ectoderm is very thin (∼1–2 µm) (Fig. 2E), comparable in thickness to respiratory epithelia of, e.g., teleost fish (23), thus enabling shallow diffusion gradients for pCO2. However, the relatively high pHe within the body cavity in our measurements could also be related to an ectoderm that is leaky for ions and small molecules. Using fluorescein-5-isothiocyanate (FITC) dextran conjugates of different molecular weights, we studied the permeability of the larval ectoderm. We exposed four-armed pluteus larvae to FITC dextran-containing seawater (Fig. 2) and found that free FITC (Mr = 0.4 kDa) equilibrated within 15 min with the extracellular matrix (ECM). Significant entry of FITC dextran conjugates of 4 kDa (15-, 60-min incubation) and 10 kDa (60-min incubation) and the absence of entry of 40-kDa particles within the 60-min incubation period (Fig. 2 A and B) indicated a comparatively high permeability of the pluteus ectoderm. Because no significant increases in FITC-derived fluorescence were measured in the cytosol of ectodermal or PMC cells (Fig. 2B), it appears that equilibration between ECM and seawater is mediated through paracellular pathways. It is likely that the pluteus ectoderm is characterized by a high permeability for ions as well. This assumption is supported by the rapid equilibration of pHe with pHsw in our experiments: a change in 0.3 pH units in the seawater medium typically resulted in stable pHe within ∼20 s (Figs. S2 and S3).
Fig. 1.
(A) S. droebachiensis pluteus larvae attached to the holding pipette and the tip of the measuring electrode inside the primary body cavity (PBC). (B) Relationship between seawater and the PBC pH (NBS scale) during acute and chronic environmental hypercapnia. Bars represent ±SD; n = 4–5.
Fig. 2.
Determination of epithelial permeability in pluteus larvae in vivo using FITC dextran conjugates of varying molecular weights and confocal microscopy. (A) Transmission image of pluteus larva; (B) confocal image of larva in a similar position as in A exposed to 4-kDa FITC dextran in seawater for 60 min; the green color in the extracellular matrix (ECM) indicates equilibration of FITC dextran between surrounding seawater (SW) and ECM; (C) confocal image of larva exposed to 40 kDa for 60 min; the dark color indicates lack of equilibration of FITC dextran between ECM and SW. (D) Equilibration of FITC dextrans of varying molecular weights between SW and ECM following 15- and 60-min incubation. Equilibration is represented as the ratio of FITC fluorescence within the ECM and the SW surrounding the larva. Low values indicate a low permeability. (E) Corresponding confocal (FM1-43-stained) and transmission images of the pluteus larva outer epithelium. Bars represent ±SD; n = 9.
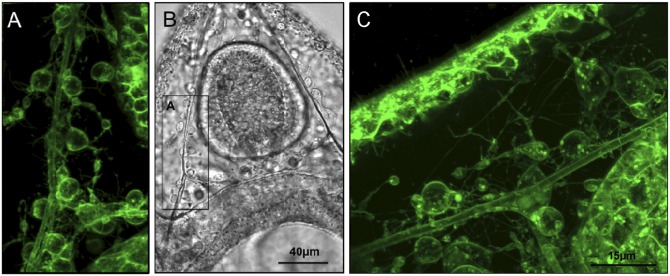
Sea urchin larvae potentially sacrifice a certain degree of control over the abiotic conditions in the extracellular space to create favorable conditions for calcification (see discussion below). Vital stains using the dye FM1-43 indicate that the mesenchymal cells (PMCs, secondary mesenchyme cells) that populate the ECM are connected via a multitude of filopodia to each other and to cells of the ectoderm and the digestive tract (Fig. 3 and Fig. S4) (24, 25). These filopodial connections, which have been suggested to primarily aid in cell–cell signaling (e.g., refs. 26, 27), might also fulfill roles related to nutrient distribution that are classically mediated by the extracellular fluid in most adult invertebrates. This suggests that sea urchin larvae shift nutrient distribution to cellular syncytia to use the extracellular space more efficiently for the demands of ionic exchange processes relevant for calcification.
Fig. 3.
In vivo confocal images of pluteus larvae using the vital dye FM1-43 that stains membranes. Primary mesenchyme cell (PMC) syncytia and their sheaths, as well as filopodia connecting them with each other and epithelial cells are visible, as are vesicles within cells and filopodial connections. A is a close-up confocal image of the region depicted in the Inset in the transmission image (B). C depicts a similar region from a different larva as shown in A and B, but rotated by 90° clockwise.
Chronically Elevated Seawater pCO2 Directly Affects the Sites of Calcification.
The present work demonstrates that, even under chronically decreased seawater pH, the primary body cavity conforms in pH with the environment. Under control conditions, a comparatively high pHe (>8.1) in comparison with other marine invertebrates (28) might be beneficial for maintenance of the endoskeleton. Because the larval spicules are surrounded by sheaths of the PMC syncytium that do not fully isolate them from the extracellular medium (8, 15), high pHe will enable a comparatively high CaCO3 saturation state in the direct vicinity of the spicules. On the other hand, uncompensated pHe during chronic acid challenge might lead to an increased energy demand for the PMCs to prevent dissolution of formed spicules within their sheaths when Ω decreases below 1. For example, Clark et al. (29) observed corrosion of larval spicules in Evechinus chloroticus and Pseudechinus huttoni pluteus larvae during exposure to pH 7.7 and 7.6, respectively. However, it has been demonstrated that sea urchin larvae are capable of maintaining calcification rates under acidified conditions when calcification rates are normalized to growth rate (4). Thus, pH conformity of the primary body cavity with the surrounding seawater suggests that PMCs themselves must have the ability to control intracellular pH, as well as pH in the microenvironment surrounding the spicules to at least partially protect the spicules from dissolution.
pHi Regulatory Abilities of PMCs.
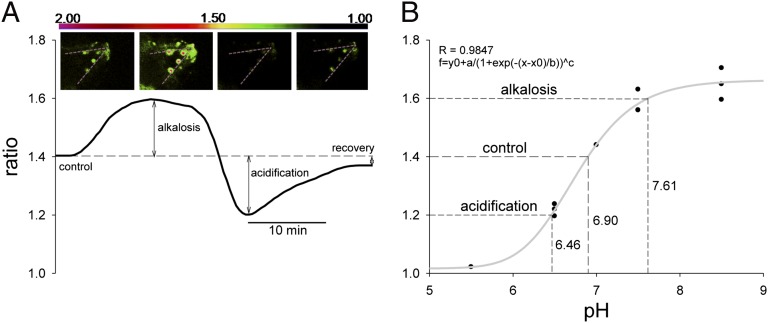
To test whether PMCs themselves are able to control pHi—necessarily linked to calcification and skeletogenesis—we measured their pHi regulatory capacity using the fluorescent pH-sensitive dye BCECF (Fig. 4 and Fig. S5). Using pulses of NH3/NH4+ solution, we demonstrated pHi regulatory abilities in PMCs (Fig. 4A). Under control conditions (pHsw 8.1), PMCs of five larvae were characterized by a control ratio of 1.41 ± 0.06, which corresponds to a pHi of 6.9 as determined by nigericine calibration (Fig. 4B). pHi values for sea urchin eggs a few minutes to hours postfertilization were reported to range between 6.8 and 7.3 depending on the method and species used (30, 31). The initial alkalization of fertilized sea urchin eggs has been hypothesized to be one of several signals inducing protein synthesis and cell functionality (32, 33). pHi values for marine invertebrates were reported to range from 6.9 to 7.4 depending on species and methods used (34–38). The 20 mM NH3/NH4+ solution induced an intracellular alkalinization leading to an increase of pHi by ∼0.2 ratio units corresponding to a ΔpH of 0.7. After washout of the NH3/NH4+ solution, pHi rapidly dropped by ∼0.7 pH units below the control value, followed by a pH compensation reaction to control levels within 15 min (Fig. 4). Buffering capacity (β) (mmol⋅L−1⋅pH unit−1, slykes) of PMCs was calculated according to Graber et al. (39) by dividing the amount of acid load by the measured change of pHi produced by this load. β values obtained for PMCs under control conditions were 20.83 ± 7.78 slykes (n = 9). This buffering capacity is comparable with that of other invertebrates, which were typically reported to be in the range of 16–40 slykes (37, 38, 40–42). Rates of pHi compensation are about five to seven times slower than recovery times reported for strong ion regulatory cells of rat epithelia [2-3 min in colon or Henle’s loop cells (22, 43–45)] but comparable with the recovery rate of crayfish neurons exposed to a similar protocol [∼20 min (46)], indicating a significant acid–base regulatory capacity in echinoid PMCs.
Fig. 4.
Ratiometric fluorimetry in PMCs using the pH-sensitive dye BCECF-AM. (A) Schematic illustration of a recording trace including ratio images (Top: the dashed lines represent the orientation of spicules). Data were obtained from the control period (control), after addition and removal of NH3/NH4+ (alkalosis and acidification; ammonium pulse), and during pHi recovery. (B) Calibration curve of BCECF-AM in PMCs fitted by a function that flattens toward more acidic or alkaline conditions allowing the translation of ratios to pH values.
Na+-Dependent pHi Regulation in PMC.
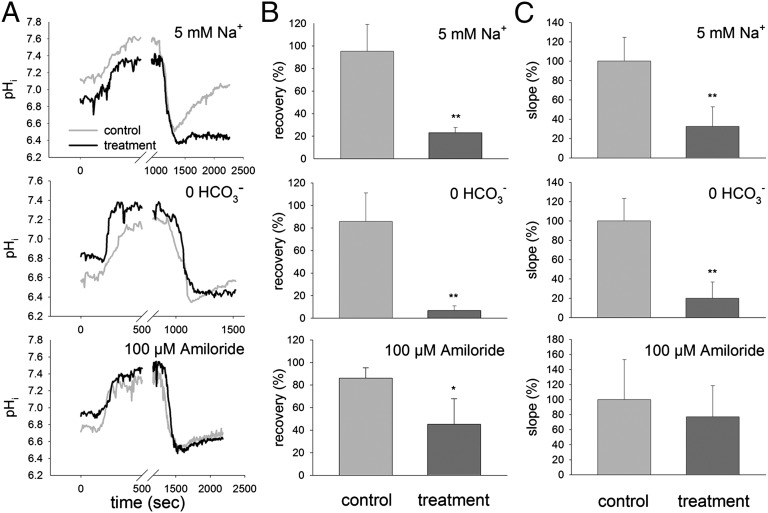
The pHi regulatory machinery has been widely characterized for various animal cell types and is essential for the maintenance of enzyme functionality and cellular processes (for a review, see ] 22). In general, cells maintain pH homeostasis by the import/export of protons and anionic buffers (e.g., HCO3−) (47). Our current data clearly indicate that the compensation reaction after NH3/NH4+-induced acidosis in PMCs is highly dependent on Na+ and HCO3− transport (Fig. 5 and Table S1, including baseline pH, pH values after NH3/NH4+ pulse, and recovery rate). Compared with control conditions, the pHi recovery rate was reduced by 68% in the presence of 5 mM Na+ and by 74% in a HCO3−-free solution (Fig. 5 A and C and Table S1). This suggests the involvement of Na+-dependent HCO3− import mechanisms during pH recovery, which are an indirect sink of ATP. Transporters of the SLC4 family, including Na+/HCO3− cotransporters and Na+-dependent Cl−/HCO3− exchangers were widely identified as key players in this regulatory process (21, 48–50). The sea urchin PMC transcriptome contains a large number of genes coding for ion transporters including Na+/K+-ATPase (NKA), Na+/HCO3− cotransporters (NBC), H+-ATPases (HA), and NHEs (51). This indicates that PMCs possess the necessary molecular machinery to regulate pHi by means of proton secretion and HCO3− accumulation. However, besides the importance of Na+-dependent HCO3− import in PMCs, the present work also suggests that amiloride-sensitive proton extrusion mechanisms are probably not the major compensation pathway in response to an intracellular acidosis. Although a significant reduction in the extent of recovery in pHi was observed in the presence of 100 µM amiloride (to 45% of control pHi/ratio) (Fig. 5B), the slope of the recovery reaction was not significantly different from that under control conditions (Fig. 5C). Because nonmammalian NHEs were demonstrated to be less sensitive to amiloride, with some NHEs being insensitive up to concentrations of 500 µM (52), this experiment cannot provide definitive answers to the role of NHE proteins in PMC pH regulation. However, because protons are generated during the calcification process within the PMCs (20, 53), the identification and characterization of other acid secretion mechanisms involving transporters such as the V-type H+-ATPase or H+/K+-ATPase is an important task in further investigations.
Fig. 5.
Acid–base regulatory abilities of PMCs in the presence of low (5 mM) Na+, without HCO3− and with 100 µM amiloride. (A) Original recordings of control and treatment traces representing average values of four to five single measurements. Presence of NH3/NH4+-induced alkalosis and the washout-induced acidosis under the three experimental conditions. (B) The recovery given in percentage of the initial value, before alkalosis in response to control and treatment conditions. (C) Recovery rate represented by the compensatory slope after acidosis given in percentage of the control (100%) value, in response to control and treatment conditions. Bars represent ±SD; n = 4–5.
Conclusion.
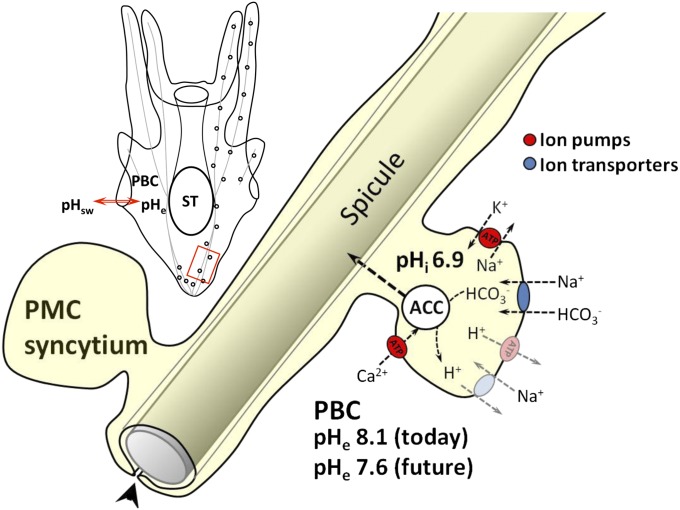
Gene expression analysis of sea urchin larvae reared under acidified conditions revealed an increased expression of NKA α-subunit, the key enzyme that provides the electrochemical gradient for most secondary active transport processes (4, 54, 55). This up-regulation of NKA is accompanied by an increase in metabolic rate, indicating a higher energy demand during environmental hypercapnia (10, 54). Because up to 77% of larval metabolism in sea urchin pluteus larvae is required to fuel the energetic demands of NKA under control conditions (56), it is likely that additional acid–base stress will significantly impact the organism’s energy budget. We suggest that a higher fraction of energy is spent on PMC acid–base regulation because these have to maintain full cell functionality in a more acidified extracellular medium. Thus, calcification during environmental hypercapnia could impact energy allocation. Because vesicular ACC precipitation occurs intracellularly in PMCs (8), the processes of pHi regulation and calcification are intrinsically linked: membrane transporters that are involved in ACC precipitation depend on H+ and HCO3− concentrations in the cytosol, which in turn depend on the action of the pHi regulatory machinery (summarized in Fig. 6). In this way, our data support the concept that maintenance of calcification rates in sea urchin larvae under elevated seawater pCO2 is primarily an energetic, rather than a physicochemical problem. This helps to explain the observed phenomenon of an altered energy allocation under acidified conditions. Decreases in larval developmental rates and delays in metamorphosis will likely result in a higher mortality during the planktonic life phase (57–59). Furthermore, because the energy reserves required by the juvenile sea urchin to support the first weeks after metamorphosis are accumulated by the larvae (60), energetic compromises faced by larval stages may translate into juvenile fitness problems and thus will define the long-term fate of these keystone species in future marine habitats.
Fig. 6.
Schematic model summarizing the interplay of calcification, pH regulation, and energetic costs in sea urchin larvae during environmental acidification. The primary body cavity (PBC) pH conforms to the surrounding seawater pH (pHsw). A decrease in pHe directly affects the calcifying primary mesenchyme cell (PMC) syncytia challenging the intracellular pH (pHi) regulatory machinery due to decreased proton gradients. The vesicular precipitation of amorphous calcium carbonate (ACC) within PMCs is intrinsically linked to pHi regulation. pH homeostasis is maintained by ion transporters, which either directly or indirectly depend on the consumption of energy. Increased energetic costs due to decreased proton gradients lead to a shift in the larvae’s energy budget, which decreases scope for growth and may also translate into juvenile impaired fitness. ST, stomach; putative transporters are in gray.
Materials and Methods
Sea Urchin Larvae Culture and CO2 Perturbation Experiment.
All animal experiments were performed according to the German law for animal welfare and were approved by the animal welfare officer of the Christian Albrechts University, Kiel. Adult Strongylocentrotus droebachiensis were collected in Winter 2010 and 2011 in the Kattegat (Dröbak, Norway) by divers. Spawning was induced by injection of 2 mL of 0.5 mM KCl into the coelomic cavity. For each experiment, eggs of one to two females were collected in separate 1-L beakers, washed, and fertilized by adding dry sperm (20 µL) of two males. Fertilization was followed by monitoring the fertilization-induced elevation of the oocyte membrane under a stereomicroscope (fertilization rates, >95%). Zygotes were allowed to divide once before they were pooled, concentrated in 25 mL, and split into 2-L (three replicates per pCO2 treatment, Erlenmeyer flasks) culture vessels, which were preequilibrated at three different pCO2, control (∼0.05 kPa, pH 8.1), intermediate (0.12 kPa, pH 7.7), and high pCO2 (∼0.24 kPa, pH 7.4) by a central automatic gas mixing facility (Linde Gas, HTK Hamburg, Germany). Water pHNBS, salinity, and temperature were monitored daily in the setup during the entire incubation period. Seawater samples (0.5 L) for determination of total dissolved inorganic carbon, CT, were collected weekly. CT was determined using an AIRICA analyzer (Marianda). CT was corrected using certified reference material provided by Andrew Dickson (Scripps Institution of Oceanography, La Jolla, CA) (61). Seawater carbonate system speciation was calculated from pHNBS and CT with the open-source program CO2SYS (62) using the dissociation constants by Mehrbach et al. (63) as refitted by Dickson and Millero (64). Water parameters measured during the experimental period are summarized in Table S2.
Larval culturing and monitoring was essentially conducted as described in previous studies (10, 54).
Selective Ion Electrode Technique.
Selective ion electrode measurements were essentially performed after Kuehl and Revsbech (65) to measure H+ concentrations in the extracellular gel matrix (ECM) of sea urchin larvae. Glass capillary tubes (borosilicate; inner diameter, 1.2 mm; outer diameter, 1.5 mm; Hilgenberg) were pulled on a DMZ-Universal puller (Zeitz Instruments) into micropipettes with tip diameters of 2–3 µm. These were then baked at 200 °C for 4 h and vapor-silanized with dimethyl chlorosilane (Sigma-Aldrich) overnight. The micropipettes were front-loaded with a 200-µm column of liquid ion exchanger mixture (H+ ionophore III; Sigma-Aldrich) diluted in 2-nitrophenyl ether at a concentration of 10.5 mg⋅mL−1. Additionally, micropipettes were again front-loaded with a 100-µm column of the ionophore mixture containing a polyvinylchloride/tetrahydrofuran (330 mg⋅mL−1) solution with a ratio of 1:3 to seal the opening of the electrode tip. The micropipette was backfilled with a 4-cm column of pH electrolyte solution (300 mM KCl, 50 mM NaPO4, pH 7) to create an ion selective microelectrode (probe). To calibrate the ion-selective probe, the Nernstian property of each microelectrode was measured by placing the microelectrode in a series of artificial seawater (ASW) solutions (pH 6, 7, 8, and 9) with reference to an Ag/AgCl electrode. By plotting the voltage output of the probe against log [H+] values, a linear regression yielded a Nernstian slope of 49.8 ± 2.3 mV (n = 12) for 1 pH unit. With this method, we were able to resolve a minimum difference of 0.1 pH units (corresponding to a change in pCO2 of ∼0.02–0.04 kPa). Electrode response times in the perfusion solution (0.79 ± 0.21 s⋅nM [H+]−1) and within the primary body cavity (1.75 ± 0.42 s⋅nM [H+]−1) were calculated by the time needed to reach 95–100% of the fully stabilized value after a pH change (usually 0.3 or 0.4 pH units between pH 8.1, 7.7, and 7.4) in the perfusion bath.
Measurement of Extracellular H+ Concentrations.
The electrode measurements were performed at 10 °C on an inverse microscope (Axiovert 135; Zeiss) equipped with a temperature-controlled perfusion system, allowing the quick exchange of different pH solutions inside the perfusion chamber. The pH of the bath solutions was adjusted by equilibrating ASW with pure CO2 to the desired pH. The larva was placed inside the perfusion bath and kept in position via a holding pipette (30- to 40-µm tip diameter) to which a slight vacuum was applied. The ion-selective probe was mounted on a remote-controlled micromanipulator (Phytron) and was introduced into the ECM from the base of the arms at the oral side of the larvae (10–20 µm inside the ECM) to record the ionic activities (Fig. 1A). The epidermis and basal lamina formed a seal around the entrance point of the electrode, preventing fluid exchange between seawater and the primary body cavity. pH recordings were performed on pluteus larvae [8–10 d postfertilization (dpf)] reared under control conditions, which were exposed to acute changes in seawater pH ranging from 6.9 to 8.2. Furthermore, the same measurements were also performed on pluteus larvae (8–10 dpf) from the CO2 perturbation experiment to address the effects of chronically elevated seawater pCO2 on extracellular pH homeostasis.
Visualization of Mesenchyme Cell Filopodial Network.
Four-armed pluteus larvae (96 h) were incubated for 15 min in control (pH 8.1) seawater with 20 µM FM1-43 at 10 °C. Following incubation, larvae were placed on microscopy slides suspended in the incubation solution and covered with glass coverslips supported by ∼100-µm-thick fibers as spacers. Larvae were then imaged with a Zeiss LSM510 using a Plan Neofluar 40×/1.3 objective and a 505-nm long-pass filter at an excitation wavelength of 488 nm. Images were collected within 20 min (n = 20) at room temperature (20 °C).
Epithelial Permeability Measurements with FITC Dextran.
To measure epithelial permeability, four-armed pluteus larvae (96 h) were incubated for 15 and 60 min in control (pH 8.1) seawater with 0.4 mM FITC dextran (free FITC, 0.4, 4, 10, 40 kDa; Sigma-Aldrich) at 10 °C. FITC dextran solutions were dialyzed overnight in ASW in MWCO-dialysis tubing (Roth) before experiments. Following incubation, larvae were imaged on a Zeiss LSM510 as described above. Images were collected within 10 min (n = 9 larvae per treatment) at room temperature (20 °C).
Bag Isolation Procedure and Dye (BCECF-Acetoxymethylester) Loading.
To measure PMC intracellular pH (pHi), epidermal cells of 8–10 dpf larvae were removed according to Harkey and Whiteley (66). Using this protocol, both epithelial as well as PMCs can be maintained viable for hours (66). Larvae were incubated at 10 °C with a final concentration of 50 μmol L−1 BCECF-acetoxymethylester (BCECF-AM) for 30 min to allow sufficient uptake and cleavage of the esterified dye by intracellular esterases. Thereafter, only larvae, which were firmly attached to the perfusion bath bottom, were used for measurement. The flow rate of the perfusion solutions was 2–3 mL⋅min−1. With this dye-loading procedure, we achieved a signal-to-noise ratio for the emission signal of >10 throughout an experimental period of >2 h without appreciable loss of signal intensity.
Solutions.
ASW solutions were mixed according to Zeebe and Wolf-Gladrow (67). Osmolality (980 ± 10 mosm⋅kg−1) and salinity (31 ± 1) were chosen to match the natural seawater used in the larval cultures. All ingredients of the experimental solutions are given in Table S3. Amiloride was added at a final concentration of 100 μM to the ASW. DMSO did not exceed a concentration of 0.1%.
Microfluorimetry.
Fluorescence was monitored with an imaging system (Visitron). The dye was alternatively excited at a rate of 0.2 Hz at 486 and 440 nm (±10-nm bandwidth) for 24 and 60 ms, respectively. Emission was recorded at 525 nm (±13-nm bandwidth), and the integrated ratio of the emission intensities at the two excitation wavelengths over the whole cell was calculated after subtraction of system immanent camera offset and background signal (MetaFluor Software 7.6.1; Molecular Devices). From each larva, the recordings of four to five PMCs were averaged and treated as one replicate (n = 1). Four to five control and treatment larvae were measured in an alternate mode and used for further analysis. Nigericin was used to calibrate pHi of living cells as previously described by Suffrian et al. (68). PMCs of pluteus larvae were exposed to 10 μmol⋅L−1 nigericin in the presence of 150 mmol⋅L−1 K+ at pH 5.5, 6.5, 7, 7.5, and 8.5. This [K+]i is in the same range reported for fertilized and unfertilized sea urchin eggs (69) and intestinal tissues of the sea urchin S. droebachiensis (70). The calibration curve (Fig. 4) allowed estimation of the relationship between detected emission ratio of BCECF and the respective pHi. A change of 0.1 ratio units corresponded to a change of 0.35 pH units.
For all experiments, the bath was exchanged at a rate of 2–3 mL⋅min−1 at 10 °C, and pHi was continuously monitored. All larvae were exposed to ASW followed by the 20 mM NH3/NH4+ prepulse. Acidosis was induced by NH3/NH4+ washout using the respective solutions, 5 mM Na+,HCO3−-free solutions and ASW plus 100 μM amiloride or control solutions (e.g., ASW or ASW plus DMSO), which were directly applied to the larvae via the perfusion system.
Traces of intracellular pH measurements were analyzed according to the parameters depicted in Fig. 3A. To determine the ability of PMCs to recover from an NH3/NH4+-induced acidosis, we calculated the ratio of the recovery value (in the stationary phase of the recovery phase) and the initial control value. Additionally, the slope of the compensation reaction was used to characterize the rate of recovery after an induced acidosis by determining the change in the ratio as a function of time.
Statistical Analysis.
Statistical differences between response times of ion-selective electrode measurements and pH regulatory abilities of PMCs were analyzed with Student t test with significance levels of P < 0.05 (*) and P < 0.01 (**).
Supplementary Material
Acknowledgments
We thank U. Panknin, S. Syré, and A. Cipriano for valuable laboratory help and D. de Beer for advice on pH microsensor techniques. This work was funded by Bundesministerium für Bildung und Forschung Grant 03F0608M [Biological Impacts of Ocean ACIDification (BIOACID) 3.1.4] (to F.M and M.B.); by the Linnaeus Centre for Marine Evolutionary Biology, University of Gothenburg (S.T.D.); by a Linnaeus grant from the Swedish Research Councils Vetenskapsrådet and Formas (to M.C.T.); and by Knut and Alice Wallenberg’s minnen and the Royal Swedish Academy of Sciences (M.S. and M.C.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1209174109/-/DCSupplemental.
References
- 1.Dupont S, Ortega-Martínez O, Thorndyke MC. Impact of near-future ocean acidification on echinoderms. Ecotoxicology. 2010;19(3):449–462. doi: 10.1007/s10646-010-0463-6. [DOI] [PubMed] [Google Scholar]
- 2.Kurihara H, Shirayama Y. Effects of increased atmospheric CO2 on sea urchin early development. Mar Ecol Prog Ser. 2004;274:161–169. [Google Scholar]
- 3.O’Donnell MJ, Hammond LM, Hofmann GE. Predicted impact of ocean acidification on a marine invertebrate: Elevated CO2 alters response to thermal stress in sea urchin larvae. Mar Biol. 2009;156(3):439–446. [Google Scholar]
- 4.Martin S, et al. Early development and molecular plasticity in the Mediterranean sea urchin Paracentrotus lividus exposed to CO2-driven acidification. J Exp Biol. 2011;214(Pt 8):1357–1368. doi: 10.1242/jeb.051169. [DOI] [PubMed] [Google Scholar]
- 5.Cao L, Caldeira K. Atmospheric CO2 stabilization and ocean acidification. Geophys Res Lett. 2008;35:1–5. [Google Scholar]
- 6.Caldeira K, Wickett ME. Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J Geophys Res. 2005;110:1–12. [Google Scholar]
- 7.Raz S, Hamilton PC, Wilt FH, Weiner S, Addadi L. The transient phase of amorphous calcium carbonate in sea urchin larval spicules: The involvement of proteins and magnesium ions in its formation and stabilization. Adv Funct Mater. 2003;13(6):480–486. [Google Scholar]
- 8.Beniash E, Aizenberg J, Addadi L, Weiner S. Amorphous calcium carbonate transforms into calcite during sea urchin larval spicule growth. Proc Biol Sci. 1997;264:461–465. [Google Scholar]
- 9.Thomsen J, Melzner F. Moderate seawater acidification does not elicit long-term metabolic depression in the blue mussel Mytilus edulis. Mar Biol. 2010;157(12):2667–2676. [Google Scholar]
- 10.Stumpp M, Wren J, Melzner F, Thorndyke MC, Dupont ST. CO2 induced seawater acidification impacts sea urchin larval development I: Elevated metabolic rates decrease scope for growth and induce developmental delay. Comp Biochem Physiol A Mol Integr Physiol. 2011;160(3):331–340. doi: 10.1016/j.cbpa.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Sikes CS, Okazaki K, Fink RD. Respiratory CO2 and the supply of inorganic carbon for calcification of sea urchin embryos. Comp Biochem Physiol A. 1981;70(3):285–291. [Google Scholar]
- 12.Politi Y, et al. Transformation mechanism of amorphous calcium carbonate into calcite in the sea urchin larval spicule. Proc Natl Acad Sci USA. 2008;105(45):17362–17366. doi: 10.1073/pnas.0806604105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakano E, Okazaki K, Iwamatsu T. Accumulation of radioactive calcium in the larvae of the sea urchin Pseudecentrotus depressus. Biol Bull. 1963;125(1):125–136. [Google Scholar]
- 14.Wilt FH. Biomineralization of the spicules of sea urchin embryos. Zoolog Sci. 2002;19(3):253–261. doi: 10.2108/zsj.19.253. [DOI] [PubMed] [Google Scholar]
- 15.Decker GL, Morrill JB, Lennarz WJ. Characterization of sea urchin primary mesenchyme cells and spicules during biomineralization in vitro. Development. 1987;101(2):297–312. doi: 10.1242/dev.101.2.297. [DOI] [PubMed] [Google Scholar]
- 16.Politi Y, Arad T, Klein E, Weiner S, Addadi L. Sea urchin spine calcite forms via a transient amorphous calcium carbonate phase. Science. 2004;306(5699):1161–1164. doi: 10.1126/science.1102289. [DOI] [PubMed] [Google Scholar]
- 17.Hwang SP, Lennarz WJ. Studies on the cellular pathway involved in assembly of the embryonic sea urchin spicule. Exp Cell Res. 1993;205(2):383–387. doi: 10.1006/excr.1993.1101. [DOI] [PubMed] [Google Scholar]
- 18.Jayantha Gunaratne H, Vacquier VD. Sequence, annotation and developmental expression of the sea urchin Ca2+-ATPase family. Gene. 2007;397(1-2):67–75. doi: 10.1016/j.gene.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 19.McConnaughey TA, Whelan JF. Calcification generates protons for nutrient and bicarbonate uptake. Earth Sci Rev. 1996;41(1–2):95–117. [Google Scholar]
- 20.Buitenhuis ET, de Baar HJW, Veldhuis MJW. Photosythesis and calcification by Emiliania huxleyi (Prymnesiophyceae) as a function of inorganic carbon species. J Phycol. 1999;35(5):949–959. [Google Scholar]
- 21.Romero MF, Fulton CM, Boron WF. The SLC4 family of HCO3− transporters. Pflugers Arch. 2004;447(5):495–509. doi: 10.1007/s00424-003-1180-2. [DOI] [PubMed] [Google Scholar]
- 22.Boron WF. Regulation of intracellular pH. Adv Physiol Educ. 2004;28(1-4):160–179. doi: 10.1152/advan.00045.2004. [DOI] [PubMed] [Google Scholar]
- 23.Evans DH, Piermarini PM, Choe KP. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev. 2005;85(1):97–177. doi: 10.1152/physrev.00050.2003. [DOI] [PubMed] [Google Scholar]
- 24.Malinda KM, Fisher GW, Ettensohn CA. Four-dimensional microscopic analysis of the filopodial behavior of primary mesenchyme cells during gastrulation in the sea urchin embryo. Dev Biol. 1995;172(2):552–566. doi: 10.1006/dbio.1995.8044. [DOI] [PubMed] [Google Scholar]
- 25.Miller J, Fraser SE, McClay D. Dynamics of thin filopodia during sea urchin gastrulation. Development. 1995;121(8):2501–2511. doi: 10.1242/dev.121.8.2501. [DOI] [PubMed] [Google Scholar]
- 26.Röttinger E, et al. FGF signals guide migration of mesenchymal cells, control skeletal morphogenesis [corrected] and regulate gastrulation during sea urchin development. Development. 2008;135(2):353–365. doi: 10.1242/dev.014282. [DOI] [PubMed] [Google Scholar]
- 27.Ettensohn CA. Cell interactions in the sea urchin embryo studied by fluorescence photoablation. Science. 1990;248(4959):1115–1118. doi: 10.1126/science.2188366. [DOI] [PubMed] [Google Scholar]
- 28.Melzner F, et al. Physiological basis for high CO2 tolerance in marine ectothermic animals: Pre-adaptation through lifestyle and ontogeny. Biogeoscience. 2009;6(10):2313–2331. [Google Scholar]
- 29.Clark D, Lamare M, Barker M. Response of sea urchin pluteus larvae (Echinodermata: Echinoidea) to reduced seawater pH: A comparison among tropical, temperate and polar species. Mar Biol. 2009;156(6):1125–1137. [Google Scholar]
- 30.Johnson CH, Epel D. Intracellular pH of sea urchin eggs measured by the dimethyloxazolidinedione (DMO) method. J Cell Biol. 1981;89(2):284–291. doi: 10.1083/jcb.89.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson JD, Epel D. Intracellular pH and activation of sea urchin eggs after fertilisation. Nature. 1976;262(5570):661–664. doi: 10.1038/262661a0. [DOI] [PubMed] [Google Scholar]
- 32.Rees BB, Patton C, Grainger JL, Epel D. Protein synthesis increases after fertilization of sea urchin eggs in the absence of an increase in intracellular pH. Dev Biol. 1995;169(2):683–698. doi: 10.1006/dbio.1995.1179. [DOI] [PubMed] [Google Scholar]
- 33.Grainger JL, Winkler MM, Shen SS, Steinhardt RA. Intracellular pH controls protein synthesis rate in the sea urchine egg and early embryo. Dev Biol. 1979;68(2):396–406. doi: 10.1016/0012-1606(79)90213-6. [DOI] [PubMed] [Google Scholar]
- 34.Aickin CC, Thomas RC. Micro-electrode measurement of the internal pH of crab muscle fibres. J Physiol. 1975;252(3):803–815. doi: 10.1113/jphysiol.1975.sp011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venn AA, et al. Imaging intracellular pH in a reef coral and symbiotic anemone. Proc Natl Acad Sci USA. 2009;106(39):16574–16579. doi: 10.1073/pnas.0902894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wheatly MG, Henry RP. Extracellular and intracellular acid-base regulation in crustaceans. J Exp Zool. 1992;263(2):127–142. [Google Scholar]
- 37.Wisemann RW, Ellington WR. Intracellular buffering capacity in molluscan muscle: Superfused muscle versus homogenates. Physiol Zool. 1989;62(2):541–558. [Google Scholar]
- 38.Pörtner H-O. Determination of intracellular buffer values after metabolic inhibition by fluoride and nitrilotriacetic acid. Respir Physiol. 1990;81(2):275–288. doi: 10.1016/0034-5687(90)90051-y. [DOI] [PubMed] [Google Scholar]
- 39.Graber M, DiPaola J, Hsiang FL, Barry C, Pastoriza E. Intracellular pH in the OK cell. I. Identification of H+ conductance and observations on buffering capacity. Am J Physiol. 1991;261(6 Pt 1):C1143–C1153. doi: 10.1152/ajpcell.1991.261.6.C1143. [DOI] [PubMed] [Google Scholar]
- 40.Zange J, Grieshaber MK, Jans AWH. The regulation of intracellular pH estimated by 31P-NMR spectroscopy in the anterior byssus retractor muscle of Mytilus edulis L. J Exp Biol. 1990;150:95–109. [Google Scholar]
- 41.Thomas RC. The effect of carbon dioxide on the intracellular pH and buffering power of snail neurones. J Physiol. 1976;255(3):715–735. doi: 10.1113/jphysiol.1976.sp011305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burton RF. Intracellular buffering. Respir Physiol. 1978;33(1):51–58. doi: 10.1016/0034-5687(78)90083-x. [DOI] [PubMed] [Google Scholar]
- 43.Bleich M, Köttgen M, Schlatter E, Greger R. Effect of NH4+/NH3 on cytosolic pH and the K+ channels of freshly isolated cells from the thick ascending limb of Henle’s loop. Pflugers Arch. 1995;429(3):345–354. doi: 10.1007/BF00374149. [DOI] [PubMed] [Google Scholar]
- 44.Boron WF, De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hasselblatt P, Warth R, Schulz-Baldes A, Greger R, Bleich M. pH regulation in isolated in vitro perfused rat colonic crypts. Pflugers Arch. 2000;441(1):118–124. doi: 10.1007/s004240000377. [DOI] [PubMed] [Google Scholar]
- 46.Moody WJ., Jr The ionic mechanism of intracellular pH regulation in crayfish neurones. J Physiol. 1981;316:293–308. doi: 10.1113/jphysiol.1981.sp013788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boron WF. Transport of H+ and of ionic weak acids and bases. J Membr Biol. 1983;72(1-2):1–16. doi: 10.1007/BF01870311. [DOI] [PubMed] [Google Scholar]
- 48.Virkki LV, Choi I, Davis BA, Boron WF. Cloning of a Na+-driven Cl/HCO3 exchanger from squid giant fiber lobe. Am J Physiol Cell Physiol. 2003;285(4):C771–C780. doi: 10.1152/ajpcell.00439.2002. [DOI] [PubMed] [Google Scholar]
- 49.Piermarini PM, Choi I, Boron WF. Cloning and characterization of an electrogenic Na/HCO3− cotransporter from the squid giant fiber lobe. Am J Physiol Cell Physiol. 2007;292(6):C2032–C2045. doi: 10.1152/ajpcell.00544.2006. [DOI] [PubMed] [Google Scholar]
- 50.Henriksen K, Bollerslev J, Everts V, Karsdal MA. Osteoclast activity and subtypes as a function of physiology and pathology—implications for future treatments of osteoporosis. Endocr Rev. 2011;32(1):31–63. doi: 10.1210/er.2010-0006. [DOI] [PubMed] [Google Scholar]
- 51.Zhu X, et al. A large-scale analysis of mRNAs expressed by primary mesenchyme cells of the sea urchin embryo. Development. 2001;128(13):2615–2627. doi: 10.1242/dev.128.13.2615. [DOI] [PubMed] [Google Scholar]
- 52.Harada K, Fukuda E, Hirohashi N, Chiba K. Regulation of intracellular pH by p90Rsk-dependent activation of an Na+/H+ exchanger in starfish oocytes. J Biol Chem. 2010;285(31):24044–24054. doi: 10.1074/jbc.M109.072553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fujino Y, Mitsunaga K, Yasumasu I. Inhibitory effects of omeprazole, a specific inhibitor of H+, K+-ATPase, on spicule formation in sea urchin embryos and in cultured micromere-derived cells. Dev Growth Differ. 1987;29(6):591–597. doi: 10.1111/j.1440-169X.1987.00591.x. [DOI] [PubMed] [Google Scholar]
- 54.Stumpp M, Dupont S, Thorndyke MC, Melzner F. CO2 induced acidification impacts sea urchin larval development II: Gene expression patterns in pluteus larvae. Comp Biochem Physiol A Mol Integr Physiol. 2011;160(3):320–330. doi: 10.1016/j.cbpa.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 55.Hu MY, et al. Elevated seawater PCO2 differentially affects branchial acid-base transporters over the course of development in the cephalopod Sepia officinalis. Am J Physiol Regul Integr Comp Physiol. 2011;300(5):R1100–R1114. doi: 10.1152/ajpregu.00653.2010. [DOI] [PubMed] [Google Scholar]
- 56.Leong PKK, Manahan D. Metabolic importance of Na+/K+-ATPase activity during sea urchin development. J Exp Biol. 1997;200(Pt 22):2881–2892. doi: 10.1242/jeb.200.22.2881. [DOI] [PubMed] [Google Scholar]
- 57.Allen JD. Size-specific predation on marine invertebrate larvae. Biol Bull. 2008;214(1):42–49. doi: 10.2307/25066658. [DOI] [PubMed] [Google Scholar]
- 58.Dupont S, Dorey N, Thorndyke M. What meta-analysis can tell us about vulnerability of marine biodiversity to ocean acidification? Estuar Coast Shelf Sci. 2010;89(2):182–185. [Google Scholar]
- 59.Hare JA, Cowen RK. Size, growth, development, and survival of the planktonic larvae of Pomatomus saltatrix (Pisces: Pomatomidae) Ecology. 1997;78(8):2415–2431. [Google Scholar]
- 60.Byrne M, et al. Maternal provisioning for larvae and larval provisioning for juveniles in the toxopneustid sea urchin Tripneustes gratilla. Mar Biol. 2008;155(5):473–482. [Google Scholar]
- 61.Dickson AG, Afghan JD, Anderson GC. Reference materials for oceanic CO2 analysis: A method for the certification of total alkalinity. Mar Chem. 2003;80(2–3):185–197. [Google Scholar]
- 62.Lewis E, Wallace D. Program Developed for CO2 System Calculations. Oak Ridge, TN: Oak Ridge National Laboratory; 1998. [Google Scholar]
- 63.Mehrbach C, Culberson CH, Hawlwy JE, Pytkowicz RM. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol Oceanogr. 1973;18(6):897–907. [Google Scholar]
- 64.Dickson AG, Millero FJ. A comparison of the equilibrium-constants for the dissociation of carbonic-acid in seawater media. Deep-Sea Res. 1987;34(10):1733–1743. [Google Scholar]
- 65.Kuehl M, Revsbech NP. Microsensors for the Study of Interfacial Biogeochemical Processes. Oxford: Oxford Univ Press; 2001. [Google Scholar]
- 66.Harkey MA, Whiteley AH. Isolation, culture, and differentiation of echinoid primary mesenchyme cells. Wilhelm Raux’s Arch. 1980;189(2):111–122. doi: 10.1007/BF00848500. [DOI] [PubMed] [Google Scholar]
- 67.Zeebe RE, Wolf-Gladrow DA. CO2 in Seawater: Equilibrium, Kinetics, Isotopes. Amsterdam: Elsevier; 2001. p. 346. [Google Scholar]
- 68.Suffrian K, Schulz KG, Gutowska MA, Riebesell U, Bleich M. Cellular pH measurements in Emiliania huxleyi reveal pronounced membrane proton permeability. New Phytol. 2011;190(3):595–608. doi: 10.1111/j.1469-8137.2010.03633.x. [DOI] [PubMed] [Google Scholar]
- 69.Shen SS, Sui A-L. K+ activity and regulation of intracellular pH in the sea urchin egg during fertilization. Exp Cell Res. 1989;183(2):343–352. doi: 10.1016/0014-4827(89)90395-9. [DOI] [PubMed] [Google Scholar]
- 70.Lange R. The osmotic adjustment in the echinoderm, Strongylocentrotus droebrachiensis. Comp Biochem Physiol. 1964;13(3):205–216. doi: 10.1016/0010-406x(64)90117-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.