Abstract
A major physiologic sign in Parkinson disease is the occurrence of abnormal oscillations in cortico-basal ganglia circuits, which can be normalized by l-DOPA therapy. Under normal circumstances, oscillatory activity in these circuits is modulated as behaviors are learned and performed, but how dopamine depletion affects such modulation is not yet known. We here induced unilateral dopamine depletion in the sensorimotor striatum of rats and then recorded local field potential (LFP) activity in the dopamine-depleted region and its contralateral correspondent as we trained the rats on a conditional T-maze task. Unexpectedly, the dopamine depletion had little effect on oscillations recorded in the pretask baseline period. Instead, the depletion amplified oscillations across delta (∼3 Hz), theta (∼8 Hz), beta (∼13 Hz), and low-gamma (∼48 Hz) ranges selectively during task performance times when each frequency band was most strongly modulated, and only after extensive training had occurred. High-gamma activity (65–100 Hz), in contrast, was weakened independent of task time or learning stage. The depletion also increased spike-field coupling of fast-spiking interneurons to low-gamma oscillations. l-DOPA therapy normalized all of these effects except those at low gamma. Our findings suggest that the task-related and learning-related dynamics of LFP oscillations are the primary targets of dopamine depletion, resulting in overexpression of behaviorally relevant oscillations. l-DOPA normalizes these dynamics except at low-gamma, linked by spike-field coupling to fast-spiking interneurons, now known to undergo structural changes after dopamine depletion and to lack normalization of spike activity following l-DOPA therapy.
Loss of the dopamine-containing innervation of the basal ganglia is a primary pathology in Parkinson disease, resulting, in addition to its behavioral effects, in abnormal local field potential (LFP) oscillations within cortico-basal ganglia circuits (1–4). Clinical evidence suggests that successful therapies for Parkinson disease reduce these abnormal LFP oscillations (3–6), establishing them as a central feature of Parkinson disease. In particular, abnormally strong beta-range oscillations (12–30 Hz) and weakened high-frequency gamma oscillations (>70 Hz) have been found in basal ganglia structures. The “antimovement” beta-band oscillations are reduced by both l-DOPA therapy and by deep brain stimulation (DBS) (3–6). How these observations relate to the proposed network functions of oscillatory neural activity is not yet clear. LFP oscillations have been linked not only to motor control but also to sensory perception, attention, learning, memory formation, and interregional communication (7–11). In Parkinson disease models, abnormal patterns of synchrony have been found in rest and locomotion (12–14), but the effect of dopamine loss on LFP oscillations during complex tasks requiring learning and decision making has not been explored.
Here we report that dopamine depletion in the sensorimotor striatum has striking effects both on oscillatory power in multiple frequency ranges and on spike-field synchrony, but that the abnormal patterns of synchronization are behaviorally regulated and are not omnipresent features of the dopamine-depleted state.
Results
Sixteen rats were given unilateral 6-hydroxydopamine (6-OHDA) injections to produce local dopamine depletion in the dorsolateral striatum. Fast-scan cyclic voltammetric measurements were made in 4 other rats; these demonstrated that the localized 6-OHDA lesions reduced evoked dopamine release by about 75% relative to levels in the contralateral striatum or ipsilateral striatal regions outside of the injected zone†. After a 5-wk lesion stabilization period, the rats were trained on a T-maze task while we recorded spike and LFP activity in the dopamine-depleted dorsolateral striatum and the contralateral intact dorsolateral striatum (Fig. 1A). The spike activity patterns are reported in a companion study†. The T-maze task began with a click signal indicating the trial initiation, followed by the opening of the gate allowing rats to run down the maze toward one of the two end arms. Before the rats reached the choice point, one of two auditory cues instructed which maze goal was baited with chocolate reward (Fig. 1B). The localized 6-OHDA lesions did not produce apparent difficulties with learning of the task: the rats reached the 72.5% correct learning criterion in an average of 8.3 sessions, a rate comparable to that of normal rats in a similar maze task (15). The percentage of correct trials rose from chance levels to above 85% late in training (P < 0.0001, ANOVA; Fig. 1C). Running times (from start to goal-reaching), which early in training were slightly longer than those observed for normal rats, decreased significantly as the rats acquired the T-maze task (Fig. 1D, P < 0.0001) and became comparable to those of normal rats (15).
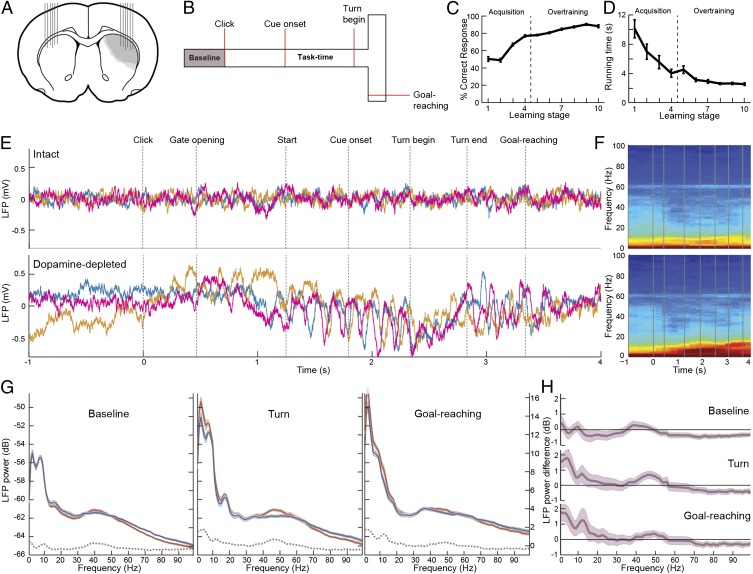
Fig. 1.
Dopamine loss amplifies LFP oscillations during performance of a T-maze task. (A) Extent of the local, unilateral dopamine depletion and location of bilateral recording sites in the dorsolateral striatum (DLS). (B) Cue-instructed turning task in a T-maze. Baseline period began 2 s before a click to indicate trial start. (C and D) Mean percentage of correct responses (C) and running time (D) across training (n = 16 rats). (E) Raw LFP trace from three trials in a single training session recorded simultaneously by tetrodes in the intact (Upper) and dopamine-depleted (Lower) DLS. (F) Trial average spectrograms for LFP activity shown in E. (G) Aggregate session average spectra (single taper), calculated from overtraining sessions of 16 rats. Each plot shows power of oscillations during 1-s periods during pretrial baseline (n = 114 sessions), around turn beginning (n = 112 sessions), and around goal-reaching (n = 107 sessions) recorded in the intact (blue) and dopamine-depleted (red) DLS. Shading indicates SEM. Dashed lines show power difference between the intact and depleted sides (scale at Right). (H) Session average differences in LFP power between the intact and dopamine-depleted DLS. Positive values indicate depleted side > intact side. Shading indicates ±2 SEM (95% confidence interval for difference from zero). Only sessions with simultaneous recordings in both sides were used.
Dopamine Depletion Amplifies Oscillations Selectively only During Task Times in Which They Are Actively Modulated.
Confirming earlier observations (16), task-related modulation of oscillatory LFP activity was apparent in the sensorimotor striatum of the intact hemisphere. Oscillations in the theta range (6–9 Hz) strengthened as the animals started to run down the maze, subsided at the end of the run near goal, and were often replaced by a slightly more rapid low-beta oscillation (11–15 Hz) at goal-reaching (Fig. 1 E and F). Delta (2–5 Hz) and gamma oscillations (40–53 Hz) also occurred during task execution (Fig. 1 F and G), as well as a harmonic of the 8-Hz theta oscillation at 16 Hz (Fig. 1G, Middle and Fig. S1).
Similar task-related oscillatory dynamics were present in the dopamine-depleted sensorimotor striatum, but these were markedly enhanced only at specific task points and in select task-relevant ranges of frequencies (Fig. 1 G and H). We focused on the prominent delta, theta, beta, and low-gamma oscillations to determine the role of dopamine depletion on modulations of these rhythms.
For all but the high-gamma oscillations, the oscillatory patterns were strongly enhanced as the rats ran in the maze (delta, theta, and low-gamma rhythms, P < 0.01 for all, ANOVA), or at goal-reaching (low-beta rhythm, P < 0.03; Figs. 1H and 2D). Remarkably, no significant differences in power were observed during the pretrial baseline period in any of these frequency bands (P > 0.3 for all; Figs. 1G and H and 2D), a period directly prior to the warning click and gate-opening during which the rats were at rest and were waiting for the trial to begin. LFP power in the high-gamma range (65–100 Hz) was reduced by dopamine depletion across the entire task and pretask baseline periods (P < 0.05 at all task periods; Figs. 1 G and H and 2).
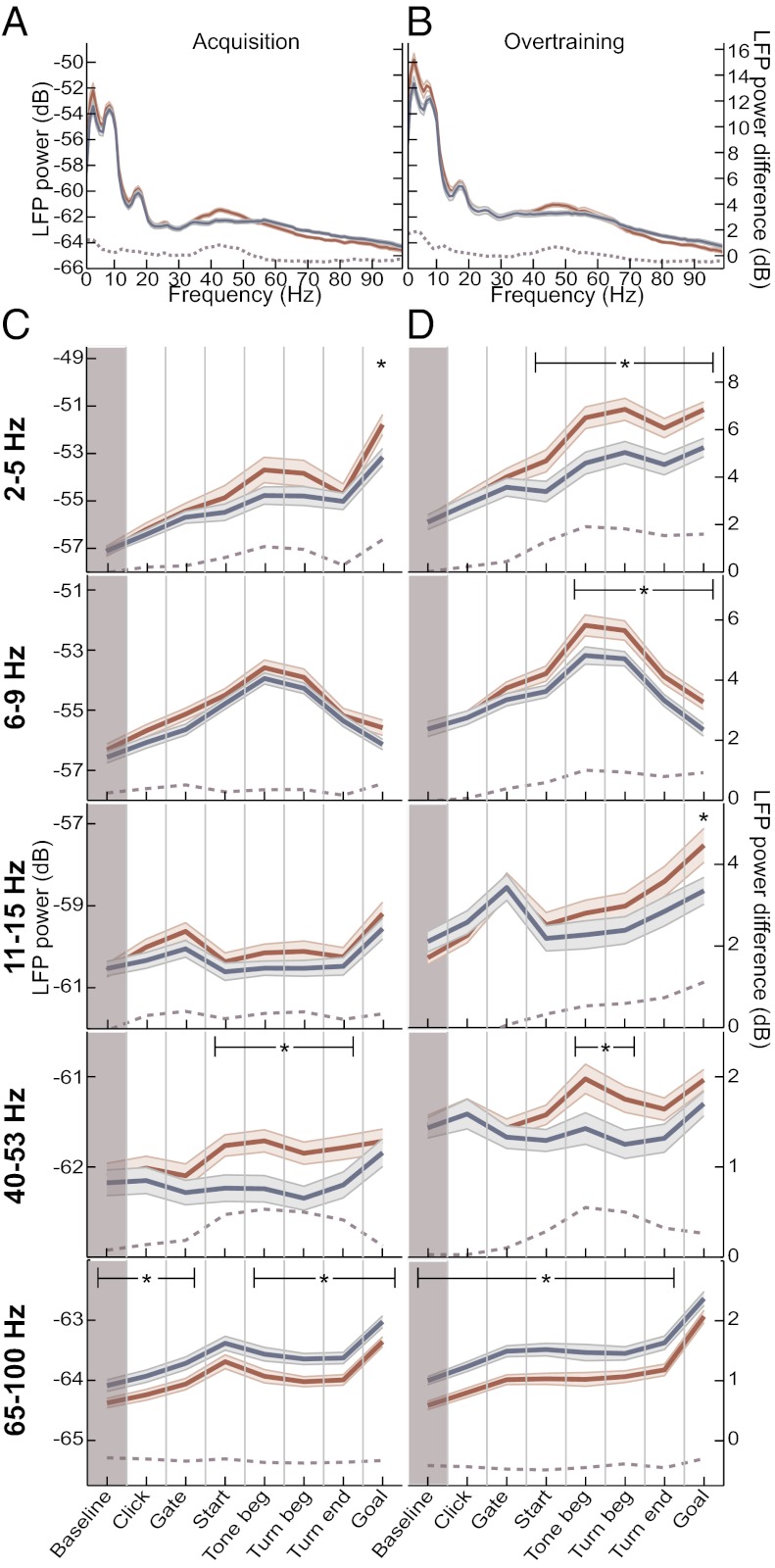
Fig. 2.
Effects of dopamine depletion on LFP oscillations are dependent on learning phase and task time. (A and B) Session average spectra (single taper) for LFPs recorded in the intact (blue) and dopamine-depleted (red) DLS during a 1-s window around turn beginning in sessions before (A, n = 65 sessions from 10 rats) and after (B, n = 90 sessions from the same 10 rats) acquisition criterion was reached. Shading indicates ±SEM. Dashed lines show power difference between depleted and intact sides (scale at Right). (C and D) Session average power (single taper) of oscillations in the delta (2–5 Hz), theta (6–9 Hz), low-beta (11–15 Hz), low-gamma (40–53 Hz), and high-gamma (65–100 Hz) bands recorded in the intact (blue) and dopamine-depleted (red) DLS during acquisition (C) and overtraining (D) sessions. Each plot shows power during 1-s windows centered on successive task events. Brown shading indicates the baseline period. Red and blue shading indicates SEM. Dashed lines show differences between the two sides (scale at Right). Only sessions with simultaneous recordings in both sides were used. *P < 0.05.
Effects of Dopamine Depletion on LFP Oscillations Emerge After Learning on the Associative T-Maze Task.
During training, power rose for the delta, theta, and beta bands on both the intact side and dopamine-depleted sides (P < 0.05 for all, ANOVA; Fig. S2), indicating that the strength of the oscillations increases with task experience. However, this process was significantly augmented in the absence of dopamine. During acquisition training, power in these bands was similar on the two sides (P > 0.1, ANOVA; Fig. 2 A and C). During the overtraining period, however, the power in each of these frequency bands was enhanced on the dopamine-depleted side, relative to the intact side (P < 0.05; Fig. 2 B and D). Exceptionally, low-gamma rhythms were elevated from early in training and remained so throughout training (P < 0.05 for each phase). High-gamma oscillations were reduced in all training stages and all trial periods (P < 0.02 for all).
l-DOPA Normalizes Power in All Oscillations with the Exception of the Low-Gamma Oscillation.
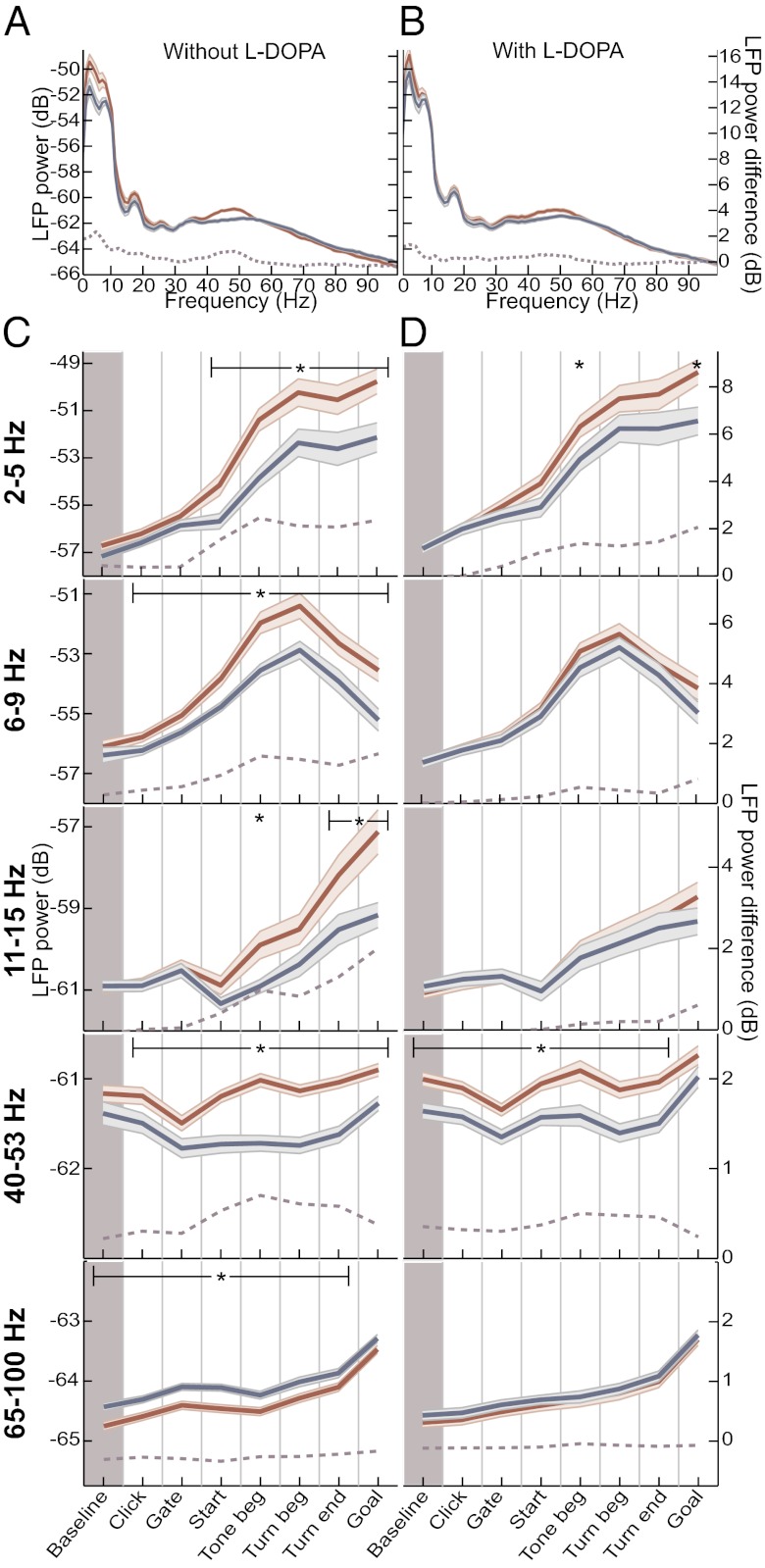
We compared the effects of systemic l-DOPA treatment on the LFP oscillations after extended overtraining. After l-DOPA administration, power in theta, beta, and high-gamma frequencies was no longer different from that in the simultaneously recorded intact hemisphere in any of the task periods (P > 0.1, ANOVA), and delta band power was nearly normalized (Fig. 3). However, low-gamma oscillations remained significantly elevated after l-DOPA administration (P < 0.005). This normalization of power in the low frequencies occurred due to a decrease in oscillation strength in the depleted dorsolateral striatum by l-DOPA (P < 0.05; Fig. S3) and not due to changes to LFP power in the intact dorsolateral striatum (P > 0.1). The power differences at high frequencies could have reflected a decrease in high-frequency power in the intact sensorimotor striatum, or a combination of effects in the intact and depleted dorsolateral striatum (Fig. S3). l-DOPA treatment produced no significant differences in low-gamma power in the dopamine-depleted dorsolateral striatum (P > 0.2), further indicating that this oscillation was not corrected by the treatment (Fig. S3).
Fig. 3.
l-DOPA treatment normalizes LFP oscillations in the dopamine-depleted DLS, with the exception of the low-gamma oscillation. (A and B) Session average spectra calculated and shown as in Fig. 2 A and B, for overtraining sessions before (A, n = 61 sessions from 10 rats) and during (B, n = 70 sessions from same 10 rats) daily l-DOPA treatment. (C and D) Session average power of oscillations in different frequency bands, as in Fig. 2 C and D, for sessions before (C) and during (D) l-DOPA treatment. *P < 0.05.
Spike–LFP Relationships Are Selectively Affected by Dopamine Depletion.
Single-unit spiking was recorded along with the LFP oscillations, and recorded units were putatively separated into neuronal subtypes according to previously used criteria (15, 17). The average firing rate of medium spiny projection neurons (MSNs) and fast-spiking interneurons (FSIs) was increased by the dopamine depletion† as previously seen (18, 19). To address the effect of dopamine depletion on the spike–LFP relationships in the dorsolateral striatum, we analyzed the phase coupling of spikes to LFP oscillations, measured during the maze runs in overtraining sessions—the time during which we found strongest effects of dopamine depletion on LFP power. We exclude the high-gamma oscillations in this analysis due to the likelihood that spike artifact (Fig. S4) is reflected in this high-frequency range; however, we did not observe any effect of dopamine depletion in the spike–LFP coupling in the high-gamma range.
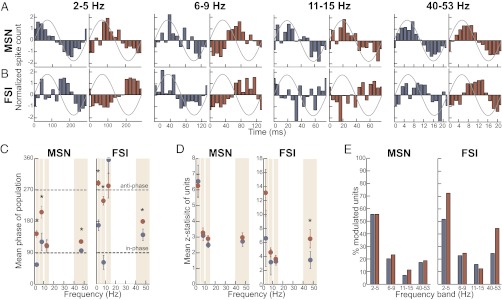
The population spiking of MSNs and FSIs in the control and depleted striatum displayed distinct phase preferences with respect to the ongoing LFP oscillations in the relevant frequency ranges (P < 0.02, Rayleigh’s test, for all except FSI–low beta; Fig. 4 A and B). Phase locking of MSNs and FSIs was strongest to delta oscillations (Fig. 4D). After normalizing for firing rate, spike–LFP coupling in individual units was subtly, but not significantly, stronger in the dopamine-depleted hemisphere (P > 0.05, Mann–Whitney test; Fig. 4D). Notably, however, FSIs displayed significantly stronger entrainment to low-gamma oscillations in the dopamine-depleted hemisphere relative to the intact hemisphere (P < 0.02, Mann–Whitney test; Fig. 4D). The percentage of significantly phase-modulated individual MSNs and FSIs (P < 0.05, Rayleigh's test) was further consistent with these findings, as the lesion produced a substantial increase in the proportion of FSIs entrained to the low-gamma rhythm from 33 to 57% (Fig. 4E).
Fig. 4.
Dopamine-depletion shifts phase preferences of MSN and FSI spiking and strengthens FSI spike coupling to low-gamma oscillations. (A and B) Normalized population spike phase histograms for MSNs (A) and FSIs (B) in the intact (blue, all spikes from 662 MSNs and 66 FSIs) and dopamine-depleted (red, all spikes from 712 MSNs and 82 FSIs) DLS. LFP activity was recorded during 3-s windows centered around cue onset in overtraining sessions and filtered for frequencies for each band. (C) Mean phase of population spiking of MSNs and FSIs in the intact (blue) and dopamine-depleted (red) DLS. Error bars indicate 75% confidence interval. *P < 0.05 (ANOVA for circular distributions) between intact and depleted sides. (D, Left) Average Z statistic (strength of phase locking) from Rayleigh's test of individual MSNs in the intact (blue) and dopamine-depleted (red) DLS. Only units with at least 100 spikes in analyzed windows were used and were normalized to 100 spikes each (n = 227 in intact DLS, n = 317 units in depleted DLS). (Right) FSI average Z statistics for FSI units similarly normalized to 1,000 spikes each (n = 57 units and 82 units, respectively). Error bars indicate SEM. *P < 0.05; Mann–Whitney test between the two sides. (E) Proportions of MSNs and FSIs shown in D that are significantly phase modulated (P < 0.01, Rayleigh's test) by oscillations in the given frequency bands.
During the baseline period, we found weaker, but still significant, spike–LFP coupling (Fig. S5), and small significant increases in MSN spike–LFP coupling due to dopamine depletion (Fig. S5D). Largely different sets of MSNs were active during the baseline period and the task time†, such that the MSN population included in the baseline analysis was different from that included for the in-task analysis. This difference could account for differences in baseline and in-task coupling effects. After l-DOPA treatment, similar trends in the MSNs and FSIs remained (Fig. S6). The low number of FSIs in this recording stage made it difficult to assess the effect of l-DOPA on spike–LFP coupling for FSIs, but we continued to see a trend of an increased proportion of FSIs modulated by the low-gamma oscillations in the dopamine-depleted hemisphere (Fig. S6E). Despite the lack of strong global effects of the local dopamine depletion on the strength of phase locking, dopamine depletion did result in a broadband forward shift in the preferred phase of firing of MSNs and FSIs in task (P < 0.01 for delta, theta, and low gamma in MSNs and FSIs, ANOVA for circular distributions; Fig. 4C) and at baseline (Fig. S5C), which persisted with l-DOPA treatment (Fig. S6C).
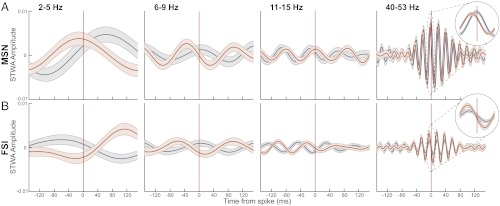
We constructed spike-triggered waveform averages (STWAs) with LFP traces filtered in the same frequency ranges and normalized for amplitude differences (SI Materials and Methods). The STWAs displayed prominent oscillations at frequencies in the range of the filtered LFPs, verifying the presence of significant spike–LFP relationships (Fig. 5). The delta band STWAs had the highest amplitude waveforms, indicating strong spike–LFP coupling in that range as seen in the spike-phase histograms. In most frequency bands, the preferred phase of the MSNs and FSIs tended to shift forward on the dopamine-depleted side (i.e., the spike occurred at a later phase of the LFP; Fig. 5), also consistent with the spike-phase histograms. Finally, the amplitude of the FSI STWA in the low-gamma band was significantly larger in the dopamine-depleted dorsolateral striatum than the intact dorsolateral striatum, indicating stronger phase locking of the FSIs to low-gamma oscillations (Fig. 5B).
Fig. 5.
Spike–LFP coupling is altered by dopamine depletion. Average LFP oscillations aligned to MSN (A) and FSI (B) spikes, calculated for activity in the intact (blue, n = 227 MSNs and 57 FSIs) and dopamine-depleted (red, n = 317 MSNs and 81 FSIs) DLS during a ±1.5-s window around cue onset in overtraining sessions. Spike-triggered waveform averages were calculated after randomly selecting 100 spikes for each MSN and 1,000 spikes for each FSI to normalize for firing rate differences. Shading indicates SEM.
Discussion
Our findings suggest that dopamine depletion in the sensorimotor striatum does not abolish normal oscillatory patterns nor create oscillations at new frequencies, but instead, amplifies intrinsically occurring oscillations in a selective manner. Enhancement of low-frequency (<55 Hz) oscillations occurred only in particular task-modulated frequency bands, only during periods in which those oscillations were actively task-modulated and only after the behavior had become highly trained. By contrast, high-frequency (>65 Hz) oscillations were statically and uniformly diminished with dopamine depletion. l-DOPA therapy normalized the oscillations in most frequency ranges but did not reduce the elevated low-gamma oscillations, suggesting that certain aspects of compromised circuit function may not be correctable by l-DOPA treatment. Further, the effects of dopamine depletion on spike–LFP coupling were also highly frequency-band and cell-type specific. With these findings, we begin building a link between the abnormal LFP oscillations found in Parkinson disease and the field of research characterizing the behavioral relevance of oscillatory activity and dopamine to behavior in normal organisms.
Dopamine Depletion Amplifies Low-Frequency LFP Oscillations only During the Performance of a Well-Learned Task.
Our findings support those of other electrophysiological studies, in human Parkinson patients and in rodent models, in which low-frequency oscillatory activity in basal ganglia circuits were increased by dopamine depletion (1, 2, 14, 20). However, previous observations were primarily made in freely behaving animals in at-rest conditions. By using a specific learned task to engage striatal circuits, we found that this effect is strongest when performing a well-trained task and that the increases are most evident during the task times in which specific oscillations are active. These findings accord with studies in which the effects of dopamine depletion on LFP oscillations in the substantia nigra pars reticulata and motor cortex were different during inattentive rest and treadmill walking, demonstrating state dependency of the effect of dopamine depletion (12, 13).
We extend previous observations by addressing the effect of dopamine depletion on LFP oscillations occurring during a complex learned task involving action initiation, decision making, and reward. We demonstrate that these effects are highly dependent on learning and task time. We suggest that these remarkably selective effects reflect previously described synaptic plasticity of dopamine-dependent responses during learning and the desynchronizing influence that dopamine appears to have on the basal ganglia networks (19, 21–24). The lack of this desynchronizing influence, by this view, could be most evident late in learning and during task performance times at which oscillations in the sensorimotor striatum are strongest. This task selective effect of dopamine loss may be related to the proposed role of dopamine in behavioral effort and incentive salience (25, 26) whereby the most pronounced effects of dopamine loss could be seen in situations where learned salient stimuli are present, motivation is high, and effort is required. The lack of significant effect of the depletion during the baseline period could thus reflect the level of engagement of these circuits, as well as the levels of dopamine depletion achieved by our local intrastriatal intervention, or to the fact that these were local, not global depletions.
l-DOPA Fails to Normalize Elevated Low-Gamma Oscillations, Which May Be Specifically Linked to FSI Firing in the Dopamine-Depleted Dorsolateral Striatum.
l-DOPA treatment almost completely restored normal LFP oscillatory power in all of the frequency bands analyzed, with the clear exception of the low-gamma (40–53 Hz) oscillation. Notably, FSI, but not MSN, spike coupling to low-gamma oscillations, as measured by the strength of spike–LFP coupling, percentage of modulated units, and amplitude of STWAs, was enhanced by dopamine depletion. Moreover, the low-gamma oscillation was the only prominent rhythm that was elevated in early learning stages, indicating that network alterations affecting the oscillations likely occurred during the 5 wk from the 6-OHDA injection to the initiation of recording. Dopamine depletion has been found to increase dendritic arborization of FSIs, a structural change which may not be readily reversed by acute l-DOPA treatment (24). We suggest that remodeling in FSI circuits may produce chronic, treatment-resistant alterations in striatal network function, which may result in elevated synchrony in the low-gamma range.
Dopamine Depletion Alters the Phase Relationships Between Spiking and LFPs.
With the notable exception of the low-gamma range, the degree of spike–LFP coupling for MSNs and FSIs was not strongly affected by the dopamine depletion after we controlled for the effects of increased firing rate in our measures. Surprisingly, however, we found a broadband shift in the preferred phase of spiking of the MSNs and FSIs on the dopamine-depleted side. We favor the possibility that this is a small forward shift (<90°) in the preferred phase (the spikes occur slightly later with respect to the LFP) rather than the alternative of a large backward shift (>270°). The causal relationship between spiking and LFP oscillations is not yet clear (27), so we cannot ascertain whether this change may represent a shift in spike timing with respect to equivalent LFP signals or a shift in the LFPs themselves. l-DOPA treatment did not reverse this phase shift, despite its reversal of the LFP power differences in the same frequency bands. These findings suggest, along with previous work (19, 28) that the effects of dopamine depletion and l-DOPA on spike–LFP coupling are not a direct reflection of their effects on LFP power.
Dopamine Depletion in Dorsolateral Striatum Affects a Broad Range of Behaviorally Relevant LFP Oscillations in a Dynamic Task- and Learning-Dependent Manner.
We explored the effect of dopamine depletion during the acquisition of a conditional T-maze task with the goal of assessing the full range of changes in striatal LFP oscillations brought about by the loss of dopamine. The effects we found were widespread, and all task-related LFP oscillations were affected in a dynamic manner. We conclude that the loss of dopamine has widespread effects on LFP oscillations beyond the prominent increase in beta-range oscillations and decrease in high-gamma power as extensively studied in the subthalamic nucleus, globus pallidus, and substantia nigra in animal models of Parkinson disease (2, 3, 12). The unifying pattern we observe is an exaggeration of the response profile of task-related LFP oscillations that exist in the normal system. These changes could reflect temporally restricted increases in network synchrony or increased responsiveness to task-modulated inputs. The striatum, as a primary source of input to downstream regions in basal ganglia including subthalamic nucleus, globus pallidus, and substantia nigra, is well placed to induce and propagate such oscillations, and has been proposed as a potential source of the abnormal oscillations in Parkinson disease (29, 30). Finally, we find that l-DOPA therapy normalizes these oscillations with the exception of the gamma oscillation, which could reflect long-term structural changes that occur in the fast-spiking interneurons (24). The failure of l-DOPA to normalize the low-gamma oscillations makes this oscillation an important potential target for Parkinson disease treatments and early interventions to prevent potentially irreversible structural changes.
Materials and Methods
Procedures were approved by the Massachusetts Institute of Technology Committee on Animal Care and accorded with the National Research Council's Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley rats (n = 16) received injections of 6-OHDA in the dorsolateral striatum unilaterally. Four weeks later, a recording drive was implanted, targeting the dorsolateral striatum bilaterally, each side with six tetrodes. Rats were then trained on a T-maze task (∼40 trials per session), in which the rats were instructed to turn left or right in response to a tone to receive a chocolate reward at the end of the indicated end arm. Rats were trained to a criterion of 72.5% correct trials and then overtrained for 10 consecutive days. They were then tested in 10 additional sessions with daily systemic l-DOPA administration. Spike and LFP activity was recorded throughout training. LFP spectral analysis was conducted using Chronux algorithms, the MATLAB CircStat Toolbox, and in-house MATLAB software. Detailed methods are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Christine Keller-McGandy and Henry F. Hall for their help. This work was supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke P50 NS-38372, the National Parkinson Foundation, a Parkinson's Disease Foundation fellowship, a National Institute of Mental Health graduate student fellowship, and the Stanley H. and Sheila G. Sydney Fund.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1216403109/-/DCSupplemental.
†Hernandez LF, et al. Dopamine depletion and L-DOPA therapy affect learning-related firing dynamics of striatal neurons, under review.
References
- 1.Eusebio A, Brown P. Oscillatory activity in the basal ganglia. Parkinsonism Relat Disord. 2007;13(Suppl 3):S434–S436. doi: 10.1016/S1353-8020(08)70044-0. [DOI] [PubMed] [Google Scholar]
- 2.Brown P. Oscillatory nature of human basal ganglia activity: Relationship to the pathophysiology of Parkinson’s disease. Mov Disord. 2003;18(4):357–363. doi: 10.1002/mds.10358. [DOI] [PubMed] [Google Scholar]
- 3.Jenkinson N, Brown P. New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci. 2011;34(12):611–618. doi: 10.1016/j.tins.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Jenkinson N, Kühn AA, Brown P. Gamma oscillations in the human basal ganglia. Exp Neurol. July 31, 2012 doi: 10.1016/j.expneurol.2012.07.005. 10.1016/j.expneurol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Eusebio A, Cagnan H, Brown P. Does suppression of oscillatory synchronisation mediate some of the therapeutic effects of DBS in patients with Parkinson’s disease? Front Integr Neurosci. July 10, 2012 doi: 10.3389/fnint.2012.00047. 10.3389/fnint.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ray NJ, et al. Local field potential beta activity in the subthalamic nucleus of patients with Parkinson’s disease is associated with improvements in bradykinesia after dopamine and deep brain stimulation. Exp Neurol. 2008;213(1):108–113. doi: 10.1016/j.expneurol.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Wang XJ. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev. 2010;90(3):1195–1268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 9.Buzsáki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegel M, Donner TH, Engel AK. Spectral fingerprints of large-scale neuronal interactions. Nat Rev Neurosci. 2012;13(2):121–134. doi: 10.1038/nrn3137. [DOI] [PubMed] [Google Scholar]
- 11.Engel AK, Fries P. Beta-band oscillations—signalling the status quo? Curr Opin Neurobiol. 2010;20(2):156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Avila I, et al. Beta frequency synchronization in basal ganglia output during rest and walk in a hemiparkinsonian rat. Exp Neurol. 2010;221(2):307–319. doi: 10.1016/j.expneurol.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brazhnik E, et al. State-dependent spike and local field synchronization between motor cortex and substantia nigra in hemiparkinsonian rats. J Neurosci. 2012;32(23):7869–7880. doi: 10.1523/JNEUROSCI.0943-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuentes R, Petersson P, Siesser WB, Caron MG, Nicolelis MA. Spinal cord stimulation restores locomotion in animal models of Parkinson’s disease. Science. 2009;323(5921):1578–1582. doi: 10.1126/science.1164901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437(7062):1158–1161. doi: 10.1038/nature04053. [DOI] [PubMed] [Google Scholar]
- 16.DeCoteau WE, et al. Oscillations of local field potentials in the rat dorsal striatum during spontaneous and instructed behaviors. J Neurophysiol. 2007;97(5):3800–3805. doi: 10.1152/jn.00108.2007. [DOI] [PubMed] [Google Scholar]
- 17.Howe MW, Atallah HE, McCool A, Gibson DJ, Graybiel AM. Habit learning is associated with major shifts in frequencies of oscillatory activity and synchronized spike firing in striatum. Proc Natl Acad Sci USA. 2011;108(40):16801–16806. doi: 10.1073/pnas.1113158108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen MT, Morales M, Woodward DJ, Hoffer BJ, Janak PH. In vivo extracellular recording of striatal neurons in the awake rat following unilateral 6-hydroxydopamine lesions. Exp Neurol. 2001;171(1):72–83. doi: 10.1006/exnr.2001.7730. [DOI] [PubMed] [Google Scholar]
- 19.Burkhardt JM, Jin X, Costa RM. Dissociable effects of dopamine on neuronal firing rate and synchrony in the dorsal striatum. Front Integr Neurosci. 2009;3:28. doi: 10.3389/neuro.07.028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dejean C, et al. Evolution of the dynamic properties of the cortex-basal ganglia network after dopaminergic depletion in rats. Neurobiol Dis. 2012;46(2):402–413. doi: 10.1016/j.nbd.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30(5):211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Costa RM. Plastic corticostriatal circuits for action learning: What’s dopamine got to do with it? Ann N Y Acad Sci. 2007;1104:172–191. doi: 10.1196/annals.1390.015. [DOI] [PubMed] [Google Scholar]
- 23.Cruz AV, Mallet N, Magill PJ, Brown P, Averbeck BB. Effects of dopamine depletion on information flow between the subthalamic nucleus and external globus pallidus. J Neurophysiol. 2011;106(4):2012–2023. doi: 10.1152/jn.00094.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gittis AH, et al. Rapid target-specific remodeling of fast-spiking inhibitory circuits after loss of dopamine. Neuron. 2011;71(5):858–868. doi: 10.1016/j.neuron.2011.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berridge KC. The debate over dopamine’s role in reward: The case for incentive salience. Psychopharmacology (Berl) 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 26.Salamone JD, Correa M, Nunes EJ, Randall PA, Pardo M. The behavioral pharmacology of effort-related choice behavior: Dopamine, adenosine and beyond. J Exp Anal Behav. 2012;97(1):125–146. doi: 10.1901/jeab.2012.97-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 2012;13(6):407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parr-Brownlie LC, et al. Dopamine lesion-induced changes in subthalamic nucleus activity are not associated with alterations in firing rate or pattern in layer V neurons of the anterior cingulate cortex in anesthetized rats. Eur J Neurosci. 2007;26(7):1925–1939. doi: 10.1111/j.1460-9568.2007.05814.x. [DOI] [PubMed] [Google Scholar]
- 29.McCarthy MM, et al. Striatal origin of the pathologic beta oscillations in Parkinson’s disease. Proc Natl Acad Sci USA. 2011;108(28):11620–11625. doi: 10.1073/pnas.1107748108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar A, Cardanobile S, Rotter S, Aertsen A. The role of inhibition in generating and controlling Parkinson’s disease oscillations in the Basal Ganglia. Front Syst Neurosci. 2011;5:86. doi: 10.3389/fnsys.2011.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.