Abstract
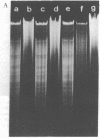
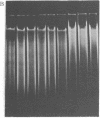
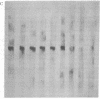
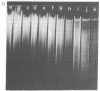
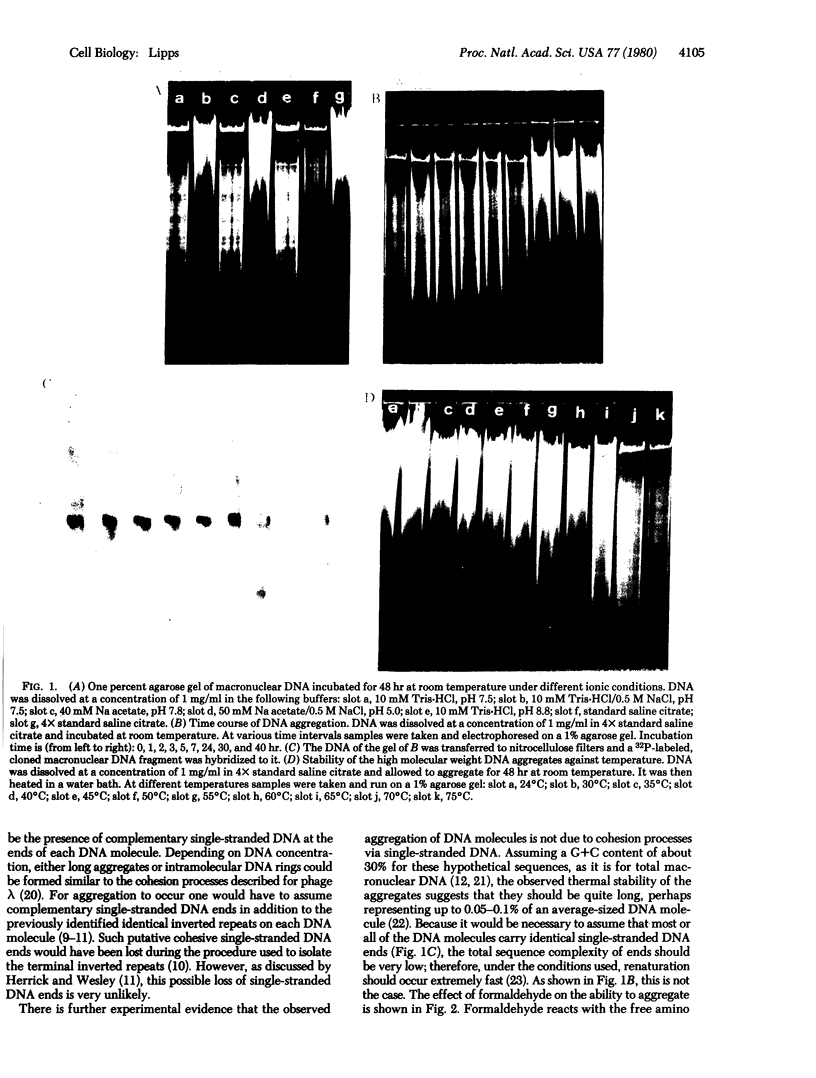
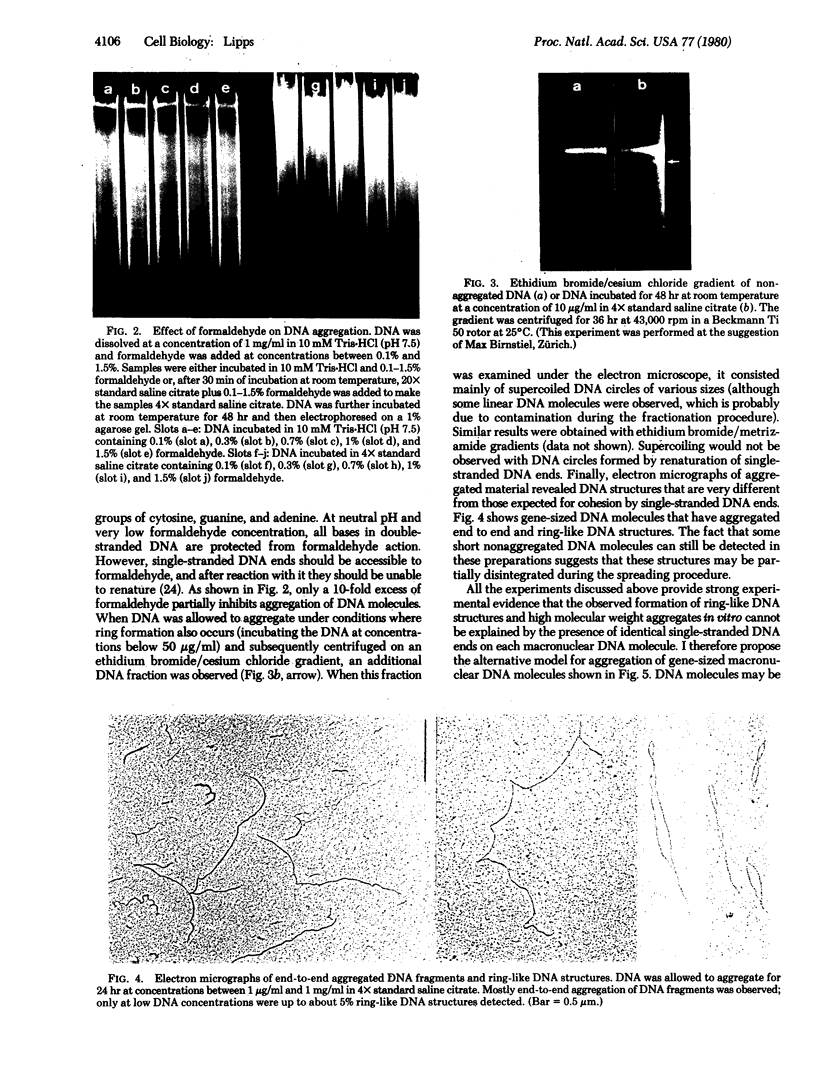
Macronuclear DNA of hypotrichous ciliates exists in the form of gene-sized DNA molecules. It can be resolved on agarose gels into a continuum of sizes upon which is imposed a set of characteristic DNA bands. Most or all of the DNA molecules carry identical terminal inverted repeat sequences. By incubating macronuclear DNA under increasingly stronger ionic conditions, high molecular weight DNA aggregates and ring-like DNA structures are formed. Experimental evidence is presented that this aggregation is not due to the presence of identical single-stranded DNA ends on each macronuclear DNA fragment, and an alternative model for DNA aggregation is discussed.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammermann D. Morphology and development of the macronuclei of the ciliates Stylonychia mytilus and Euplotes aediculatus. Chromosoma. 1971;33(2):209–238. doi: 10.1007/BF00285634. [DOI] [PubMed] [Google Scholar]
- Ammermann D., Steinbrück G., von Berger L., Hennig W. The development of the macronucleus in the ciliated protozoan Stylonychia mytilus. Chromosoma. 1974 May 10;45(4):401–429. doi: 10.1007/BF00283386. [DOI] [PubMed] [Google Scholar]
- Bauer W., Vinograd J. The interaction of closed circular DNA with intercalative dyes. I. The superhelix density of SV40 DNA in the presence and absence of dye. J Mol Biol. 1968 Apr 14;33(1):141–171. doi: 10.1016/0022-2836(68)90286-6. [DOI] [PubMed] [Google Scholar]
- Birnie G. D., Rickwood D., Hell A. Buoyant densities and hydration of nucleic acids, proteins and nucleoprotein complexes in metrizamide. Biochim Biophys Acta. 1973 Dec 7;331(2):283–294. doi: 10.1016/0005-2787(73)90441-3. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Elsevier S. M., Lipps H. J., Steinbrück G. Histone genes in macronuclear DNA of the ciliate Stylonychia mytilus. Chromosoma. 1978 Dec 6;69(3):291–306. doi: 10.1007/BF00332133. [DOI] [PubMed] [Google Scholar]
- Herrick G., Wesley R. D. Isolation and characterization of a highly repetitious inverted terminal repeat sequence from Oxytricha macronuclear DNA. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2626–2630. doi: 10.1073/pnas.75.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey A. D., Burgi E., Ingraham L. COHESION OF DNA MOLECULES ISOLATED FROM PHAGE LAMBDA. Proc Natl Acad Sci U S A. 1963 May;49(5):748–755. doi: 10.1073/pnas.49.5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. The rabbit beta-globin gene contains a large large insert in the coding sequence. Cell. 1977 Dec;12(4):1097–1108. doi: 10.1016/0092-8674(77)90172-6. [DOI] [PubMed] [Google Scholar]
- Lawn R. M. Gene-sized DNA molecules of the Oxytricha macronucleus have the same terminal sequence. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4325–4328. doi: 10.1073/pnas.74.10.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn R. M., Heumann J. M., Herrick G., Prescott D. M. The gene-size DNA molecules in Oxytricha. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):483–492. doi: 10.1101/sqb.1978.042.01.051. [DOI] [PubMed] [Google Scholar]
- Lipps H. J., Nock A., Riewe M., Steinbrück G. Chromatin structure in the macronucleus of the ciliate Stylonychia mytilus. Nucleic Acids Res. 1978 Dec;5(12):4699–4709. doi: 10.1093/nar/5.12.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipps H. J., Sapra G. R., Ammermann D. The histones of the ciliated protozoan Stylonychia mytilus. Chromosoma. 1974 Apr 9;45(3):273–280. doi: 10.1007/BF00283411. [DOI] [PubMed] [Google Scholar]
- Lipps H. J., Steinbrück G. Free genes for rRNAs in the macronuclear genome of the ciliate Stylonychia mytilus. Chromosoma. 1978 Oct 20;69(1):21–26. doi: 10.1007/BF00327378. [DOI] [PubMed] [Google Scholar]
- McGavin S. Models of specifically paired like (homologous) nucleic acid structures. J Mol Biol. 1971 Jan 28;55(2):293–298. doi: 10.1016/0022-2836(71)90201-4. [DOI] [PubMed] [Google Scholar]
- Meyer G. F., Lipps H. J. Chromatin elimination in the hypotrichous ciliate Stylonychia mytilus. Chromosoma. 1980;77(3):285–297. doi: 10.1007/BF00286054. [DOI] [PubMed] [Google Scholar]
- Prescott D. M., Murti K. G., Bostock C. J. Genetic apparatus of Stylonychia sp. Nature. 1973 Apr 27;242(5400):576, 597-600. doi: 10.1038/242576a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Swanton M. T., Heumann J. M., Prescott D. M. Gene-sized DNA molecules of the macronuclei in three species of hypotrichs: size distributions and absence of nicks. DNA of ciliated protozoa. VIII. Chromosoma. 1980;77(2):217–227. doi: 10.1007/BF00329546. [DOI] [PubMed] [Google Scholar]
- Wesley R. D. Inverted repetitious sequences in the macronuclear DNA of hypotrichous ciliates. Proc Natl Acad Sci U S A. 1975 Feb;72(2):678–682. doi: 10.1073/pnas.72.2.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Wilson J. H. Nick-free formation of reciprocal heteroduplexes: a simple solution to the topological problem. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3641–3645. doi: 10.1073/pnas.76.8.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]