Abstract
Naïve T cells continually recirculate between blood and secondary lymphoid organs, scanning dendritic cells (DC) for foreign antigen. Despite its importance for understanding how adaptive immune responses are efficiently initiated from rare precursors, a detailed quantitative analysis of this fundamental process has not been reported. Here we measure lymph node (LN) entry, transit, and exit rates for naïve CD4+ and CD8+ T cells, then use intravital imaging and mathematical modeling to relate cell–cell interaction dynamics to population behavior. Our studies reveal marked differences between CD4+ vs. CD8+ T cells. CD4+ T cells recirculate more rapidly, homing to LNs more efficiently, traversing LNs twice as quickly, and spending ∼1/3 of their transit time interacting with MHCII on DC. In contrast, adoptively transferred CD8+ T cells enter and leave the LN more slowly, with a transit time unaffected by the absence of MHCI molecules on host cells. Together, these data reveal an unexpectedly asymmetric role for MHC interactions in controlling CD4+ vs. CD8+ T lymphocyte recirculation, as well as distinct contributions of T cell receptor (TCR)-independent factors to the LN transit time, exposing the divergent surveillance strategies used by the two lymphocyte populations in scanning for foreign antigen.
Keywords: trafficking, migration, immune recognition
Peripheral naïve CD4+ and CD8+ T cells lead a nomadic existence, circulating between blood, secondary lymphoid organs (SLOs), and lymph in search of foreign antigen and survival signals (1, 2). Factors affecting T-cell entry into lymph nodes (LN) across vascular endothelium are well characterized. l-Selectin (CD62L) enables the initial rolling of T cells along high endothelial venules (HEV), whereas chemokine (C-C motif) receptor 7 (CCR7) signaling upon binding of its ligands CCL19 and CCL21 activates the integrins αLβ2 (LFA-1) and α4β1 (VLA-4), facilitating their firm adhesion and transendothelial migration (1). T cells are extremely dynamic after entering SLOs, moving along the fibroblastic reticular cell network at average speeds of ∼11 μm/min (3, 4). In contrast, the temporal and spatial dynamics of distinct T-cell populations as they traverse SLOs after such endothelial transmigration, the influence of this migratory behavior on efficient scanning of the body for invasion by infectious agents, and the factors that influence motility, residence time, and egress rates from SLOs of these highly motile T cells are largely unexplored or just beginning to be understood (5).
A key step in developing a dynamic understanding of lymphocyte percolation through LNs is to measure how long different T-cell subsets spend in an SLO before egressing into lymph. To date, only expression of CCR7 and Sphingosine-1-phosphate receptor 1 (S1PR1) has been shown to affect the time spent by T cells within LNs. CCR7 promotes retention, whereas S1PR1 expression is essential to overcome this retention signal and promote egress into the efferent lymph (6). In addition to these chemotactic cues (7–9), the LN transit of T cells might be impacted by the numerous cell–cell contacts they make (4, 10), but this issue has not been experimentally investigated. Furthermore, it has been assumed without direct evidence that the factors determining the SLO transit time, scanning behavior, and hence recirculation dynamics are the same for CD4+ and CD8+ T cells.
Engagement of the antigen receptors (TCR) on naïve T cells may also play a role in recirculation dynamics. T cells recognize foreign antigen via interaction with peptides bound by cell surface MHC-encoded molecules (pMHC). The encounter of a naïve T cell with its cognate foreign pMHC on presenting cells in an SLO leads to migration arrest and activation (11). Naïve T cells also make functional, weaker affinity interactions with self-peptides presented by MHC (self-pMHC). In the absence of MHCI, naïve CD8+ T-cell populations decay with a half-life of 10–19 d, implying that interactions with MHCI play a role in their maintenance (12, 13). Similarly, loss of MHCII leads to reduced survival of naïve CD4+ T cells (13–15), although the impact of the absence of MHCII on naïve CD4+ T cells is less acute than that of MHCI loss on CD8+ T cells (16). Nonetheless, intraclonal competition among naïve CD4+ T cells suggests that specific self-pMHC cues may be a limiting resource (17). Finally, for both naïve CD4+ and CD8+ T cells, contact with self-pMHC results in partial phosphorylation of the TCR ζ-chain, maintaining T cells in a state of greater sensitivity for responses to foreign antigen (16, 18). However, it remains unknown how long contacts involved in self-pMHC recognition last, where they take place, or whether they influence the bulk trans-LN migration of T cells, and hence the overall dynamics of the search for foreign antigen.
To develop a better understanding of the factors that regulate naïve T-cell dynamics in SLO, we have now carefully quantified the time that naïve CD4+ and CD8+ T cells spend traversing LNs. We also asked whether, in the absence of antigen, TCR–self-pMHC contacts measurably impact T–dendritic cell (DC) interaction time as assessed by intravital 2-photon (2P) microscopy and whether the cumulative impact of self-recognition contributes to SLO residence time. Our data reveal that the two naïve T-cell subsets transit LN at strikingly different rates based on factors distinct from the classically considered CCR7 or S1PR1 control mechanisms influencing exit likelihood, and further, that there is a substantial asymmetry in the effect of self-recognition on CD4+ vs. CD8+ T-cell dynamics in LN. In combination, these observations highlight important and previously unrecognized differences between CD4+ and CD8+ T cells in their repertoire scanning strategies.
Results
Impact of the Absence of MHCII on T–DC Interaction Duration.
Only thymocytes weakly interacting with self-pMHC are positively selected and released into the periphery. However, the reduced naïve T-cell survival in the absence of self-pMHC (12–14) and a subthreshold level of partial TCR ζ-chain phosphorylation rapidly lost when T cells are deprived of MHC (16, 18) indicate that interactions of naïve T cells with self-pMHC do not end in the thymus. We have used the maintenance of CD5 expression, which is proportional to the strength of TCR signaling (19), to test the expectation that functional T-cell interactions with self-pMHC take place primarily in SLOs. Upon transfer of congenic T cells into WT, MHCII−/−, or MHCI−/− mice there is a significant decrease in CD5 expression on CD4+ T cells in MHCII−/− and on CD8+ T cells in MHCI−/− mice 20 h later (Fig. S1A). Thus, CD5 expression levels are actively maintained by interactions with MHC. When splenectomized mice were treated with neutralizing antibodies to αL and α4 integrins, blocking T-cell entry into LNs, and with FTY720, an inhibitor of lymphocyte LN egress, to trap T cells either in blood or LN, there was a significant reduction in CD5 surface levels in the blood but not the LN (Fig. S1B). These data suggest that functional contacts of naïve T cells with self-MHC take place primarily in SLOs, raising the possibility that such interactions might affect the dynamics of T-cell migration in these organs.
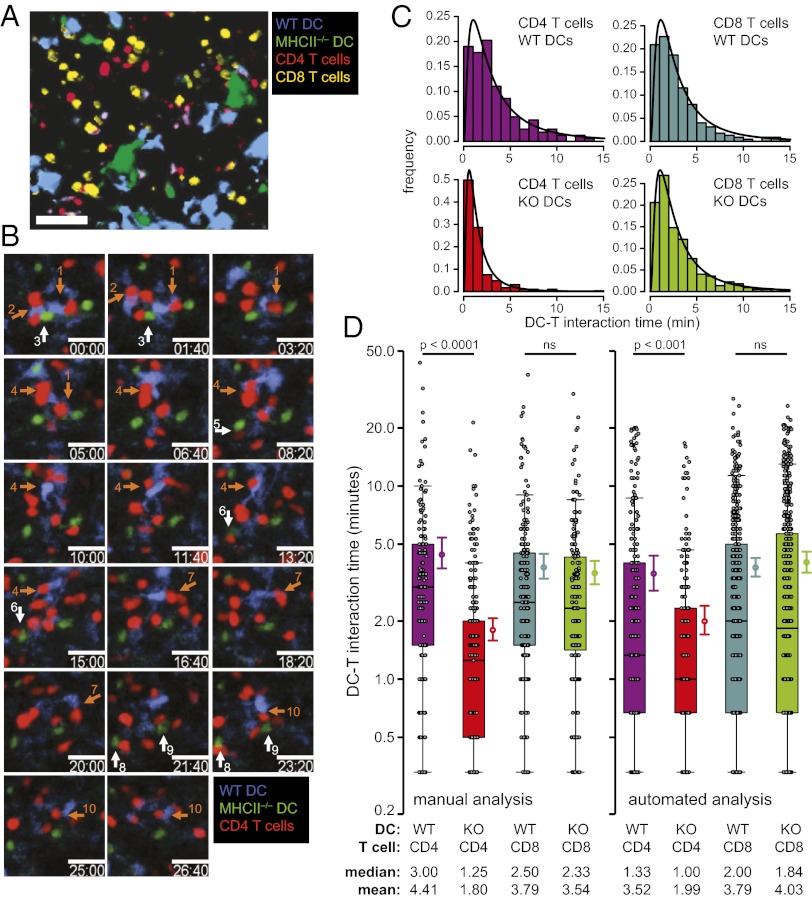
To examine directly whether this recognition influences the duration of T-cell interactions with DC in vivo, we used 2P intravital microscopy. We focused on CD4+ T cells, because this enabled us to examine a specific cell population, DCs, which are likely key in providing MHCII signals in SLOs (20). Bone marrow-derived DCs obtained from WT and MHCII−/− mice were transferred with naïve CD4+ and CD8+ T cells into MHCII−/− recipients and T–DC interaction durations quantified (Fig. 1 A and B and Movie S1). This setup enabled comparison of the contact duration of both CD4+ and CD8+ T cells with the two DC populations within the same mouse. CD8+ T cells served as internal controls, because we did not expect them to interact differentially with DCs differing only in MHCII expression. In the absence of foreign pMHC, T–DC interactions were best approximated by a log normal distribution, with the majority of T–DC contacts being very short (<5 min) and only few contacts lasting longer than 10 min (Fig. 1C), in accord with published data (21). There was a significant reduction in the mean and median interaction duration of CD4+ T cells with DCs that lacked MHCII compared with WT DCs (Fig. 1D) but no such difference in CD8+ T-cell interaction times. To avoid manual scoring bias, we used newly developed automated image analysis software to analyze the same data set, with similar results (Fig. 1D).
Fig. 1.
Reduced steady-state contact times of naïve CD4+ T cells with MHCII−/− DCs. (A) Representative xyzt intravital 2P microscopy image showing four dye-labeled transferred cell populations in popliteal LN: WT or MHCII−/− DCs, CD4+, and CD8+ T cells. (Scale bar, 50 μm.) (B) Time-lapse images from a representative 2P microscopy z-stack of CD4+ T cells interacting with WT and MHCII−/− DCs in vivo (Movie S1). Individual DC–T-cell contacts are denoted by numbered arrows (contacts with WT DCs, orange arrows; MHCII−/− DCs, white arrows). (Scale bars, 20 μm.) Time shown in minutes and seconds. (C) Histograms of contact durations of CD4+ and CD8+ T cells with WT and MHCII−/− DCs estimated by manual analyses. Black lines show log-normal curve fits. (D) Contact durations of CD4+ and CD8+ T cells with WT and MHCII−/− DCs estimated by manual and automated analyses of four independent experiments. Black circles denote individual contact durations shown with superimposed 25%, 50% (median), and 75% quartiles for each group. Colored circles to right of whisker plots represent mean contact durations with bootstrapped 95% confidence interval.
Distinct CD4+ and CD8+ T-Cell LN Transit Times.
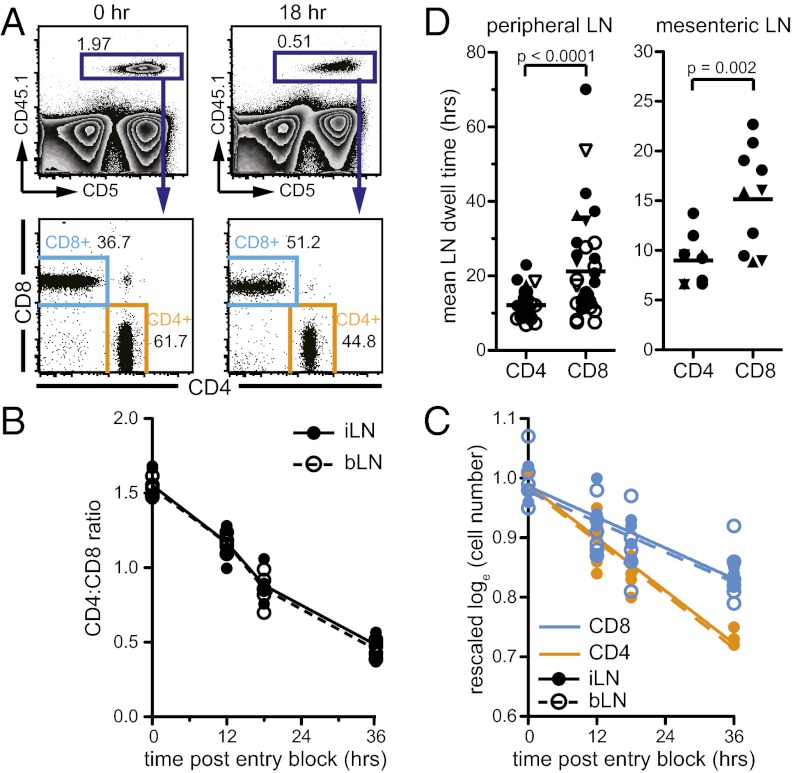
To put this finding in the context of T-cell trafficking dynamics and quantify the cumulative time of CD4+ T-cell LN residency accounted for by interactions with MHCII, we first measured LN transit times of naïve CD4+ and CD8+ T cells in WT mice. We transferred marked T cells into mice, blocked further LN entry, and counted transferred cells in multiple LNs at different times after entry blockade. The CD4:CD8 T-cell ratio rapidly inverted when LN entry was inhibited, suggesting that CD4+ T cells egressed LNs faster than CD8+ T cells (Fig. 2 A and B). The egress kinetics of both CD4+ and CD8+ T cells in sampled peripheral LNs (pLN) and mesenteric LN (mLN) were well described by an exponential decay process (Fig. 2C). This corresponds to a model in which T cells leave with a constant probability per unit time, independent of the total number of T cells in the LN. By fitting this exponential decay model to the T-cell number decay curves, we estimated egress rates (r) of transferred cells and mean LN dwell times, E(t) (Fig. 2D and Fig. S2A). Whereas CD4+ T cells resided in pLN for a mean of 12.2 h, CD8+ T cells remained in pLNs for 21.2 h (Fig. 2D). Transit through mLNs was slightly faster, with E(t) = 9.6 h for CD4+ T cells and E(t) = 17.0 h for CD8+ T cells. Carboxyfluorescein succinimidyl ester (CFSE)-labeled T cells gave similar estimates, and we saw no division of T cells during the assay period. We also estimated egress rates of endogenous naïve T-cell populations, finding mean transit rates in pLNs of 12.1 h and 18.8 h for CD4+ and CD8+ T cells, respectively, similar to the results obtained using cell transfers (Fig. S2B).
Fig. 2.
CD4+ T cells have shorter LN transit times than CD8+ T cells. (A) Representative flow cytometry plots of transferred congenic CD4+ and CD8+ T cells 0 and 18 h after blocking LN entry in WT recipients. Numbers on plots denote percentage of cells in gate. (B) CD4:CD8 ratio of transferred cells in inguinal and brachial LNs (i/bLN) at various times after LN entry blockade. (C) Exponential decay model fit (lines) to transferred CD4+ and CD8+ T-cell numbers (circles) after LN entry blockade. Data from B and C are pooled from two independent representative experiments, each with three mice per time point. (D) Mean dwell time estimates for transferred naïve CD4+ and CD8+ T cells. Points denote egress estimates from one experiment (as shown in C). Lines represent means. Closed symbols, iLN; open symbols, bLN; ●, B6 mice; ▲, B10.A mice; ▼, B10 mice; egress estimates are from 8 to 19 independent experiments.
Despite the longer transit time of naïve CD8+ T cells, we did not detect any gross differences in the anatomic localization of CD4+ and CD8+ T cells within the LN (Fig. S3A); nor did we find significant differences in the intranodal migration behavior of CD4+ and CD8+ T cells that would explain the distinct residence times (Fig. S3 B and C and Movie S2). Given existing data on factors controlling T-cell exit from LN, we asked whether CD4+ T cells are more sensitive to sphingosine-1-phosphate (S1P), perhaps enabling them to more efficiently egress into lymph. However, CD4+ T cells were not more responsive to S1P in vitro than CD8+ T cells (Fig. S3D). Similarly, the fraction of CD69+ cells, which lack surface S1PR1 expression (22), was greater among CD4+ than CD8+ T cells and thus does not explain the faster egress rates of the CD4+ cohort. These studies reveal that CD4+ T cells spend a significantly shorter time in an LN after entry compared with CD8+ T cells, that this is true for endogenous as well as transferred cells, and that differential functional expression of a major regulator of LN egress, S1PR1, did not seem to explain this difference.
Distinct LN Entry Rates of CD4+ and CD8+ T Cells.
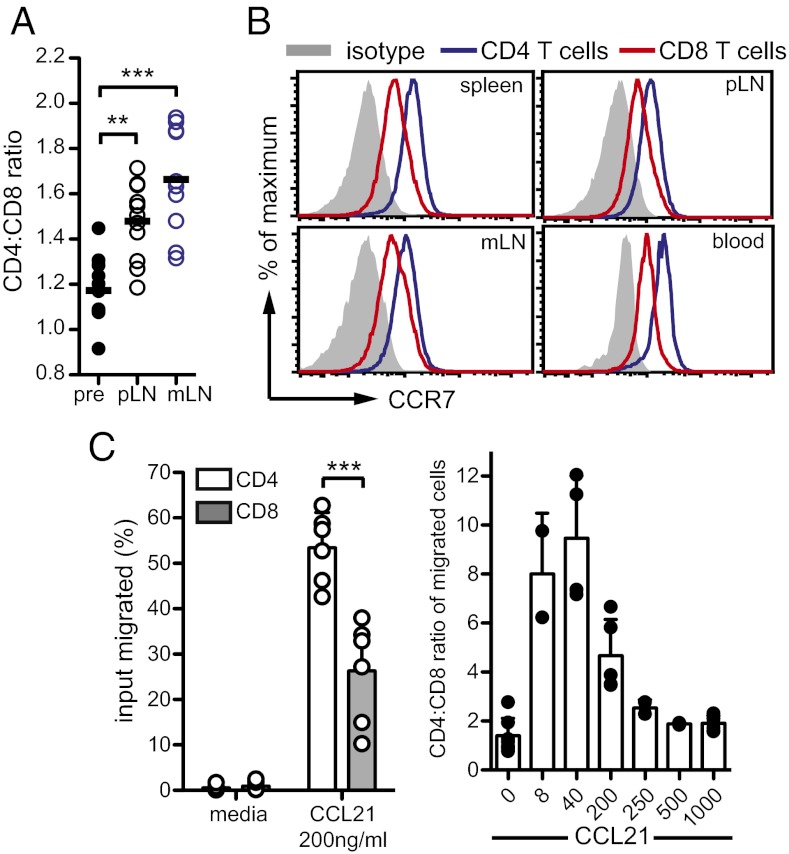
To maintain equilibrium, the greater LN egress rates of CD4+ T cells must be matched by increased LN entry rates. To investigate this prediction, we determined whether, without in vivo manipulations, the LN entry rate of naïve CD4+ T cells was faster than that of CD8+ T cells. T cells with a known CD4:CD8 ratio were transferred into recipient mice and the CD4:CD8 ratio of transferred cells measured 2 h later (Fig. 3A). The CD4:CD8 ratio of entered cells 2 h after transfer was significantly increased compared with the preinjection ratio. Thus, CD4+ T cells were indeed more efficient at entering LNs than CD8+ T cells, as expected from the egress rate differences.
Fig. 3.
CD4+ T cells have greater LN entry rates than CD8+ T cells. (A) To assess homing of CD4+ and CD8+ T cells, marked cells were injected into WT mice and LNs harvested 2 h later. The CD4:CD8 ratio among transferred T cells before (pre) was compared with the ratio after injection in LNs. Each open circle is a mean of 3 mice. Data are from 11 independent experiments. (B) CCR7 expression on naïve CD4+ and CD8+ T cells. Data representative of three independent experiments. (C) Responsiveness of CD4+ and CD8+ T cells to indicated concentrations of CCL21 (ng/mL) in transwell migration assays. (Left) Mean percentage migration of spleen T cells summarized from six mice, two independent experiments. (Right) CD4:CD8 ratio among migrated cells. Each circle represents mean from an individual mouse; data were pooled from two to five mice, two independent experiments. **P < 0.005; ***P < 0.0001.
To investigate the mechanism underlying the entry efficiency of naïve CD4+ T cells, we analyzed surface molecules that impact T-cell SLO homing (1, 23). The expression of αL and β1 integrins, P-selectin glycoprotein ligand-1 (PSGL-1), chemokine (C-X-C motif) receptor 4 (CXCR4), or CD62L was not greater on CD4+ compared with CD8+ T cells (Fig. S4A). However, naïve CD4+ T cells had ∼twofold greater CCR7 surface levels than CD8+ T cells (Fig. 3B). CCR7 has two chemokine ligands, CCL21 and CCL19, but only CCL21 is expressed by HEVs and is the primary ligand facilitating CCR7-dependent LN homing of T cells (24). In chemotaxis assays, CD4+ T cells were significantly more sensitive to CCL21 than CD8+ T cells (Fig. 3C), especially at lower CCL21 concentrations (Fig. 3D). Because CCR7 interactions with its ligands promote LN retention of T cells, and because greater CCR7 expression by CD4+ T cells would therefore be expected to result in a longer, not shorter, LN transit time in the absence of other factors, we also investigated whether CCR7 was down-regulated on CD4+ T cells after LN entry. Although we observed changes in CCR7 expression over time, these changes were equivalent in CD4+ and CD8+ T cells (Fig. S4B).
Self-pMHC Interactions Retain CD4+ but Not CD8+ T Cells in LNs.
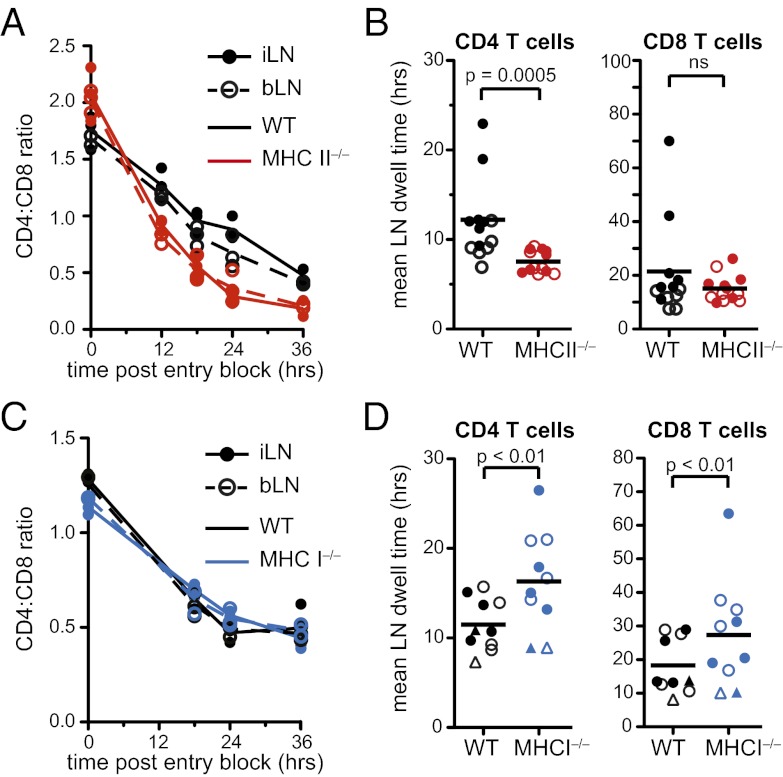
Apart from S1PR1 and CCR7 expression, it is currently unknown what other factors impact T-cell transit time through SLOs. Given our data that CD4+ T-cell contacts with DCs are of significantly shorter duration in the absence of MHCII, we asked whether interactions of T cells with MHC act as a retention signal. In addition, we examined whether the greater LN transit time of CD8+ T cells results from a greater amount of time spent interacting with MHCI, because many more cells in the LN express MHCI than MHCII. To do so, we quantified the egress rates of CD4+ and CD8+ T cells in mice deficient in either MHCII or MHCI. When T cells were transferred into MHCII−/− recipients the CD4:CD8 ratio inverted over time after LN entry blockade, as in WT mice (Fig. 4A). However, the CD4:CD8 ratio change occurred more rapidly in MHCII−/− mice, indicating that CD4+ T cells were egressing at an accelerated rate relative to CD8+ T cells (Fig. 4A). To examine whether this was explained by concomitant changes in CD8+ T-cell transit time and to quantify the difference in CD4+ T-cell transit in WT compared with MHCII−/− mice, we estimated E(t) as before. Whereas CD8+ T-cell transit was unaffected, CD4+ T cells had an increased egress rate and shorter mean transit time of 7.5 h when they did not interact with MHCII, 4 h less than in WT animals (Fig. 4B and Fig. S5). In contrast, in the absence of endogenous MHCI, we saw no changes in the rate of CD4:CD8 ratio inversion (Fig. 4C), with egress being slightly but significantly diminished for both CD4+ and CD8+ T cells (Fig. 4D and Fig. S5). Our data thus indicate that there are important differences in the role played by pMHC in retaining CD4+ or CD8+ T cells within LNs and suggest the existence of distinct processes that determine the LN dwell time of CD4+ and CD8+ T-cell populations.
Fig. 4.
Shorter LN dwell times of CD4+ T cells in the absence of MHCII but not of CD8+ T cells in the absence of MHCI. (A and C) CD4:CD8 ratio of adoptively transferred T cells in i/bLNs at various times after entry blockade in WT and MHCII−/− or MHCI−/− mice. (B and D) Mean LN dwell time estimates for transferred naïve CD4+ and CD8+ T cells. Circles denote transit time estimates from one experiment. Lines represent group means from five to seven independent experiments. Closed symbols, iLN; open symbols, bLN. In D: ●, β2M−/−- or WT-matched recipients; ▲, KbDb−/−- or WT-matched recipients.
Estimating the DC Scanning Efficiency of T Cells.
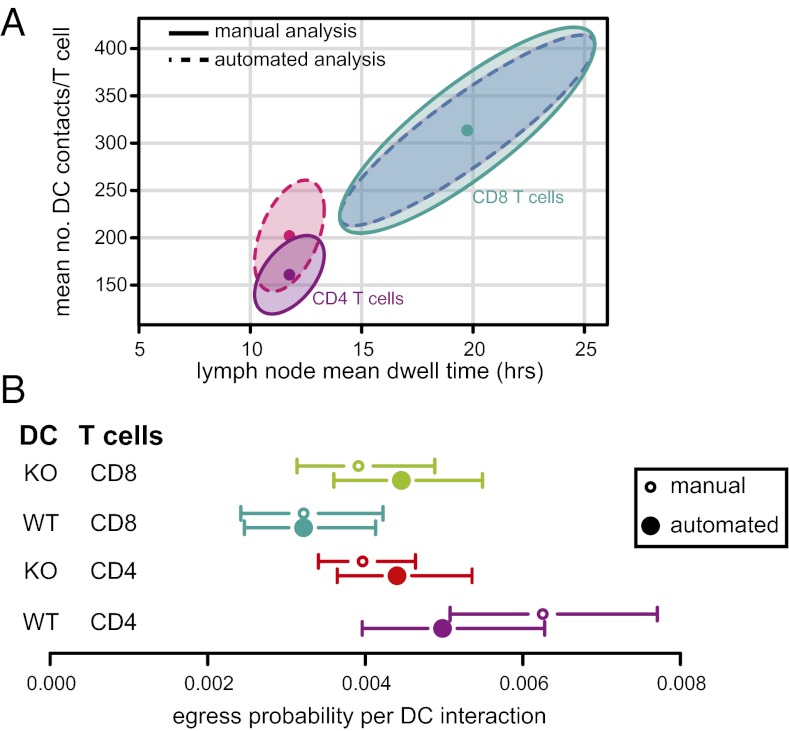
We next combined transit time and DC contact duration data to estimate DC scanning efficiency of T cells (mean number of contacts per LN transit, C; SI Materials and Methods). We estimated C for each T-cell and DC population and found a range of ∼160–320 contacts with DCs per LN transit (Fig. 5A), with good agreement for estimates made using the manual and automated datasets. There was a trend for naïve CD4+ T cells to make fewer DC contacts than CD8+ T cells. Using the manual dataset, the estimated mean number of DC contacts per transit for CD4+ T cells was 161 (95% confidence interval 128–194) vs. 314 for CD8+ T cells (227–401); using the automated dataset, 202 (155–249) vs. 313 (223–394). However, this difference reached significance (P < 0.001) only in the manual data set (Fig. 5A). Our observations that naïve CD4+ T cells have shorter transit times than CD8+ T cells but have similar DC contact durations can be explained by differences in (i) the time it takes for T cells to move between DC and/or (ii) differences in LN egress probability per DC interaction. We investigated whether the egress probability after a DC encounter, q, was different between CD4+ and CD8+ T cells. There was a trend for q to be greater for naïve CD4+ T cells compared with CD8+ T cells (this reached significance only in the manual data set, P < 0.001) after interaction with WT DCs (Fig. 5B).
Fig. 5.
Quantifying T-cell DC scanning dynamics within LNs. (A) Estimate of the mean number of WT DCs scanned by an individual CD4+ or CD8+ T cell during steady-state LN transit. Means (points) and bootstrapped joint 95% normal confidence intervals (shaded areas) are shown for DC–T-cell contacts obtained from both the manual and automated estimates of DC contact duration (Fig. 2). (B) Estimates of the probability that a T cell will egress from the LN after each DC contact, with bootstrap 95% confidence intervals.
It was possible that the faster egress rate of CD4+ T cells in MHCII−/− compared with WT recipients was primarily due to a reduced time spent being in contact with DCs and thus less mechanical restraint away from egress portals (Fig. 1D). Alternatively, cells may have an increased egress probability after interaction with MHCII−/− DCs. To distinguish these two hypotheses, we estimated q for CD4+ and CD8+ T cells after encounter with an MHCII−/− DC. We found no difference in egress probability for CD8+ T cells after interaction with WT vs. MHCII−/− DCs in either data set. However, on the basis of the manual data set, CD4+ T cells were ∼1.6 times less likely to egress after interacting with an MHCII−/− DC than WT DC (Fig. 5B). This suggests that CD4+ T cells are not obtaining MHCII-dependent signals that delay egress but that CD4+ T-cell egress rates are lower in WT compared with MHCII−/− recipients, primarily owing to longer contacts with DC that sequester these T cells away from egress sites. In fact, despite the shorter LN transit time of CD4+ T cells in the absence of MHCII, the reduced DC interaction time and egress probability per DC interaction results in an increase in the estimated mean number of DC contacts per transit in MHCII−/− recipients (Fig. S6).
Discussion
Two key aspects of T-cell biology—finding and responding to Ag and maintaining a constant naïve population size—are intimately tied to and dependent on cell recirculation among SLOs. Recent advances in our ability to track individual cells within complex tissues in live animals using 2P microscopy (25) have made it possible to begin to link individual cell-level events with population-level processes. Here we have combined such intravital imaging and mathematical modeling with more traditional immunological tools to track T-cell populations and quantify the LN transit time of naïve CD4+ and CD8+ T cells. We find substantial differences in CD4+ vs. CD8+ T-cell recirculation rates under steady-state conditions, with CD4+ T cells completing the blood to lymph transition in little more than half the time required by CD8+ T cells. Although CD4+ T-cell LN transit time is impacted by measurable interactions with self-pMHCII, self-pMHCI on host cells does not detectably retain transferred CD8+ T cells within LNs. Thus, not only are the recirculation dynamics between naïve CD4+ and CD8+ T-cell populations different, at least some of the key factors influencing the duration of their stay within SLOs are distinct. This has implications for the foreign antigen surveillance strategies used by the two T-cell populations. Whereas CD4+ T cells scan a greater number of LNs per unit time at the cost of scanning each individual LN less thoroughly, CD8+ T cells scan a given LN more meticulously but are slower to transit between LNs and hence slower to locate an LN with relevant foreign antigen. Put another way, CD4+ T cells survey the body’s entire cohort of LNs more rapidly but might miss low abundance antigen in doing so, whereas CD8+ T cells are less likely to miss antigen in an LN when it is present but may take longer to find the relevant LN.
After the initial demonstration that lymphocytes recirculate continuously between blood, SLOs, and lymph (2), early studies in rats showed that transferred T cells appear in SLOs within 30 min and take 4–18 h to passage LNs (26). Since then, LN transit times of T cells have been cited as lasting somewhere in the broad range of 8–24 h, with the rules governing CD4+ and CD8+ T-cell LN homing and dwell time assumed to be the same (9, 27). Here we estimate that naïve CD4+ and CD8+ T cells spend a mean duration of 12 and 21 h, respectively, within LNs of normal mice. The egress kinetics of T cells are approximated well by an exponential decay model, indicating that the egress probability is constant over time once T cells have acquired egress competency. This model is consistent with the wide distribution of residence times of a given T-cell population entering the LN at the same time (28). It is also consistent with microscopy data demonstrating the random walk behavior of individual T cells along fibroblastic reticular cells within LNs (3, 4) and the probabilistic nature of their egress at lymphatic sinuses (29), both of which would be difficult to reconcile with a fixed LN dwell time.
We show that the reduced entry of naïve CD8+ T cells into LNs is associated with a lower CCR7 expression on CD8+ T cells compared with CD4+ T cells. Given previous data that LN entry is sensitive to CCR7 expression levels (6, 30), this suggests that CCR7 expression plays a role in the differential naïve T-cell LN homing observed. Why the naïve CD4+ T-cell LN egress rate is greater than that of CD8+ T cells remains an open question. Our data clearly show that the balance between CCR7-mediated retention signals and S1PR1-mediated egress signals (6) cannot alone explain this difference. Not only is CCR7 expression greater on CD4+ T cells (which would predict longer LN dwell times), but sensitivity to S1P is less than for CD8+ T cells (again, predicting longer LN dwell times). Nor can differences in cell motility, displacement, or intranodal location explain their distinct LN transit kinetics. Interestingly, early studies performed in sheep (31) found that CD4+ T cells appear at greater frequencies in the lymph following cannulation of an individual LN, suggesting differences between recirculation rates of CD4+ and CD8+ T cells. This observation in another mammal makes it more likely that this is an evolutionarily conserved phenomenon and might help explain why changes in lymphoid tissue architecture in infections such as HIV in humans impact the homeostasis of one T-cell population more than others (32).
We show here that one factor differentially impacting CD4+ and CD8+ T-cell LN dwell times is cell–cell contacts, specifically those made with DCs via MHC. Data from 2P microscopy suggested that DCs make contact with 500–5,000 T cells per hour (21, 33). However, determining how many DCs one T-cell surveys is technically challenging and has only been done using in silico simulations (34). Our quantitative data showed that DC–T-cell contact durations are similar for CD4+ and CD8+ T cells, lasting on average 3–4 min, and are log-normally distributed, with most interactions being short but with rare contacts lasting >15 min. From these results we calculated that in each LN passage, CD4+ T cells scan a mean of ∼160–200 DCs, whereas CD8+ T cells scan ∼310 DCs. The low scanning coverage in the absence of antigen highlights the need for mechanisms that direct T cells toward relevant antigen-bearing DCs via, for instance, the expression of chemokines (35).
T-cell scanning of DCs is critical both for immune surveillance and acquisition of homeostatic signals. It is likely that T-cell transit times and precursor frequencies are optimized to ensure that foreign antigens are detected rapidly enough for effective pathogen clearance. If preimmune precursor population sizes were approximately equivalent between CD4+ and CD8+ T cells, the accelerated recirculation kinetics of CD4+ T cells might mean that they have an increased probability of locating an LN in which there is foreign antigen, but a reduced probability of detecting it within a given LN, compared with CD8+ T cells (36). However, precursor frequencies of CD4+ T cells may on average be lower than that of CD8+ T cells (37, 38). In this case, the shorter LN dwell time and more rapid transit between LNs of CD4+ T cells could be a mechanism to ensure that the probability of an antigen-specific precursor being present in a draining LN is similar for CD4+ and CD8+ T cells despite differences in precursor population size.
Access to SLOs impacts T-cell survival (39), and decreasing DC numbers reduces homing to SLOs through effects on chemokine signaling (40, 41). Here we show that DCs can also influence T-cell LN transit times on the basis of interactions via MHCII. T cells make ongoing TCR–MHC interactions in SLO that result in proximal TCR signaling, but only for CD4+ T cells do the TCR–MHCII scanning interactions also contribute substantially to LN retention in the absence of antigen. We estimate that one-third of the time CD4+ T cells spend in LN transit arises from prolongation of T–DC contacts via self-pMHC recognition.
Collectively, the data presented here, together with previous work, suggest that egress rates are impacted by the distribution of path lengths from entry points to egress portals, the number and duration of contacts made as cells travel through the LN, and the probability of egress at lymphatic sinuses, which result in exponentially distributed LN dwell times at the population level. Furthermore, our quantitative analysis indicates that the immune surveillance strategies of naïve CD4+ and CD8+ T cells are distinct—with CD4+ T cells scanning fewer DCs per LN and moving more rapidly between LNs. Further characterizing the processes regulating T-cell recirculation dynamics will be important in gaining insight into clinical diseases in which lymphocyte trafficking is impaired and in designing interventions to target T-cell migration to modulate T-cell homeostasis and immunocompetence.
Materials and Methods
Mice.
Animal housing, care, and research were in accordance with the Guide for the Care and Use of Laboratory Animals, and all procedures performed were approved by the National Institutes of Health and Case Western Reserve University Animal Care and Use Committee.
Estimation of LN Egress and Homing Rates.
Total T cells were purified by negative selection using MACS (Miltenyi) and 1 × 107 cells injected i.v. into recipient mice (∼90% of which were CD44lo). Two hours later, LN entry was blocked by i.p. administration of 100 μg anti-αL (clone M17/4) and anti-α4 (clone PS/2; BioXCell) in PBS, as previously described (6). Inguinal, brachial, and mesenteric LNs were harvested at time points after entry blockade, made into single-cell suspensions, counted, and cell fractions analyzed by flow cytometry. Congenic markers were used to distinguish transferred T-cell populations, or cells were labeled with 1.5 μM CFSE. Data from each independent experiment were fitted to an exponential model of egress and the egress rate (r) estimated from the best model fits. Mean dwell times were calculated as E(t) = 1/r. To estimate naïve endogenous T-cell transit times, we gated on CD44lo T cells. For LN homing rate estimates, 1 × 107 purified T cells were injected into recipients, LNs harvested 2 h later, and naive cell fractions enumerated.
Two-Photon Imaging.
WT and MHCII−/− DCs were differentially dye-labeled and an equal number (∼1 × 106 cells per mouse) of each population mixed and injected s.c. into the right dorsal footpad of MHCII−/− recipients. Eighteen to 48 h later, CD4+ and CD8+ T cells were isolated from B6 mice and dye-labeled. Equal numbers (∼1 × 107 cells per mouse) of each cell type were then coinjected i.v. into the same recipients and DC–T-cell interactions imaged in the popliteal LN 2–24 h after T-cell injection.
All other methods are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank I. Stefanova, J. Egen, and H. Qi for technical advice and I. Ifrim, P. Torabi-Parizi, D. Barkauskas, and C. Su for help with large experiments. This work was supported by the National Institutes of Health (NIH) Office of AIDS Research (J.N.M.), NIH Grant R01 AI093870-01 (to A.J.Y.), NIH Grants R21 AI092299-01 and R01 CA154656-01, the St. Baldrick’s Foundation (A.Y.H.), and the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, NIH.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1211717109/-/DCSupplemental.
References
- 1.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3(11):867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 2.Gowans JL, Knight EJ. The Route of Re-Circulation of Lymphocytes in the Rat. Proc R Soc Lond B Biol Sci. 1964;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- 3.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296(5574):1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 4.Bajénoff M, et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25(6):989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Worbs T, Bernhardt G, Förster R. Factors governing the intranodal migration behavior of T lymphocytes. Immunol Rev. 2008;221:44–63. doi: 10.1111/j.1600-065X.2008.00580.x. [DOI] [PubMed] [Google Scholar]
- 6.Pham TH, Okada T, Matloubian M, Lo CG, Cyster JG. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity. 2008;28(1):122–133. doi: 10.1016/j.immuni.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Worbs T, Mempel TR, Bölter J, von Andrian UH, Förster R. CCR7 ligands stimulate the intranodal motility of T lymphocytes in vivo. J Exp Med. 2007;204(3):489–495. doi: 10.1084/jem.20061706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okada T, Cyster JG. CC chemokine receptor 7 contributes to Gi-dependent T cell motility in the lymph node. J Immunol. 2007;178(5):2973–2978. doi: 10.4049/jimmunol.178.5.2973. [DOI] [PubMed] [Google Scholar]
- 9.Schwab SR, Cyster JG. Finding a way out: Lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8(12):1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 10.Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296(5574):1873–1876. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- 11.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427(6970):154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 12.Takada K, Jameson SC. Self-class I MHC molecules support survival of naive CD8 T cells, but depress their functional sensitivity through regulation of CD8 expression levels. J Exp Med. 2009;206(10):2253–2269. doi: 10.1084/jem.20082553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polic B, Kunkel D, Scheffold A, Rajewsky K. How alpha beta T cells deal with induced TCR alpha ablation. Proc Natl Acad Sci USA. 2001;98(15):8744–8749. doi: 10.1073/pnas.141218898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin B, Bécourt C, Bienvenu B, Lucas B. Self-recognition is crucial for maintaining the peripheral CD4+ T-cell pool in a nonlymphopenic environment. Blood. 2006;108(1):270–277. doi: 10.1182/blood-2006-01-0017. [DOI] [PubMed] [Google Scholar]
- 15.Labrecque N, et al. How much TCR does a T cell need? Immunity. 2001;15(1):71–82. doi: 10.1016/s1074-7613(01)00170-4. [DOI] [PubMed] [Google Scholar]
- 16.Dorfman JR, Stefanová I, Yasutomo K, Germain RN. CD4+ T cell survival is not directly linked to self-MHC-induced TCR signaling. Nat Immunol. 2000;1(4):329–335. doi: 10.1038/79783. [DOI] [PubMed] [Google Scholar]
- 17.Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312(5770):114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 18.Stefanová I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420(6914):429–434. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 19.Azzam HS, et al. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med. 1998;188(12):2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feuillet V, Lucas B, Di Santo JP, Bismuth G, Trautmann A. Multiple survival signals are delivered by dendritic cells to naive CD4+ T cells. Eur J Immunol. 2005;35(9):2563–2572. doi: 10.1002/eji.200526127. [DOI] [PubMed] [Google Scholar]
- 21.Miller MJ, Hejazi AS, Wei SH, Cahalan MD, Parker I. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proc Natl Acad Sci USA. 2004;101(4):998–1003. doi: 10.1073/pnas.0306407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiow LR, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440(7083):540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 23.Veerman KM, et al. Interaction of the selectin ligand PSGL-1 with chemokines CCL21 and CCL19 facilitates efficient homing of T cells to secondary lymphoid organs. Nat Immunol. 2007;8(5):532–539. doi: 10.1038/ni1456. [DOI] [PubMed] [Google Scholar]
- 24.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286(5447):2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 25.Germain RN, Miller MJ, Dustin ML, Nussenzweig MC. Dynamic imaging of the immune system: Progress, pitfalls and promise. Nat Rev Immunol. 2006;6(7):497–507. doi: 10.1038/nri1884. [DOI] [PubMed] [Google Scholar]
- 26.Smith ME, Ford WL. The recirculating lymphocyte pool of the rat: A systematic description of the migratory behaviour of recirculating lymphocytes. Immunology. 1983;49(1):83–94. [PMC free article] [PubMed] [Google Scholar]
- 27.Henrickson SE, von Andrian UH. Single-cell dynamics of T-cell priming. Curr Opin Immunol. 2007;19(3):249–258. doi: 10.1016/j.coi.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 28.Grigorova IL, Panteleev M, Cyster JG. Lymph node cortical sinus organization and relationship to lymphocyte egress dynamics and antigen exposure. Proc Natl Acad Sci USA. 2010;107(47):20447–20452. doi: 10.1073/pnas.1009968107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grigorova IL, et al. Cortical sinus probing, S1P1-dependent entry and flow-based capture of egressing T cells. Nat Immunol. 2009;10(1):58–65. doi: 10.1038/ni.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okada T, et al. Chemokine requirements for B cell entry to lymph nodes and Peyer’s patches. J Exp Med. 2002;196(1):65–75. doi: 10.1084/jem.20020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Washington EA, Kimpton WG, Cahill RN. CD4+ lymphocytes are extracted from blood by peripheral lymph nodes at different rates from other T cell subsets and B cells. Eur J Immunol. 1988;18(12):2093–2096. doi: 10.1002/eji.1830181235. [DOI] [PubMed] [Google Scholar]
- 32.Zeng M, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121(3):998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4(6):579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 34.Beltman JB, Marée AF, Lynch JN, Miller MJ, de Boer RJ. Lymph node topology dictates T cell migration behavior. J Exp Med. 2007;204(4):771–780. doi: 10.1084/jem.20061278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castellino F, et al. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440(7086):890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 36.Lee M, Mandl JN, Germain RN, Yates AJ. The race for the prize: T-cell trafficking strategies for optimal surveillance. Blood. 2012;120(7):1432–1438. doi: 10.1182/blood-2012-04-424655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moon JJ, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27(2):203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obar JJ, Khanna KM, Lefrançois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28(6):859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Link A, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8(11):1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 40.Moussion C, Girard JP. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature. 2011;479(7374):542–546. doi: 10.1038/nature10540. [DOI] [PubMed] [Google Scholar]
- 41.Wendland M, et al. Lymph node T cell homeostasis relies on steady state homing of dendritic cells. Immunity. 2011;35(6):945–957. doi: 10.1016/j.immuni.2011.10.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.