Abstract
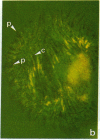
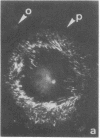
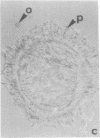
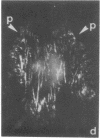
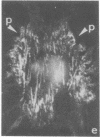
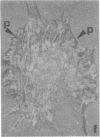
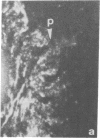
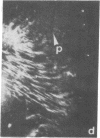
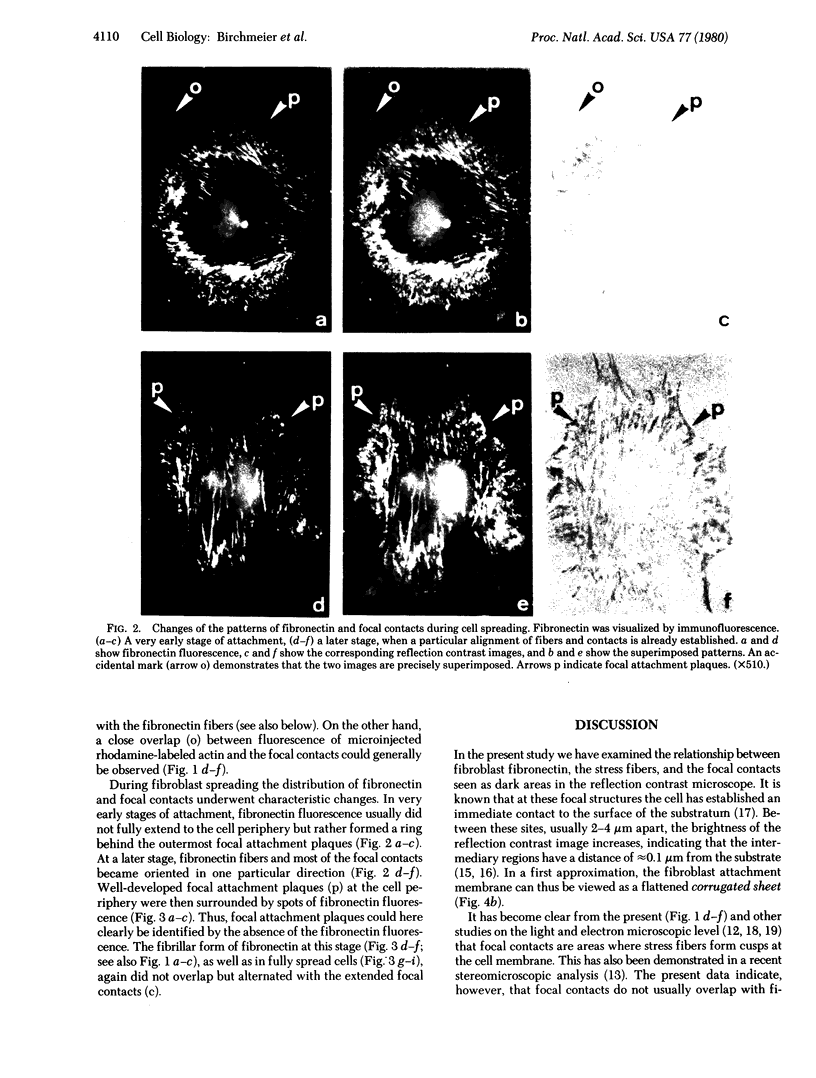
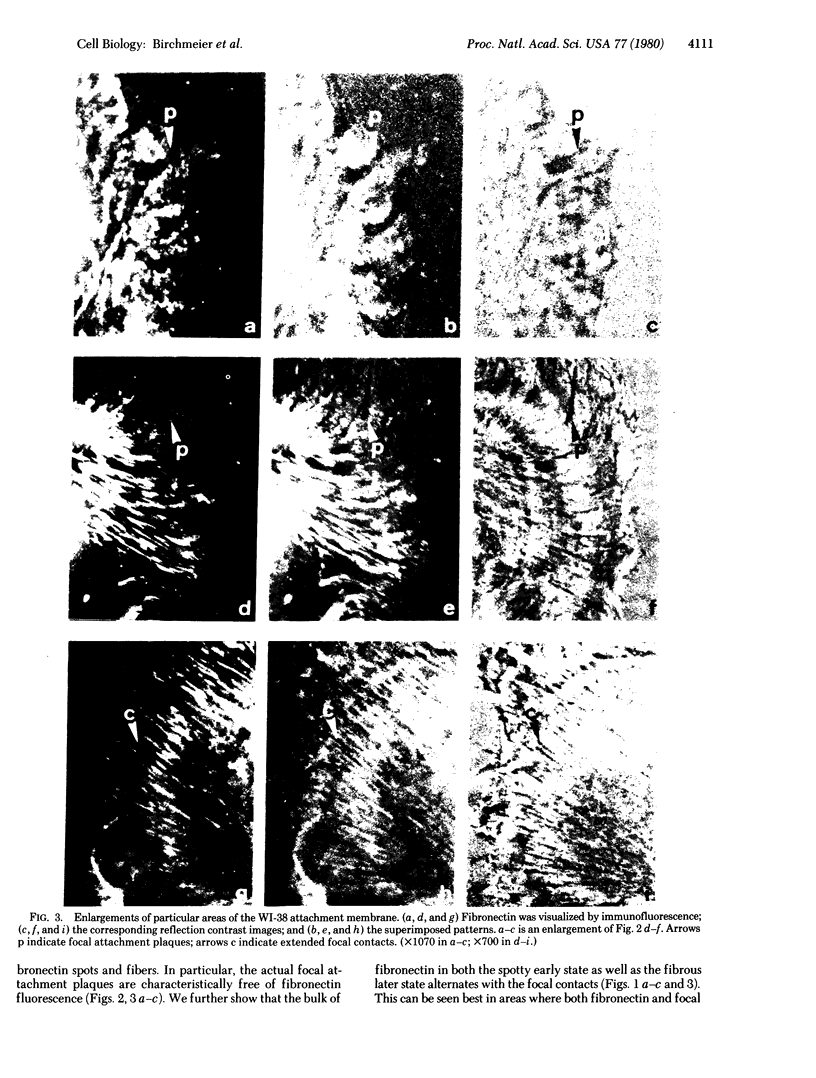
The distributions of both fibronectin (LETS, CSP) fibers and focal contacts to the substratum, as viewed by fluorescence and reflection contrast microscopy, respectively, have been compared in freshly plated WI-38 human fibroblasts. Most frequently, the actual focal attachment plaques did not contain fibronectin fluorescence and, furthermore, fibronectin spots and fibers often alternated with focal contacts. Overlap, however, was observed between focal contacts and the endings of actin-containing stress fibers [see also Wehland, J., Osborn, M. & Weber, K. (1979) J. Cell Sci. 37, 257-273]. Thus, the fibroblast attachment membrane might best be described as a corrugated sheet that undulates between alternating microfilaments and fibronectin fibers, at the points of closest and farthest distance to the substratum, respectively.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie M., Dunn G. A. Adhesions of fibroblasts to substratum during contact inhibition observed by interference reflection microscopy. Exp Cell Res. 1975 Apr;92(1):57–62. doi: 10.1016/0014-4827(75)90636-9. [DOI] [PubMed] [Google Scholar]
- Ali I. U., Hynes R. O. Effects of cytochalasin B and colchicine on attachment of a major surface protein of fibroblasts. Biochim Biophys Acta. 1977 Nov 15;471(1):16–24. doi: 10.1016/0005-2736(77)90388-1. [DOI] [PubMed] [Google Scholar]
- Ash J. F., Louvard D., Singer S. J. Antibody-induced linkages of plasma membrane proteins to intracellular actomyosin-containing filaments in cultured fibroblasts. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5584–5588. doi: 10.1073/pnas.74.12.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereiter-Hahn J., Fox C. H., Thorell B. Quantitative reflection contrast microscopy of living cells. J Cell Biol. 1979 Sep;82(3):767–779. doi: 10.1083/jcb.82.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet M., Puri E. C., Turner D. C. Fibronectin mediates attachment of chicken myoblasts to a gelatin-coated substratum. J Biol Chem. 1979 Jun 25;254(12):5475–5482. [PubMed] [Google Scholar]
- Flanagan J., Koch G. L. Cross-linked surface Ig attaches to actin. Nature. 1978 May 25;273(5660):278–281. doi: 10.1038/273278a0. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Chaponnier C., Zumbe A., Vassalli P. Actin and tubulin co-cap with surface immunoglobulins in mouse B lymphocytes. Nature. 1977 Oct 20;269(5630):697–698. doi: 10.1038/269697a0. [DOI] [PubMed] [Google Scholar]
- Geiger B. A 130K protein from chicken gizzard: its localization at the termini of microfilament bundles in cultured chicken cells. Cell. 1979 Sep;18(1):193–205. doi: 10.1016/0092-8674(79)90368-4. [DOI] [PubMed] [Google Scholar]
- Heath J. P., Dunn G. A. Cell to substratum contacts of chick fibroblasts and their relation to the microfilament system. A correlated interference-reflexion and high-voltage electron-microscope study. J Cell Sci. 1978 Feb;29:197–212. doi: 10.1242/jcs.29.1.197. [DOI] [PubMed] [Google Scholar]
- Heggeness M. H., Ash J. F., Singer S. J. Transmembrane linkage of fibronectin to intracellular actin-containing filaments in cultured human fibroblasts. Ann N Y Acad Sci. 1978 Jun 20;312:414–417. doi: 10.1111/j.1749-6632.1978.tb16822.x. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Cell surface proteins and malignant transformation. Biochim Biophys Acta. 1976 Apr 30;458(1):73–107. doi: 10.1016/0304-419x(76)90015-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. T. Relationships between fibronectin (LETS protein) and actin. Cell. 1978 Nov;15(3):875–886. doi: 10.1016/0092-8674(78)90272-6. [DOI] [PubMed] [Google Scholar]
- Izzard C. S., Lochner L. R. Cell-to-substrate contacts in living fibroblasts: an interference reflexion study with an evaluation of the technique. J Cell Sci. 1976 Jun;21(1):129–159. doi: 10.1242/jcs.21.1.129. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Martin G. R., Fishman P. H. Ganglioside inhibition of fibronectin-mediated cell adhesion to collagen. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3367–3371. doi: 10.1073/pnas.76.7.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis T. E., Winterhalter K. H., Birchmeier W. In vivo distribution and turnover of fluorescently labeled actin microinjected into human fibroblasts. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3814–3818. doi: 10.1073/pnas.76.8.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. A., Lloyd C. W., Thom D. Control of grip and stick in cell adhesion through lateral relationships of membrane glycoproteins. Nature. 1977 May 12;267(5607):124–128. doi: 10.1038/267124a0. [DOI] [PubMed] [Google Scholar]
- Singer I. I. The fibronexus: a transmembrane association of fibronectin-containing fibers and bundles of 5 nm microfilaments in hamster and human fibroblasts. Cell. 1979 Mar;16(3):675–685. doi: 10.1016/0092-8674(79)90040-0. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Ash J. F., Bourguignon L. Y., Heggeness M. H., Louvard D. Transmembrane interactions and the mechanisms of transport of proteins across membranes. J Supramol Struct. 1978;9(3):373–389. doi: 10.1002/jss.400090308. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Kurkinen M., Lehto V. P., Linder E., Timpl R. Codistribution of pericellular matrix proteins in cultured fibroblasts and loss in transformation: fibronectin and procollagen. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4944–4948. doi: 10.1073/pnas.75.10.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Rathke P. C., Osborn M., Franke W. W. Distribution of actin and tubulin in cells and in glycerinated cell models after treatment with cytochalasin B (CB). Exp Cell Res. 1976 Oct 15;102(2):285–297. doi: 10.1016/0014-4827(76)90044-6. [DOI] [PubMed] [Google Scholar]
- Wehland J., Osborn M., Weber K. Cell-to-substratum contacts in living cells: a direct correlation between interference-reflexion and indirect-immunofluorescence microscopy using antibodies against actin and alpha-actinin. J Cell Sci. 1979 Jun;37:257–273. doi: 10.1242/jcs.37.1.257. [DOI] [PubMed] [Google Scholar]