Abstract
Mechanosensitive (MS) channels are universal cellular membrane pores. Bacterial MS channels, as typified by MS channel of small conductance (MscS) from Escherichia coli (EcMscS), release osmolytes under hypoosmotic conditions. MS channels are known to be ion selective to different extents, but the underlying mechanism remains poorly understood. Here we identify an anion-selective MscS channel from Thermoanaerobacter tengcongensis (TtMscS). The structure of TtMscS closely resembles that of EcMscS, but it lacks the large cytoplasmic equatorial portals found in EcMscS. In contrast, the cytoplasmic pore formed by the C-terminal β-barrel of TtMscS is larger than that of EcMscS and has a strikingly different pattern of electrostatic surface potential. Swapping the β-barrel region between TtMscS and EcMscS partially switches the ion selectivity. Our study defines the role of the β-barrel in the ion selection of an anion-selective MscS channel and provides a structural basis for understanding the ion selectivity of MscS channels.
Keywords: crystal structure, anion selection, cytoplasmic region, electrophysiology, single channel recording
Mechanosensitive (MS) channels are the most ancient and universal membrane pores and are found in all eukaryotic and prokaryotic cells (1–3). In response to mechanical forces, MS channels open, facilitating the passive flow of particles from higher to lower concentrations (3, 4). In bacteria, MS channels serve as emergency valves that open upon membrane tension and rapidly release cytoplasmic osmolytes when cells are osmotically challenged, thus allowing the organisms to survive under hypoosmotic shock and to grow in a wide range of external osmolarities (1–3). In mammals, MS channels have been implicated in diverse biological and physiological processes, including cell growth, cell volume regulation, blood pressure control, hearing, touch, balance, and pain sensation (5). Dysregulation of MS channels has been linked to many diseases, such as neuronal and muscular degeneration, arteriosclerosis, hypertension, cardiac arrhythmia, and glaucoma (3, 5–7).
The MS channel of small conductance (MscS) channel is one of the best-characterized MS channels, with a conductance of ∼1 nS (8–11). The MscS channel consists of a transmembrane (TM) domain and a large cytoplasmic domain that is conserved in the MscK and MscM channels (1, 2, 10). Previous structural studies of the MscS channel from Escherichia coli (EcMscS) provide a framework for the interpretation of biophysical and functional data and provide clues to the molecular mechanisms that govern channel gating (10–12). As indicated by biochemical characterization (13) and confirmed by structural studies, the functional MscS channel is a homoheptamer (10–12). The structural study of the EcMscSA106V mutant showed that substantial rotational rearrangement of the TM region results in an increase in the pore size, presumably reflecting EcMscS channels in an open state, which is also consistent with electron paramagnetic resonance spectroscopy studies (11, 14). A structural comparison of EcMscS in the closed state with the EcMscSA106V mutant revealed striking conformational changes in the TM domain but not in the cytoplasmic region (11). However, subsequent physiological (15) and biophysical (16, 17) studies suggest that the cytoplasmic region also undergoes a large conformational change when the channel is open.
The MscS channels in microbes identified thus far display different ion selectivities (1, 2). For example, EcMscS has a slight anion preference (PCl/PK ∼1.5–3) (8, 13, 18), whereas its homolog, MscMJ from Archaea, prefers cations (PK/PCl ∼5.0) (19). In contrast, the MscS-like channels in plants tend to be selective for anions, as exemplified in tobacco (PCl/PK ∼9.5) and Arabidopsis (PCl/PK ∼22.5) (20, 21). The molecular mechanisms underlying the ion selectivity of these channels are still poorly understood, in large part owing to the lack of the structural studies of an MscS channel with strong ion selectivity.
In this study we identified an anion-selective MscS channel from Thermoanaerobacter tengcongensis (T. tengc). Although they share a similar overall structure, TtMscS and EcMscS channels are strikingly different with respect to the pore formed by the β-barrel region. Electrophysiological studies using chimeras and single mutants strongly support the hypothesis that the anion selectivity of TtMscS is mainly conferred by the β-barrel region. In summary, our current study identifies an anion-selective channel and defines the molecular mechanism underlying its anion selectivity.
Results
TtMscS Is an Anion-Selective Mechanosensitive Channel.
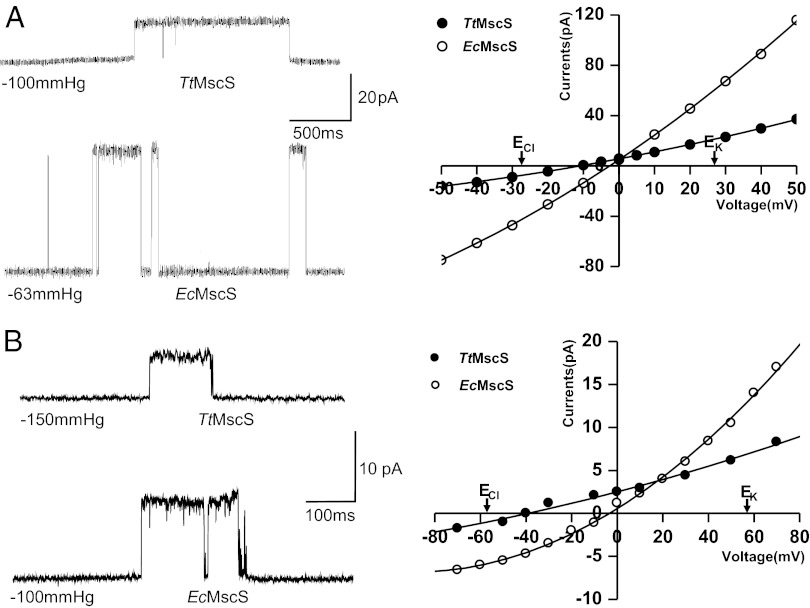
We set out to express MscS channels from different species in E. coli MJF612 (mscL::Cm, mscS−, mscK::Kan, and ybdG::apr) and/or MJF429 (mscS−, mscK::Kan) strains and to test the ion selectivity of these channels using patch-clamp methods on giant spheroplasts as described previously (8, 22, 23). One MscS channel from T. tengc (TtMscS) exhibited anion selectivity, whereas the EcMscS channels used as controls showed almost no ion preference under the same conditions (Fig. 1A). We further purified the proteins and verified their electrophysiological activities in a patch-clamp system using in vitro reconstituted giant liposomes. In asymmetric KCl solutions (150 mM/15 mM), TtMscS exhibited an average reversal potential of −39 ± 1.7 mV (n = 4 patches) compared with −4 ± 1.5 mV for EcMscS (n = 4 patches) under the same conditions (Fig. 1B; the correlative ion-permeating ratios are shown in Table S1). Under the asymmetric ion strength condition of a KNO3 solution, the average reversal potential measured from both single-channel currents and macroscopic currents was −52 ± 1.3 mV (n = 6 patches), corresponding to an anion-to-cation (PNO3:PK) permeability ratio of ∼28:1 (Fig. S1 A–C), which is close to the anion selectivity of GABA receptors (PNa:PK:PCl ∼0:0.03:1) (24, 25). Further tests of other anions showed that the relative permeability of TtMscS followed the order of NO3− > I− > Cl− > Br− (Fig. S1 D–F). Taken together, these results demonstrate that TtMscS is an anion-selective MS channel.
Fig. 1.
TtMscS Is an anion-selective MS channel. (A) Left: Single-channel traces recorded by a patch-clamp system from giant spheroplasts containing TtMscS (Upper Left) or EcMscS (Lower Left) at +20 mV. The number under the trace indicates the pressure applied to the patch during the event. Right: I-V curves for TtMscS and EcMscS channels at a 1:3 salt gradient (200 mM/600 mM); an ideal anion-selective channel with Erev = −27.7 mV according to the Nernst equation is indicated. The reversal potentials for TtMscS and EcMscS are −12 ± 1.5 mV (n = 3 patches) and −2 ± 1.3 mV (n = 3 patches), respectively. (B) Left: Single-channel open and closed traces recorded by a patch-clamp system from giant liposomes containing TtMscS (Upper Left) or EcMscS (Lower Left) at +50 mV. The number under the trace indicates the pressure applied to the patch during the event. Right: I-V curves for TtMscS and EcMscS channels at a 10:1 KCl gradient (150 mM/15 mM); an ideal anion-selective channel with Erev = −58 mV according to the Nernst equation is indicated. The reversal potentials for TtMscS and EcMscS are −39 ± 1.7 mV (n = 4 patches) and −4 ± 1.5 mV (n = 4 patches), respectively.
TtMscS Has an Overall Structural Fold Similar to That of EcMscS.
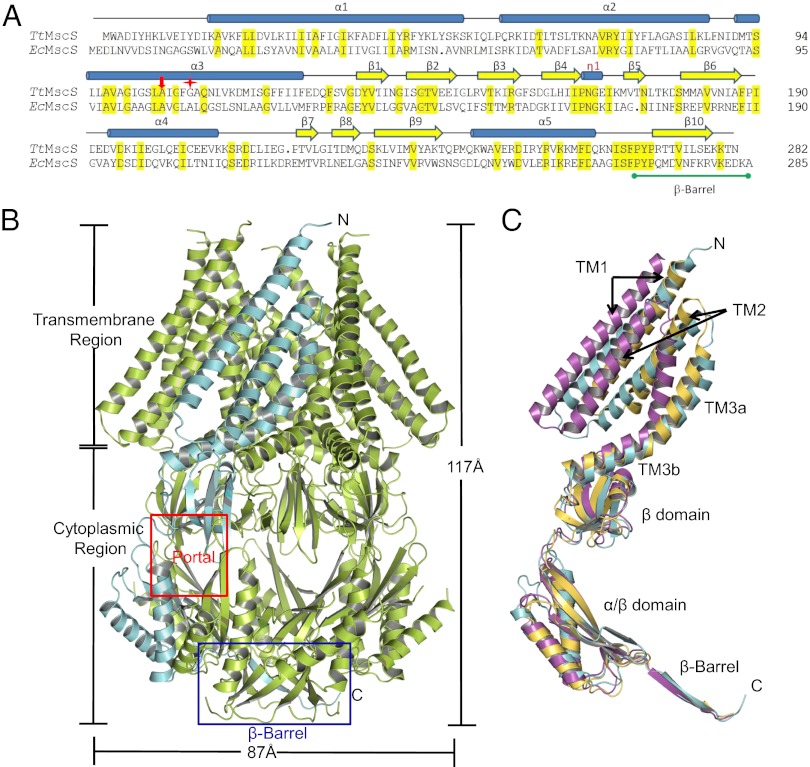
To understand the molecular mechanism of the anion selectivity of TtMscS, we solved its crystal structure at 3.44 Å resolution (Table S2). In the crystal, each asymmetric unit comprises seven TtMscS protomers, consistent with the EcMscS channel fold, forming a homoheptamer channel (Fig. 2 A and B). The heptamer extends ∼117 Å parallel to the sevenfold axis and is ∼87 Å in width in the perpendicular direction (Fig. 2B). In each TtMscS protomer, the TM region consists of three α-helices (TM1, TM2, and TM3) (Fig. 2C and Fig. S2A). The curved TM3 has a pronounced kink at TtMscSGly109 and can be further divided into TM3a and TM3b (Fig. 2A and Fig. S2A). The N termini of the three TM α-helices face the periplasm, whereas the C termini protrude into the cytoplasm. TM3 lines the channel pore (Fig. S2C).
Fig. 2.
Overall structure of TtMscS. (A) Sequence alignment of TtMscS and EcMscS. Residues highlighted in yellow are identical in the two sequences. Cylinders above the sequences designate the three transmembrane α-helices, α1–3, and the three cytoplasmic helices, 310 and α4–5, of TtMscS. Yellow arrows above the sequences denote residues in β-strands 1–10. The β-barrel region is marked with a green line. A105 is indicated with a red arrow and G109 a red star. (B) Overall structure of the TtMscS homoheptamer viewed perpendicular to the sevenfold axis. One protomer is shown in cyan, and the others are shown in lemon. The transmembrane and cytoplasmic regions are indicated. The area highlighted within the red box indicates one of the seven portals, and the blue box indicates the β-barrel region. (C) TtMscS in a closed conformation. The superposition of one single TtMscS protomer (cyan) with the open [magenta; Protein Data Bank (PDB) ID code 2VV5] and closed (olive; PDB ID code 2OAU) states of EcMscS is shown. The secondary structural elements from TtMscS are labeled. Arrows indicate the closed and open conformations of TM1 and TM2 in EcMscS.
Similar to EcMscS, TtMscS has a large cytoplasmic domain that comprises a middle β-domain, an α/β-domain, and a C-terminal extension, forming an interior chamber of ∼30 Å in diameter (Fig. 2 B and C and Fig. S2B) (10, 11). The middle β-domain mainly consists of a five-stranded antiparallel β-sheet (β1–β5) with a highly conserved 310 helix (η1) sitting on its top and the C-terminal portion of TM3b from the transmembrane region running across its opening. Immediately following the middle β-domain is a mixed α/β structure, formed by β6–β9 and α4–α5 (Fig. S2A). The isolated C terminus of β10 in each monomer forms a seven-stranded parallel β-barrel (residues 271–282), resulting in the formation of a second potential channel pore (referred to as the β-barrel pore hereafter) (Fig. 2). The structure of TtMscS closely resembles that of EcMscS in the closed conformation, with an rmsd of 2.04 Å over 103 Cα atoms of the TM domain. The structure is notably different from that of EcMscS in the open state, with the largest difference located in the TM region, which has an rmsd of 8.7 Å (Fig. 2C). These structural observations suggest that the conformation of TtMscS in the crystal reflects a nonconducting state.
TtMscS Has Smaller Equatorial Portals than EcMscS.
The cytoplasmic equatorial portals of EcMscS are proposed to be responsible for ion permeation (10–12, 16). Sequence alignment revealed that the three bulky TtMscS residues, Phe157, Met243, and Trp246, that line the portals are substituted with their smaller equivalents, Ala58, Gly244, and Gln247, respectively, in EcMscS (Fig. 3A), thus greatly reducing the size of the portals in TtMscS. The portals in TtMscS have radii of only 1.7 Å, which is much smaller than those in EcMscS (3.4 Å in radius) (Fig. 3B and Fig. S3 A and B). The smaller portals in TtMscS seem unlikely to be caused by a closed conformation, because the cytoplasmic portion of EcMscS has a similar conformation in both the closed and open states (Fig. S3C) (10, 11).
Fig. 3.
TtMscS has smaller equatorial portals than EcMscS. (A) Residues around the portals are conserved in MscS homologs from different species. Conserved residues are highlighted in dark blue, green, or light blue, according to the extent of their conservation. Conserved residues TtMscSA105 and TtMscSF108 and those lining the portals are indicated by red stars. (B) Ribbon diagrams showing close views of one of the seven portal regions of TtMscS (Left) and EcMscS (Right). Residues lining the pores are shown as sticks. One protomer is colored in cyan, and the others are colored in green.
β-Barrel Pores of TtMscS and EcMscS Differ in Size and Electrostatic Potential.
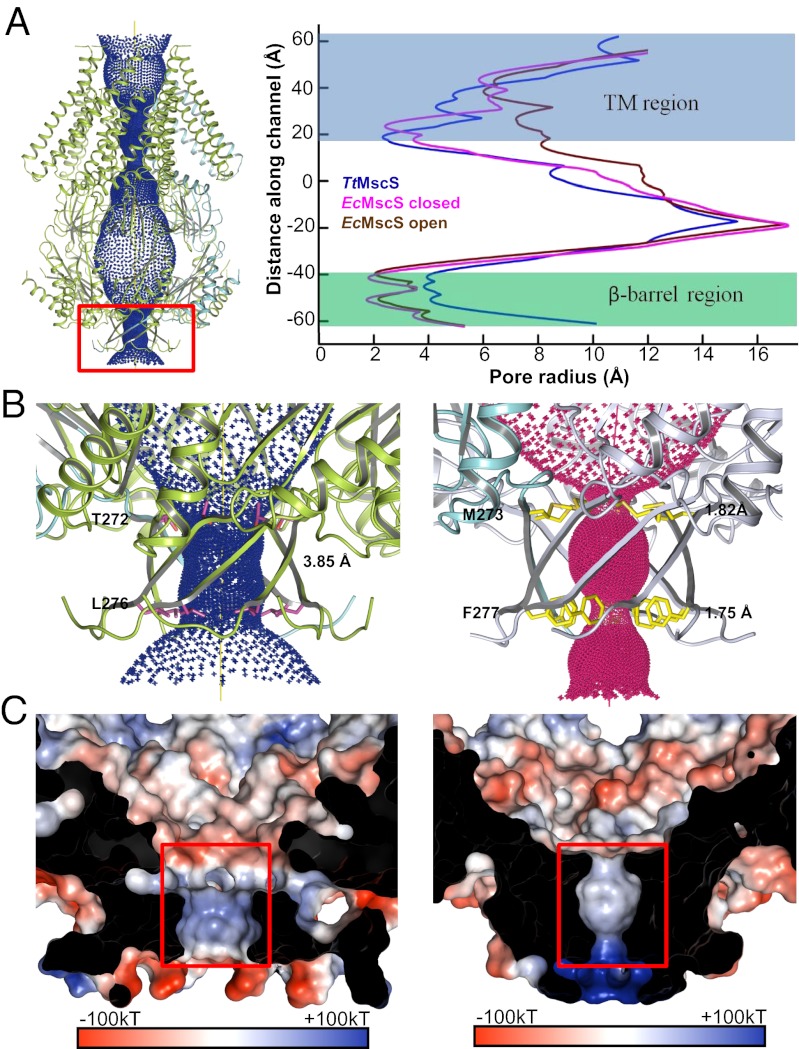
To locate the potential site of ion entry in TtMscS, we calculated the radii along its axial passage with the program HOLE (26). The following two constriction sites were found along the channel for ion passage: one in the TM region and the other in the β-barrel region (Fig. 4A). The former has a size similar to the TM constriction site in EcMscS (closed state): ∼2.4 Å in radius compared with ∼6 Å in the open state of EcMscS (Fig. 4A and Fig. S4). In contrast, the size of the C-terminal β-barrel region restriction site in TtMscS (3.85 Å in radius) is much larger than that of its equivalent in EcMscS (1.75 Å in radius). The reduced size of the β-barrel pore in EcMscS results from the two bulkier residues, EcMscSMet273, Phe277, compared with their counterparts, TtMscSThr272, Leu276, that line the inner side of the pore (Fig. 4B). In addition to the size difference in the β-barrel pore, the patterns of electrostatic potentials of TtMscS and EcMscS inside the pore and around the β-barrel region are markedly different. In TtMscS, the β-barrel region exhibits an alternating distribution of electrostatic potential starting from the inner surface (Fig. 4C). The pattern of potential distribution is nearly reversed in the corresponding regions of EcMscS (Fig. 4C).
Fig. 4.
β-Barrel pore of TtMscS differs from that of EcMscS in size and electrostatic potential. (A) The central channel in TtMscS contains two constriction sites. The channel passage (Left), calculated by HOLE (26), is indicated by blue dots along a central yellow line. The radius of the channel is plotted on the right panel and compared with those from the closed and open states of EcMscS. The β-barrel region is highlighted by a red box. (B) The β-barrel pore of TtMscS is larger than that of EcMscS. The TtMscS and EcMscS β-barrel regions are shown at Left and Right, respectively. The two residues lining the restriction sites are shown as sticks and are labeled. The radii of the β-barrel pore of both channels are indicated. (C) The β-barrel pore of TtMscS has an electrostatic surface that is distinct from that of EcMscS. Left and Right: Electrostatic potentials around the β-barrel pore inner surface of TtMscS and EcMscS, respectively. The front of the β-barrel pore is cut away to reveal the inner surface. The orientation is the same as in B.
β-Barrel Region Confers the Anion Selectivity of TtMscS.
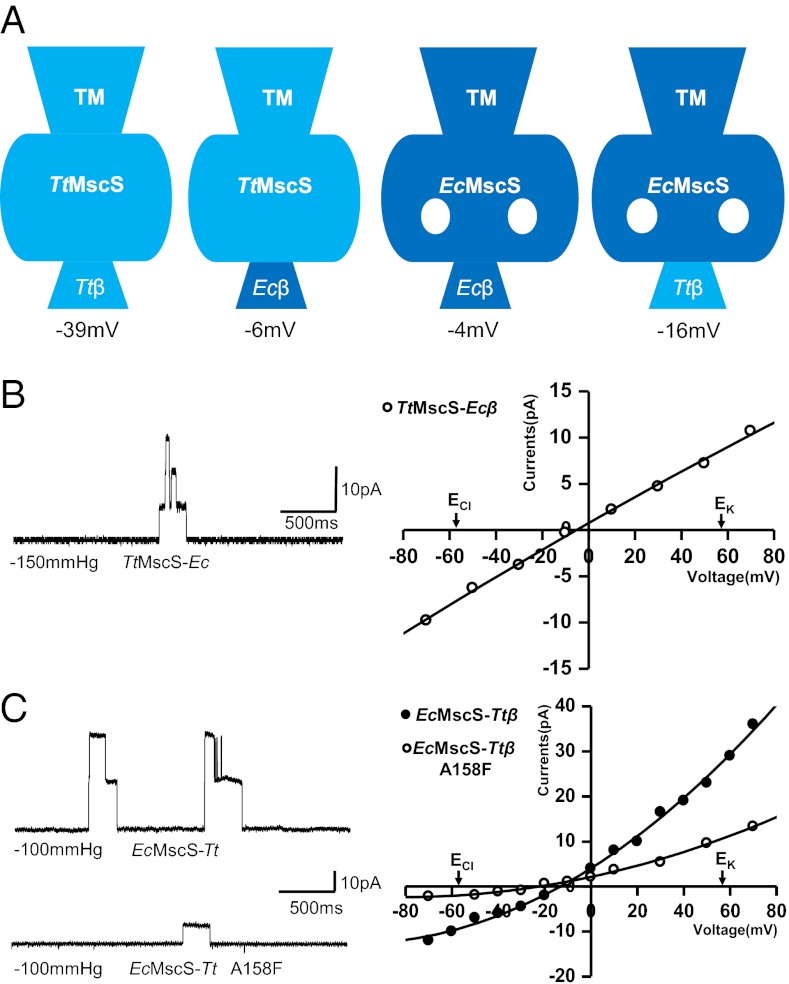
Our structural analyses suggest that the β-barrel pore of TtMscS may serve as the ion entrance and may thus play an important role in ion selection. To test this hypothesis, we substituted TtMscSLeu276 (Fig. 4B) with a bulky tyrosine residue, which is expected to shrink the size of the β-barrel pore and consequently attenuate or block channel activity. The purification profile of the TtMscSL276Y mutant protein was identical to that of the wild-type protein, according to a gel filtration assay (Fig. S5). Our electrophysiological assay showed that the resulting channel exhibited no activity, indicating that the β-barrel pore may serve as a major ion permeation site in TtMscS. To further support this conclusion, we created chimeric TtMscS and EcMscS channels with swapped β-barrel regions (after the conserved PYP motif of the C terminus; Fig. 2A) and tested their activities. The TtMscS chimera (TtMscS-Ecβ) was significantly compromised in anion preference, with a reversal potential of −6 ± 1.2 mV (n = 4; Fig. 5 A and B), which is comparable to that of the wild-type EcMscS (−4 mV) in an asymmetric KCl solution (150 mM/15 mM) (Fig. 1B). This result strongly supports a critical role for the β-barrel pore of TtMscS in ion selectivity. The striking effect generated by the chimeric mutation was not caused by a structural perturbation in the channel because the β-barrel pore in the crystal structure of TtMscS-Ecβ has a nearly identical pore size and electrostatic surface as that of EcMscS, and the other parts of the chimera are identical to those of the wild-type TtMscS (rmsd = 0.48 Å in 235 Cα atoms) (Fig. S6). The reciprocal chimera EcMscS-Ttβ was converted into an anion-selective channel with a reversal potential of −16 ± 1.6 mV (n = 6) (Fig. 5 A and C). One plausible explanation for the weaker anion selectivity of EcMscS-Ttβ compared with that of TtMscS is that the portals in the chimera may also contribute to ion permeation. If this were the case, blocking the portals in the chimera would increase ion selectivity and reduce conductance. Indeed, the A158F mutation in EcMscS-Ttβ, which is expected to reduce the radii of the portals (Fig. 3B), resulted in a channel with higher ion selectivity, as indicated by the reversal potential of −24 ± 1.7 mV (n = 3) and lower conductance (Fig. 5C). This is functional evidence for the involvement of the EcMscS portals in ion permeation, which has been widely hypothesized on the basis of structural and modeling studies (1, 2, 10–12, 16, 27).
Fig. 5.
β-Barrel region of TtMscS is a major structural determinant for ion selectivity. (A) Left to right, the wild-type TtMscS, TtMscS mutant substituted with the EcMscS β-barrel region (TtMscS-Ecβ), wild-type EcMscS, and EcMscS mutant substituted with the TtMscS β-barrel (EcMscS-Ttβ) are shown as cartoons. Ttβ and Ecβ indicate the β-barrel region of TtMscS (residues 271–282) and EcMscS (residues 272–285), respectively. The numbers below indicate their reversal potentials. (B) A TtMscS mutant with the β-barrel substituted with that of EcMscS attenuates the channel (TtMscS-Ecβ) selectivity. Left: Single- or two-channel openings recorded at +50 mV from the TtMscS-Ecβ channel. Right: I-V curve for the TtMscS-Ecβ channel. The reversal potential derived from the I-V curve is −6 ± 1.2 mV (n = 4 patches). The number under each trace indicates the pressure applied to the patch during the event. The salt gradient is same as that in Fig. 1B. (C) An EcMscS mutant with the β-barrel substituted with that from TtMscS promotes the channel (EcMscS-Ttβ) selectivity and conductance. Left: Single- or two-channel openings recorded at +50 mV from the EcMscS-Ttβ channel and its A158F mutant, with reversal potentials of −16 ± 1.6 mV (n = 6 patches) and −24 ± 1.7 mV (n = 3 patches), respectively, derived from the I-V curves shown at Right. The salt gradient is same as that in Fig. 1B.
Charge Distributions and Pore Size Are Critical for the Anion Selectivity of TtMscS.
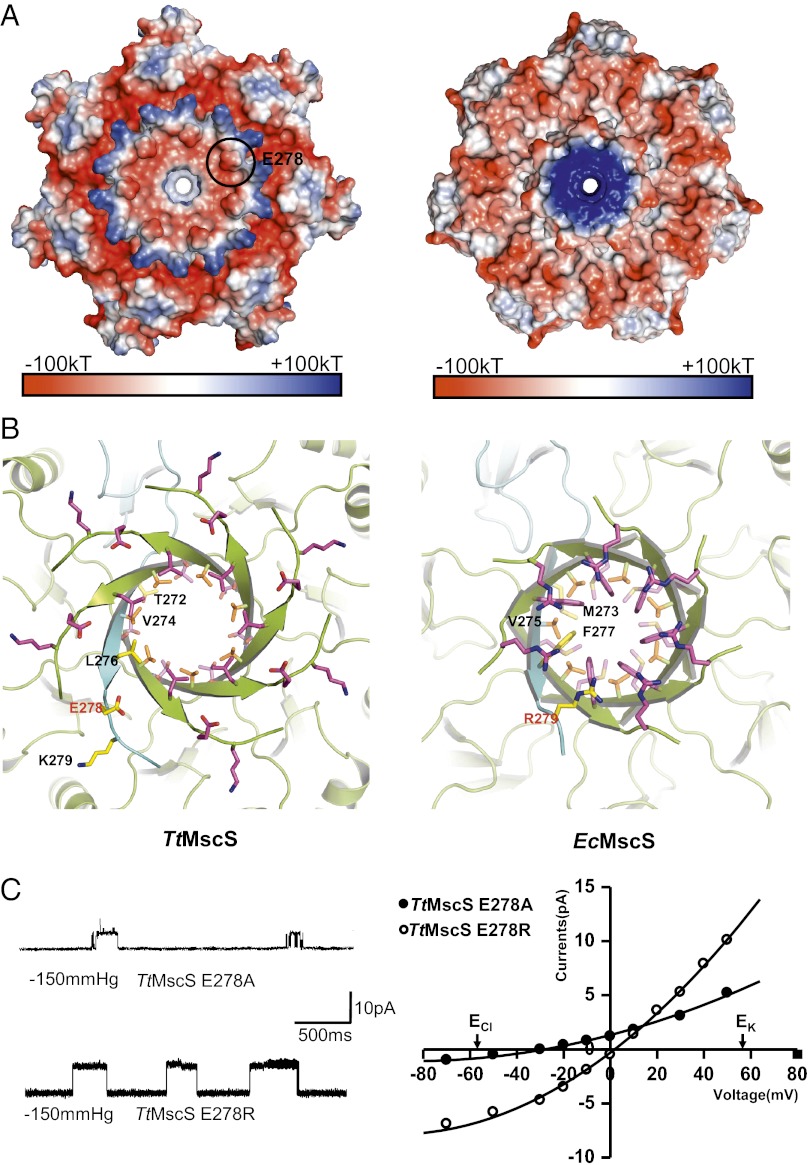
To further confirm the importance of the β-barrel region in TtMscS anion selectivity, we mutated TtMscSGlu278 to its equivalent, EcMscSArg279 (Fig. 6 A and B). The mutation was expected to alter TtMscS ion selection if the electrostatic potential around this area plays a role in this process. Remarkably, the single mutation dramatically altered the reversal potential of the channel from −39 to 2 ± 0.7 mV (n = 4) (Figs. 1B and 6C), indicating that the channel changed from being highly anion selective to having almost no selectivity. Interestingly, mutation of the same residue to alanine produced a less striking effect on channel selectivity (−30 ± 2 mV, n = 4) (Fig. 6C). Thus, the charge distributions (Fig. 6A) are critical for the anion selectivity of the channel, with pore size playing an auxiliary role. Taken together, the different patterns of the pore size and electrostatic potentials surrounding the β-barrel pore between TtMscS and EcMscS likely contribute to the distinct ion selectivity of the two channels.
Fig. 6.
Mutations in the β-barrel region of TtMscS alter the channel’s selectivity. (A) The β-barrel region of TtMscS differs from that of EcMscS in size and electrostatic surface. Shown are electrostatic potential surfaces, showing close views of the C-terminal β-barrel regions of TtMscS (Left) and EcMscS (Right) observed from the cytoplasmic side. The blue, white, and red shadings represent positively charged, neutral, and negatively charged surface regions, respectively. One of the seven TtMscSE278 residues is indicated by a black circle. (B) The residues forming the β-barrel pore of TtMscS differ from those of EcMscS. Shown are ribbon diagrams, showing close views of the C-terminal β-barrel regions of TtMscS (Left) and EcMscS (Right) observed from the cytoplasmic side. The residues lining the pores are shown as sticks. One protomer is colored in cyan, and the others are colored in lemon. TtMscSE278 and EcMscSR279 are marked in red. (C) Mutations in the β-barrel region of TtMscS alter the channel’s selectivity. The single-channel openings shown on the left were recorded at +50 mV from the mutants as indicated. The reversal potentials derived from the I-V curves for E278A and E278R are −30 ± 2 mV (n = 4 patches) and 2 ± 0.7 mV (n = 4 patches), respectively. The salt gradient is same as that in Fig. 1B.
Discussion
Ion selectivity is a common theme for ion channel studies. Typical anion-selective, ligand-gated ion channels have a small but significant permeability to cations, such as the GABA receptor (PNa:PK:PCl ∼0:0.03:1) (24) and the Gly receptor (PCl:PNa ∼25:1) (25). The voltage-dependent anion channels on the mitochondrial outer membrane are less selective with respect to anions and cations, with a permeation ratio of approximately 2:1 (28). However, the structural molecular mechanisms of these anion channels are not well defined. In the present study, we identified a type of MscS channel from T. tengc that exhibited strong anion selectivity to NO3− and moderate selectivity to Cl−, which is valuable for mechanistic studies of the ion selection of MscS channels. The striking structural and electrostatic potential differences between the TtMscS and EcMscS channels around the portals and β-barrel regions suggest that they use distinct routes for the permeating ions. Compared with EcMscS, TtMscS has smaller equatorial portals and a larger β-barrel pore (Figs. 3 and 4), suggesting that the β-barrel pore is the main entrance for ion passage in TtMscS. Our functional studies of various mutant channels, including chimera (Fig. 5), mutant chimera (Fig. 5C), and single mutant (Fig. 6C) channels, provide strong evidence that the β-barrel pore is an important structural determinant for ion passage and selection in TtMscS.
The TtMscS cylindrical β-barrel pore has a partially positive inner surface and a large size of 3.85 Å (Fig. 4). It seems to be optimal for the passage of many hydrated or partially hydrated anions, such as NO3− (3.4 Å), Cl− (3.32 Å), Br− (3.3 Å), and I− (3.31 Å in radius) (Fig. S7A) (29). Interestingly, the cylindrical β-barrel pore in TtMscS is strikingly similar to the mouth of the ion channel pore of the anion-selective eukaryotic glutamate-gated chloride (GluCl) channel in both charge distribution (partially positive charge) and size (∼3.8 Å in radius). As suggested by the structure of the GluCl channel (30), in which the iodide or chloride binding sites nestled in the electropositive pockets at the base of the pore determine the ion selectivity, we propose that the positive inner surface of the β-barrel pore region of TtMscS also forms “anion pockets” that would repel cations and attract anions. The negative electrostatic potentials external to the β-barrel pore may also contribute to ion selectivity (Fig. 6A). One possibility is that TtMscSGlu278 traps the cations in the area flanking the pore, thus blocking their entry into the pore (Fig. S7). Consistent with this hypothesis, mutations of a single acidic residue (TtMscSGlu278) outside the β-barrel pore in TtMscS compromised its ion selectivity (Fig. 6C). The observation that the TtMscSE278R mutant lost anion selectivity (Fig. 6C) is also consistent with this hypothesis. Our structural and electrophysiological data strongly support the idea that the β-barrel of TtMscS is the main constriction site critical for anion passage and selection.
Blocking the β-barrel pore with the TtMscSL276Y mutant resulted in a loss of TtMscS channel activity, suggesting that its portals may not be important for ion permeation, consistent with their smaller size in the structure (Figs. 3B and Fig. S3A). In contrast, the portals in EcMscS are likely involved in ion passage because blocking them in the chimera EcMscS-Ttβ with the EcMscS-TtβA158F mutation greatly diminished the channel conductance (Fig. 5C and Table S1). This result provides functional evidence to support the hypothesis that the portals in EcMscS are important for ion passage (1, 2, 10–12, 16). In addition to the side portals, the β-barrel pore of EcMscS seems to have a role in ion permeation, given that the TtMscS-Ecβ chimera was still active (Fig. 5B). Previous studies indicate that the β-barrel in EcMscS is important for channel activity (27, 31, 32). Comparison of the closed and open structures of EcMscS shows that there is no striking difference in the radius of the β-barrel pore size (∼2 Å in radius) (Fig. 4A, Right, and Fig. S4) (10, 11), but this does not rule out the possibility of the involvement of the β-barrel pore in EcMscS in ion permeation when the channel is in the fully open state under high mechanical pressure. Other MS channels with weak ion selectivity, such as MscL, which lacks the large cytoplasmic domain (33–35), and MscK, which has both a large cytoplasmic and an extracellular domain (36), may use different mechanisms for ion selection, with one possibility being that the transmembrane domain may also play a role in ion selection.
In summary, in the present study, we identified an anion-selective MscS channel that has strong selectivity for NO3− and moderate selectivity for other anions. Our structural and electrophysiological data support a critical role for the β-barrel region of TtMscS in anion selection. The ion-selective filter of the TtMscS channel localized at the cytoplasmic C-terminal rather than at the transmembrane domain, in contrast to most anion-selective channels. We propose that both the electrostatic potential and the size of the β-barrel pore contribute to the anion selectivity. These results expand our view of the variable ion selectivity of MscS channels and may contribute to a better understanding of the fundamental question of the mechanism of anion channel selectivity.
Methods
TtMscS and the TtMscS-Ecβ chimera were expressed and purified from E. coli strain C43. Crystals were grown at 18 °C by the hanging-drop vapor diffusion method. Diffraction data were collected at Shanghai Synchrotron Radiation Facility BL17U, and the structure was solved by molecular replacement. Data collection and structure refinement statistics are summarized in Table S2. The wild-type and mutant proteins were reconstituted into liposomes composed of 1-palmitoyl-2-oleoyl-phosphatidylethanolamine and 1-palmitoyl-2-oleoyl-phosphatidylglycerol, as previously described (13). Electrophysiological experiments were conducted using a patch-clamp system on giant liposomes and spheroplasts. The reversal potentials and permeability ratios were calculated under biionic or mixed-ionic conditions.
Supplementary Material
Acknowledgments
We thank the staff at the Shanghai Synchrotron Radiation Facility BL17U beamline for assistance with data collection. This work was supported by Ministry of Science and Technology Grants 2011CB910502 and 2012CB911101 (to M.Y.), National Natural Science Foundation Grants 31030020 and 31170679 (to M.Y.) and 31171011 (to Y.L.), and Chinese Academy of Sciences Foundation Grant 2320103112110201 (to Y.L.). I.I. and P.B. are supported by Grant RP100146 from the Cancer Prevention and Research Institute of Texas, Grant I-1420 from the Welch Foundation, and Grant GM61028 and its supplement from the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 3T9N and 3UDC).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1207977109/-/DCSupplemental.
References
- 1.Martinac B, Saimi Y, Kung C. Ion channels in microbes. Physiol Rev. 2008;88(4):1449–1490. doi: 10.1152/physrev.00005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kung C, Martinac B, Sukharev S. Mechanosensitive channels in microbes. Annu Rev Microbiol. 2010;64:313–329. doi: 10.1146/annurev.micro.112408.134106. [DOI] [PubMed] [Google Scholar]
- 3.Kung C. A possible unifying principle for mechanosensation. Nature. 2005;436(7051):647–654. doi: 10.1038/nature03896. [DOI] [PubMed] [Google Scholar]
- 4.Martinac B. Mechanosensitive ion channels: Molecules of mechanotransduction. J Cell Sci. 2004;117(Pt 12):2449–2460. doi: 10.1242/jcs.01232. [DOI] [PubMed] [Google Scholar]
- 5.Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev. 2001;81(2):685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- 6.Arnadóttir J, Chalfie M. Eukaryotic mechanosensitive channels. Annu Rev Biophys. 2010;39:111–137. doi: 10.1146/annurev.biophys.37.032807.125836. [DOI] [PubMed] [Google Scholar]
- 7.Delmas P, Hao J, Rodat-Despoix L. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat Rev Neurosci. 2011;12(3):139–153. doi: 10.1038/nrn2993. [DOI] [PubMed] [Google Scholar]
- 8.Martinac B, Buechner M, Delcour AH, Adler J, Kung C. Pressure-sensitive ion channel in Escherichia coli. Proc Natl Acad Sci USA. 1987;84(8):2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kloda A, Martinac B. Mechanosensitive channels of bacteria and archaea share a common ancestral origin. Eur Biophys J. 2002;31(1):14–25. doi: 10.1007/s002490100160. [DOI] [PubMed] [Google Scholar]
- 10.Bass RB, Strop P, Barclay M, Rees DC. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science. 2002;298(5598):1582–1587. doi: 10.1126/science.1077945. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, et al. The structure of an open form of an E. coli mechanosensitive channel at 3.45 A resolution. Science. 2008;321(5893):1179–1183. doi: 10.1126/science.1159262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perozo E, Rees DC. Structure and mechanism in prokaryotic mechanosensitive channels. Curr Opin Struct Biol. 2003;13(4):432–442. doi: 10.1016/s0959-440x(03)00106-4. [DOI] [PubMed] [Google Scholar]
- 13.Sukharev S. Purification of the small mechanosensitive channel of Escherichia coli (MscS): The subunit structure, conduction, and gating characteristics in liposomes. Biophys J. 2002;83(1):290–298. doi: 10.1016/S0006-3495(02)75169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vásquez V, Sotomayor M, Cordero-Morales J, Schulten K, Perozo E. A structural mechanism for MscS gating in lipid bilayers. Science. 2008;321(5893):1210–1214. doi: 10.1126/science.1159674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koprowski P, Grajkowski W, Isacoff EY, Kubalski A. Genetic screen for potassium leaky small mechanosensitive channels (MscS) in Escherichia coli: Recognition of cytoplasmic β domain as a new gating element. J Biol Chem. 2011;286(1):877–888. doi: 10.1074/jbc.M110.176131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gamini R, Sotomayor M, Chipot C, Schulten K. Cytoplasmic domain filter function in the mechanosensitive channel of small conductance. Biophys J. 2011;101(1):80–89. doi: 10.1016/j.bpj.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Machiyama H, Tatsumi H, Sokabe M. Structural changes in the cytoplasmic domain of the mechanosensitive channel MscS during opening. Biophys J. 2009;97(4):1048–1057. doi: 10.1016/j.bpj.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sukharev SI, Martinac B, Arshavsky VY, Kung C. Two types of mechanosensitive channels in the Escherichia coli cell envelope: Solubilization and functional reconstitution. Biophys J. 1993;65(1):177–183. doi: 10.1016/S0006-3495(93)81044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kloda A, Martinac B. Structural and functional differences between two homologous mechanosensitive channels of Methanococcus jannaschii. EMBO J. 2001;20(8):1888–1896. doi: 10.1093/emboj/20.8.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falke LC, Edwards KL, Pickard BG, Misler S. A stretch-activated anion channel in tobacco protoplasts. FEBS Lett. 1988;237(1-2):141–144. doi: 10.1016/0014-5793(88)80188-1. [DOI] [PubMed] [Google Scholar]
- 21.Qi Z, Kishigami A, Nakagawa Y, Iida H, Sokabe M. A mechanosensitive anion channel in Arabidopsis thaliana mesophyll cells. Plant Cell Physiol. 2004;45(11):1704–1708. doi: 10.1093/pcp/pch194. [DOI] [PubMed] [Google Scholar]
- 22.Blount P, Sukharev SI, Moe PC, Martinac B, Kung C. Mechanosensitive channels of bacteria. Methods Enzymol. 1999;294:458–482. doi: 10.1016/s0076-6879(99)94027-2. [DOI] [PubMed] [Google Scholar]
- 23.Schumann U, et al. YbdG in Escherichia coli is a threshold-setting mechanosensitive channel with MscM activity. Proc Natl Acad Sci USA. 2010;107(28):12664–12669. doi: 10.1073/pnas.1001405107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wotring VE, Miller TS, Weiss DS. Mutations at the GABA receptor selectivity filter: A possible role for effective charges. J Physiol. 2003;548(Pt 2):527–540. doi: 10.1113/jphysiol.2002.032045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keramidas A, Moorhouse AJ, Schofield PR, Barry PH. Ligand-gated ion channels: mechanisms underlying ion selectivity. Prog Biophys Mol Biol. 2004;86(2):161–204. doi: 10.1016/j.pbiomolbio.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Smart OS, Goodfellow JM, Wallace BA. The pore dimensions of gramicidin A. Biophys J. 1993;65(6):2455–2460. doi: 10.1016/S0006-3495(93)81293-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edwards MD, Bartlett W, Booth IR. Pore mutations of the Escherichia coli MscS channel affect desensitization but not ionic preference. Biophys J. 2008;94(8):3003–3013. doi: 10.1529/biophysj.107.123448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choudhary OP, et al. The electrostatics of VDAC: implications for selectivity and gating. J Mol Biol. 2010;396(3):580–592. doi: 10.1016/j.jmb.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volkov AG, Paula S, Deamer DW. Two mechanisms of permeation of small neutral molecules and hydrated ions across phospholipid bilayers. Bioelectrochem Bioenerg. 1997;42(2):153–160. [Google Scholar]
- 30.Hibbs RE, Gouaux E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011;474(7349):54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller S, et al. Domain organization of the MscS mechanosensitive channel of Escherichia coli. EMBO J. 2003;22(1):36–46. doi: 10.1093/emboj/cdg011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koprowski P, Kubalski A. C termini of the Escherichia coli mechanosensitive ion channel (MscS) move apart upon the channel opening. J Biol Chem. 2003;278(13):11237–11245. doi: 10.1074/jbc.M212073200. [DOI] [PubMed] [Google Scholar]
- 33.Liu Z, Gandhi CS, Rees DC. Structure of a tetrameric MscL in an expanded intermediate state. Nature. 2009;461(7260):120–124. doi: 10.1038/nature08277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maurer JA, et al. Confirming the revised C-terminal domain of the MscL crystal structure. Biophys J. 2008;94(12):4662–4667. doi: 10.1529/biophysj.107.127365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iscla I, Wray R, Blount P. An in vivo screen reveals protein-lipid interactions crucial for gating a mechanosensitive channel. FASEB J. 2011;25(2):694–702. doi: 10.1096/fj.10-170878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Moe PC, Chandrasekaran S, Booth IR, Blount P. Ionic regulation of MscK, a mechanosensitive channel from Escherichia coli. EMBO J. 2002;21(20):5323–5330. doi: 10.1093/emboj/cdf537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.