Abstract
Class I isoforms of β-1,3-glucanases (βGLU I) and chitinases (CHN I) are antifungal, vacuolar proteins implicated in plant defense. Tobacco (Nicotiana tabacum L.) βGLU I and CHN I usually exhibit tightly coordinated developmental, hormonal, and pathogenesis-related regulation. Both enzymes are induced in cultured cells and tissues of cultivar Havana 425 tobacco by ethylene and are down-regulated by combinations of the growth hormones auxin and cytokinin. We report a novel pattern of βGLU I and CHN I regulation in cultivar Havana 425 tobacco pith-cell suspensions and cultured leaf explants. Abscisic acid (ABA) at a concentration of 10 μm markedly inhibited the induction of βGLU I but not of CHN I. RNA-blot hybridization and immunoblot analysis showed that only class I isoforms of βGLU and CHN are induced in cell culture and that ABA inhibits steady-state βGLU I mRNA accumulation. Comparable inhibition of β-glucuronidase expression by ABA was observed for cells transformed with a tobacco βGLU I gene promoter/β-glucuronidase reporter gene fusion. Taken together, the results strongly suggest that ABA down-regulates transcription of βGLU I genes. This raises the possibility that some of the ABA effects on plant-defense responses might involve βGLU I.
Plant βGLU (EC 3.2.1.39) and CHN (EC 3.2.1.14) are abundant proteins widely distributed in seed-plant species (for reviews, see Meins et al., 1992; Stone and Clarke, 1992; Simmons, 1994). Both enzymes have been implicated in responses to stress, wounding, and pathogen infection (Thalmair et al., 1996; for reviews, see Boller, 1988; Linthorst, 1991). In addition, βGLU may be involved in diverse physiological and developmental processes, including cell division (Waterkeyn, 1967), microsporogenesis (Worrall et al., 1992; Bucciaglia and Smith, 1994), pollen germination (Roggen and Stanley, 1969), fertilization (Lotan et al., 1989; Ori et al., 1990), and seed germination (Cordero et al., 1994; Vögeli-Lange et al., 1994a; Leubner-Metzger et al., 1995, 1996). There is indirect evidence to suggest that CHN has a role in embryogenesis (De Jong et al., 1992).
βGLU and CHN exist as structural isoforms that differ in size, pI, cellular localization, and pattern of regulation (for review, see Meins et al., 1992). Among them are the class I vacuolar isoforms βGLU I and CHN I, the members of which are very similar in amino acid sequence and show similar patterns of regulation (Sperisen et al., 1991; van Buuren et al., 1992). There is compelling evidence that these isoforms can contribute to the defense of plants against fungal infection. βGLU I and CHN I are induced in response to microbial infection by pathogenic viruses, bacteria, and fungi (for review, see Meins et al., 1992) and can hydrolyze β-1,3-glucans and chitin, respectively, which are major components of the cell walls of pathogenic and potentially pathogenic fungi (for review, see Wessels and Sietsma, 1981). Combinations of βGLU I and CHN I have potent antifungal activity in vitro (Mauch et al., 1988), and expression of CHN I genes alone or in combination with βGLU I genes in transgenic plants can increase resistance to fungus infection (Zhu et al., 1994; Jongedijk et al., 1995). Recent studies with βGLU I-deficient mutants provide evidence that βGLU I may also be important in viral pathogenesis (Beffa et al., 1996).
Regulation of βGLU I and CHN I in tobacco (Nicotiana tabacum L.) is usually tightly coordinated, i.e. the tissue-specific distributions of the enzymes in the plant are very similar (Keefe et al., 1990; Neale et al., 1990); both enzymes are induced in leaves treated with ethylene and ozone or infected with pathogens and both show very similar kinetics of down-regulation by combinations of auxin and cytokinin in culture (Thalmair et al., 1996; for reviews, see Boller, 1988; Linthorst, 1991; Meins et al., 1992). In contrast, during the germination of tobacco seeds, βGLU I, but not CHN I, is induced specifically in the micropylar region of the endosperm where the radicle will penetrate (Vögeli-Lange et al., 1994a; Leubner-Metzger et al., 1995).
The plant hormone ABA modulates the expression of many genes in the course of growth and development, particularly during seed formation and germination and in the response of plants to certain environmental stresses (for review, see Chandler and Robertson, 1994). We have shown previously that ABA treatment of tobacco seeds inhibited induction of βGLU I in the micropylar endosperm and greatly delayed endosperm rupture during germination (Leubner-Metzger et al., 1995). This prompted us to explore the possibility that down-regulation of βGLU I by ABA is a more general feature of tobacco cells. The present study shows that physiological concentrations of ABA down-regulate βGLU I at the level of transcription in cultured tobacco pith cells and leaf explants but have little or no effect on the expression of CHN I. This novel pattern of regulation provides a cell-culture system amenable for studying ABA signal transduction and raises the possibility that ABA might affect some plant defense responses by down-regulating βGLU I.
MATERIALS AND METHODS
Tobacco (Nicotiana tabacum L. cv Havana 425) and cultured material from this cultivar were used. Plants were grown in a greenhouse or raised axenically from surface-sterilized seeds (Felix and Meins, 1986). The transformants Glb-GUS and 35S-GUS were described previously (Hart et al.,1993; Vögeli-Lange et al., 1994a). Glb-GUS is homozygous for a chimeric GUS gene (GUS) consisting of the 1.6-kb promoter region of the tobacco βGLU I-B gene (Glb) fused immediately downstream of the start of transcription to the GUS-reporter gene. 35S-GUS is a similar transformant with the Glb promoter replaced by the cauliflower mosaic virus 35S RNA promoter. S275N is a suspension-cultured line obtained from cloned pith parenchyma and was cultured as described previously (Felix and Meins, 1987).
Suspension cultures of Glb-GUS and 35S-GUS were obtained from leaves of axenically grown plants 10 weeks after germination. Discs of leaf tissue 8 mm in diameter were cultured for 21 d as described by Meins and Lutz (1980). The basal medium contained salts, Suc, myo-inositol, and thiamine concentrations recommended by Linsmaier and Skoog (1965), 5.0 mg L−1 chlorophenol red (Kodak) as a pH indicator, and 10 g L−1 purified agar. Callus pieces 5 mm in diameter were subcultured twice at 21-d intervals on basal LS medium supplemented with 11 μm NAA and 1.4 μm kinetin. Cell-suspension lines were started from the callus and maintained as described by Meins and Binns (1977). The medium used was S3 (basal LS medium without agar, containing 40 g L−1 Suc, 11 μm NAA, and 1.4 μm kinetin). Shake cultures (125 rpm) were subcultured every 14 d by transferring 2 mL of the culture into 40 mL of fresh medium. Suspension-cultured lines were first used after 6 months of subculturing. All tissues and cell suspensions were cultured at 24 ± 1°C in continuous light (80 μE m−2 s−1).
ABA Treatments
ABA (Sigma) was added to cultures as a filter-sterilized 10−3 m stock solution in 12 mm KPi, pH 6.0. Equal volumes of the same buffer were added to control cultures. For experiments with cells incubated in NAA- and kinetin-containing medium, cells were subcultured in fresh S3 medium for 4 d and then ABA was added. For experiments with cells incubated in the absence of NAA and kinetin, cell suspensions were filtered through a 500-μm sieve 8 d after subculture in S3 medium. The cells were then washed with 80 mL of S0 medium (S3 medium without NAA and kinetin), 4 g of the cells was transferred to S0 medium, and ABA was added. For experiments with explants of leaves, 8-mm-diameter discs of surface-sterilized leaves were cultured individually for 4 d in 1 mL of S0 in multiwell plates (Falcon 3047, Becton Dickinson).
Preparation of Protein Extracts
Cells from 1- to 5-mL aliquots of suspensions were collected by filtration on paper (MN 713, Whatman) under gentle vacuum. Leaf discs were dried between two layers of filter paper (3MM, Whatman). The samples were then weighed, frozen on dry ice, and stored at −70°C. For βGLU and CHN assays, extracts of the frozen material were prepared in 2 volumes of an extraction buffer containing 0.1 mm EDTA, 1 mm DTT, 1 mm PMSF, and 200 mm Tris-HCl, pH 8.0, as described previously (Felix and Meins, 1986). Samples assayed for GUS activity were extracted in the same way using the buffer recommended by Jefferson et al. (1987).
Assays of Proteins
CHN activity and endo-type βGLU activity were measured radiometrically, with [3H]chitin and the algal β-1,3-glucan [3H]laminarin reduced with NaBH3 as the substrates (Boller et al., 1983; Keefe et al., 1990). Activity is expressed in microgram equivalents of tobacco CHN I and βGLU I. CHN I and βGLU I protein was measured by ELISA with antibodies specific for the class I isoforms of the tobacco enzymes (Keefe et al., 1990; Sticher et al., 1992). Immunoblot analyses with antibodies directed against tobacco βGLU I , βGLU II, CHN I, and CHN II were as described previously (Beffa et al., 1993).
GUS activity was measured by a modification of the fluorometric method of Jefferson et al. (1987) described by Vögeli-Lange et al. (1994b) and is expressed as picomoles of 4-methylumbelliferol produced per minute. Protein concentration was measured by the Bradford (1976) method with bovine γ-globulin as the standard.
RNA-Blot Hybridization
Total RNA was isolated from cells by the method of Nagy et al. (1988). RNA was fractionated by electrophoresis in a formaldehyde gel containing 1.2% (w/v) agarose and transferred onto a Hybond nylon membrane (Amersham) by standard methods (Sambrook et al., 1989). RNA was bound to the dried membranes by UV cross-linking (0.5 J m−2). Hybridization was done with digoxigenin-labeled probes as described by the manufacturer (Boehringer Mannheim). The PstI inserts of the tobacco βGLU I cDNA clone pGL43 (Mohnen et al., 1985) and the tobacco CHN I cDNA clone pCHN50 (Shinshi et al., 1987) were used to prepare hybridization probes. The probes were labeled by PCR using a digoxigenin DNA-labeling mixture (no. 1277065, Boehringer Mannheim). The primers used were 5′-CCGTTTCCAAAAGACCTCTGG-3′ and 5′-GTTGGTGTGGTAACACCAATG-3′ for CHN I and 5′-GGAAGACTGAGGCTTTATGATC-3′ and 5′-CTGAACTG TCCCAAACACCAC-3′ for βGLU I. The PCRs were carried out in a reaction mixture containing 7.5 units of Taq-polymerase, 10 μL of 10× Taq-polymerase incubation buffer (Boehringer Mannheim), 600 ng of each nucleotide, and 2 ng of DNA template adjusted to a final volume of 100 μL with water. The reaction cycle started with a prewarming of 5 min at 94°C, followed by 30 cycles consisting of 1 min at 94°C, 1.5 min at 58°C, 3 min at 72°C, and 15 min at 72°C. The final probe concentration in the hybridization solution was 25 ng/mL.
RESULTS
ABA Inhibits Accumulation of βGLU I in Cell Suspensions
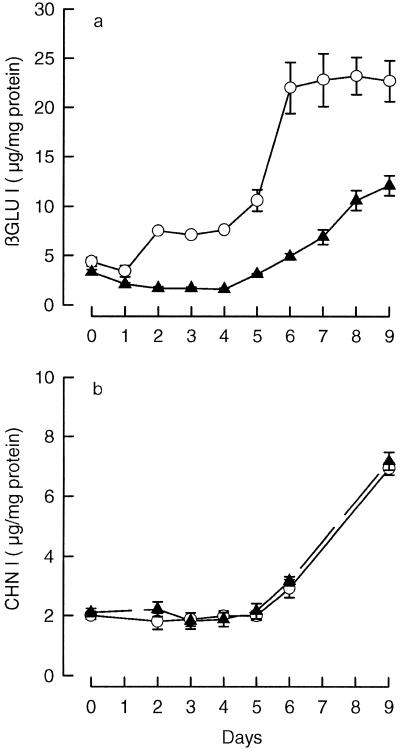
In our initial experiments we examined the effects of ABA on the accumulation of βGLU I and CHN I in suspensions of tobacco pith cells. S275N cells were subcultured on the standard auxin- and cytokinin-containing medium used for serial propagation of this cell line. After 4 d of preculture, 10 μm ABA was added to one set of cultures. The time course of βGLU I- and CHN I-antigen accumulation in control and ABA-treated cultures was measured by an ELISA assay using antibodies specific for class I isoforms (Keefe et al., 1990). The results are presented as micrograms of antigen per milligram of protein.
Figure 1 shows that the content of both βGLU I and CHN I in control cultures increased with time, i.e. by approximately 4- to 5-fold by d 9 when the experiment was ended. Treatment with ABA markedly inhibited the accumulation of βGLU I antigen by up to approximately 80% but had no detectable effect on the accumulation of CHN I antigen. In the concentration range tested, 10−8 to 10−5 m, inhibition of βGLU I accumulation depended on ABA dose. The concentration of ABA giving a 50% inhibition of accumulation was about 7 μm (data not shown). Similar results were obtained when the data were expressed on a fresh weight basis and when endo-type βGLU and CHN activities were measured (data not shown). Control and ABA-treated cultures grew at similar rates and did not substantially differ in protein content (data not shown). These findings and the fact that CHN I accumulation was not affected by ABA treatment indicate that the inhibition of βGLU I accumulation by ABA is not a general inhibitory effect of the hormone.
Figure 1.
The effect of ABA on the time course of βGLU I and CHN I accumulation in S275N suspension cultures. The content of βGLU I (a) and CHN I (b) antigens of cells cultured in standard S3 medium containing auxin and cytokinin with (▴) and without (○) 10 μm ABA added on d 0 after 4 d of preculture. The values presented are the mean antigen contents ± se of six to eight replicate cultures.
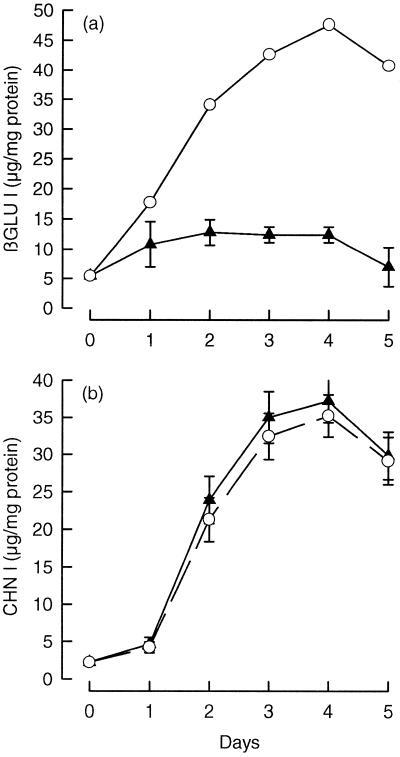
βGLU I and CHN I are strongly induced when tobacco callus tissues are subcultured on medium without auxin and cytokinin added (Felix and Meins, 1986; Shinshi et al., 1987). To determine whether ABA is effective in the absence of added auxin and cytokinin, we measured the time course of βGLU I and CHN I accumulation in S275N cells cultured in auxin- and cytokinin-free medium. Figure 2 shows that in control cultures the content of βGLU I and CHN I antigen increased by up to approximately 10-fold after 4 d. Treatment with 10 μm ABA markedly inhibited the induction of βGLU I but had no significant effect on CHN I induction. These results, which were confirmed when the data were expressed on a fresh weight basis and by measurements of enzyme activity (data not shown), indicate that added auxin and cytokinin are not required for the inhibition of βGLU I induction by ABA.
Figure 2.
The effect of ABA on the time course of βGLU I and CHN I accumulation in S275N suspensions cultured without added auxin or cytokinin. The content of βGLU I (a) and CHN I (b) antigens of cells cultured in auxin- and cytokinin-free S0 medium with (▴) and without (○) 10 μm ABA added on d 0 after 8 d of preculture. The values presented are the mean antigen contents ± se of six to eight replicate cultures.
Immunoblot Analysis and RNA-Blot Hybridization
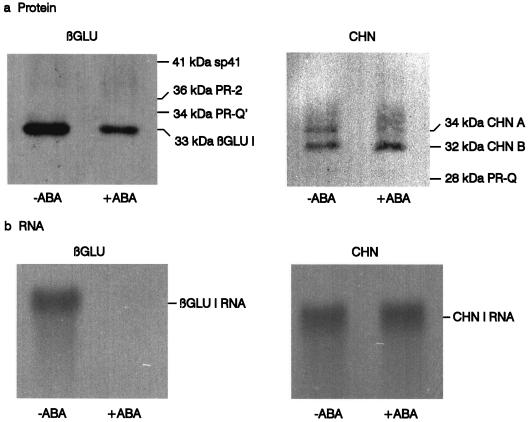
The ELISA assays used for the time-course studies are specific for βGLU I and CHN I. Immunoblot analyses were performed to determine whether additional, structurally related classes of the enzymes are expressed and regulated by ABA in suspension cultures. S275N cells were grown in auxin- and cytokinin-containing medium with and without the addition of 10 μm ABA. Immunoblots of extracts were probed with antibodies directed against tobacco βGLU I shown to cross-react with all of the known βGLU II and βGLU III (Beffa et al., 1993). βGLU II includes the approximately 36-kD proteins PR-2, PR-N, and PR-O (Kauffmann et al., 1987; Payne et al., 1990b; Ward et al., 1991) and the approximately 41-kD stylar glycoproteins Sp41a and Sp41b (Ori et al., 1990); βGLU III includes the approximately 34-kD protein PR-Q' (Payne et al., 1990b). The immunoblots were also probed with a mixture of antibodies specific for the approximately 32- and 34-kD tobacco CHN I (Shinshi et al., 1987) and the approximately 28-kD tobacco CHN II (Payne et al., 1990a).
A time course for the appearance of immunoreactive material was recorded. Figure 3a shows representative results obtained on d 6 when the inhibition of βGLU I by ABA was maximal. Immunoblots of extracts prepared from control and ABA-treated cells probed for βGLU antigens gave a signal corresponding to the 33-kD βGLU I. No signals were detected at the approximately 41-kD position or in the 34- to 36-kD region, indicating that there was no appreciable expression of βGLU II and βGLU III under the conditions tested. Similarly, only 32- and 34-kD CHN I (van Buuren et al., 1992) were detected; no signals were detected for the approximately 28-kD CHN II isoforms. Thus, only βGLU I and CHN I were detectable in cell suspensions under the conditions tested.
Figure 3.
Immunoblot analysis of βGLU and CHN protein (a) and hybridization of βGLU I and CHN I mRNAs (b) in S275N cells. Protein extracts and total RNA were prepared from cells harvested 6 d after treatment with (+ABA) and without (−ABA) 10 μm ABA. Cells were precultured for 4 d in auxin- and cytokinin-containing S3 medium. Immunoblots were stained with antibodies recognizing all known tobacco class I to III βGLU and CHN I and II isoforms. Equal amounts of protein (2 μg) were applied to each lane. The positions of βGLU I, PR-2 and sp41 (βGLU II), PR-Q' (βGLU III), CHN I-A and CHN I-B, and PR-Q (CHN II) are indicated. RNA blots with equal amounts of total RNA (11 μg) in each lane were hybridized with probes specific for βGLU I and CHN I. Note that the small differences between the +ABA and −ABA treatments apparent on the CHN immunoblot are not consistently observed.
RNA blots of total RNA prepared from S275N cells cultured in auxin- and cytokinin-containing medium with and without ABA were hybridized with probes specific for βGLU I and CHN I transcripts (Shinshi et al., 1987). Figure 3b shows that ABA treatment markedly lowered the content of βGLU I mRNA but did not appreciably affect the content of CHN I mRNA. The down-regulation of βGLU I protein (Fig. 3a) was less pronounced than the down-regulation of βGLU I mRNA. This probably reflects the low turnover rate of βGLU I protein in suspension-cultured cells (Sperisen, 1993). We conclude that ABA acts at least in part at the steady-state mRNA level to inhibit βGLU I expression in cell suspensions.
GUS-Reporter Gene Studies
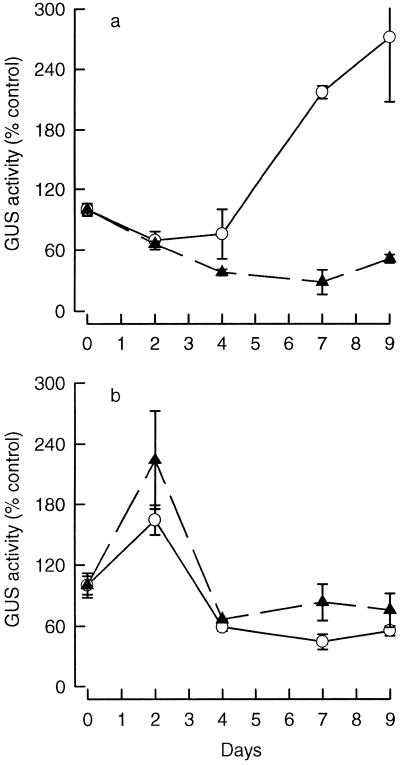
More detailed studies of βGLU I regulation were made with cell suspensions and cultured leaf explants of a Glb-GUS transformant carrying a chimeric Glb-promoter/GUS-reporter gene. The 1.6-kb 5′ sequence of Glb used in the construct had been shown earlier to contain the elements needed for organ-specific expression, induction by ethylene and TMV infection, and down-regulation in cultured tissues by auxin and cytokinin (Vögeli-Lange et al., 1994b). A 35S-GUS transformant carrying the GUS-reporter gene and driven by a 528-bp cauliflower mosaic virus 35S RNA promoter was used as a positive control (Hart et al., 1993). Cell suspensions were established from Glb-GUS and 35S-GUS leaf tissues and were precultured for 4 d in medium containing auxin and cytokinin. ABA (10 μm) was added to the suspensions and GUS activity was measured in extracts prepared at the times indicated. The data are expressed as percentages of the GUS activity found in cultures on d 0 (before ABA was added).
Figure 4a shows that ABA treatment markedly inhibited accumulation of GUS activity in cultures of the Glb-GUS transformant. The 35S-GUS cultures accumulated GUS activity at a maximum on d 2; however, the kinetics and magnitude of accumulation were essentially unaffected by ABA treatment (Fig. 4b). Taken together, these results indicate that ABA treatment inhibited Glb-promoter activity in cell suspensions.
Figure 4.
The effect of ABA treatment on GUS expression in suspensions of Glb-GUS (a) and 35S-GUS (b) cells. Cells were precultured for 4 d in auxin- and cytokinin-containing S3 medium with (▴) and without (○) 10 μm ABA added on d 0. The values presented are the mean percentages ± se of GUS activity relative to the cultures on d 0 for three to four replicates. The values obtained for the cultures on d 0, expressed in femtokatals of GUS per milligram of protein were: Glb-GUS cells without ABA, 118 ± 5.4; Glb-GUS cells with ABA, 184 ± 11.8; 35S-GUS cells without ABA, 45.9 ± 5.5; and 35S-GUS cells with ABA 19.1 ± 1.71.
We also investigated the effects of auxin, cytokinin, and ABA on βGLU, CHN, and GUS activity in discs cultured from Glb-GUS leaves. The results shown in Table I confirm the basic pattern of regulation of βGLU I and CHN I by auxin and cytokinin reported previously (Shinshi et al., 1987; Vögeli-Lange et al., 1994b): the activity of both βGLU and CHN was high in auxin- and cytokinin-free medium, reduced in medium containing auxin or cytokinin alone, and markedly reduced in medium containing both hormones. The important point is that ABA effectively inhibited the induction of βGLU I but did not affect induction of CHN I in the presence of different combinations of auxin and cytokinin. The inhibition of βGLU activity by ABA was usually appreciably larger than the inhibition of GUS activity. This probably reflects differences in the stability of the two mRNAs or proteins.
Table I.
Regulation of βGLU, CHN, and βGLU-promoter activity by auxin, cytokinin, and ABA in cultured leaf discs
| Culture Mediuma | βGLU
|
CHN
|
GUS
|
|||
|---|---|---|---|---|---|---|
| Control | ABAb | Control | ABA | Control | ABA | |
| μg equivalents g−1 fresh wtc | pmol methylumbelliferol min−1g−1fresh wt | |||||
| −Auxin, −Cytokinin | 95.9 ± 5.4d | 8.4 ± 3.2 | 115 ± 10.7 | 123 ± 11.4 | 58.9 ± 7.4 | 7.1 ± 2.8 |
| +Auxin, −Cytokinin | 56.4 ± 6.1 | 0.7 ± 0.3 | 62.0 ± 7.4 | 67.6 ± 4.2 | 34.6 ± 5.8 | 3.8 ± 2.1 |
| −Auxin, +Cytokinin | 47.6 ± 5.4 | 2.1 ± 1.4 | 59.7 ± 6.2 | 62.3 ± 7.1 | 24.7 ± 6.4 | 4.2 ± 3.7 |
| +Auxin, +Cytokinin | 10.5 ± 3.2 | 0.2 ± 0.1 | 12.4 ± 2.8 | 10.1 ± 3.4 | 7.1 ± 2.4 | 0.5 ± 0.5 |
Discs explanted from leaves of the Glb-GUS transformant were incubated for 4 d on S0 medium supplemented as indicated with auxin (11 μm NAA) and cytokinin (1.4 μm kinetin).
10 μm final concentration.
Enzyme activity is expressed as microgram equivalents of the class I isoforms.
Mean values ± se for 12 replicate samples.
DISCUSSION
Little is known about the effects of ABA on βGLU and CHN expression. In an early report, before the different classes of endo-type βGLU were recognized, Moore and Stone (1972) showed that treatment with 190 μm ABA increased the rate of senescence and decreased the βGLU activity in leaf discs of Nicotiana glutinosa incubated for 4 d in water. The specificity of this effect is unclear, since over the same time interval, ABA treatment resulted in an approximately 5-fold decrease in total protein content. More recently, Leubner-Metzger et al. (1995) showed that treatment of tobacco seeds with ABA, which is known to induce and maintain seed dormancy (for review, see Bewley and Black, 1994), delays endosperm rupture and both delays and inhibits the highly localized induction of βGLU I in the micropylar endosperm. ABA has also been found to up-regulate expression of a CHN II gene in the wild tomato Lycopersicon chilense (Chen et al., 1994).
In most studies tobacco βGLU I and CHN I were found to be coordinately regulated. For example, they show similar patterns of expression in response to auxins, cytokinins, and ethylene (for review, see Meins et al., 1992), ozone (Thalmair et al., 1996) and infection by microbial pathogens (for reviews, see Linthorst, 1991; Meins et al., 1992). Our most important finding was that ABA down-regulates βGLU I expression but does not appreciably affect CHN I expression in suspension cultures and leaf explants of tobacco. ABA treatment of cells in suspension markedly inhibited the accumulation of βGLU activity measured using a substrate specific for endo-type hydrolysis and βGLU I protein but not the accumulation of CHN activity and CHN I protein. The similar effects of ABA on the accumulation of βGLU I protein and mRNA and on the activity of the Glb promoter strongly suggest that ABA transcriptionally down-regulates βGLU I expression.
The promoter of genes encoding high-pI α-amylases in barley contains a negative-acting TAACAAA box important for down-regulation of endosperm expression by ABA (Gubler and Jacobsen, 1992). Both cloned members of the small tobacco βGLU I family, gla and glb, have distal and proximal copies of this box in their promoters (Leubner-Metzger et al., 1995). The CHN I isoforms in tobacco are encoded by the genes CHN48 and CHN50 (van Buuren et al., 1992). The TAACAAA box is not present in the CHN48 promoter and only a single, inverted copy is present in the CHN50 promoter. This suggests that the differential effect of ABA on βGLU I and CHN I expression could be due to the absence of this ABA-responsive element in the CHN I genes.
The signaling pathways important for hormonal regulation of βGLU I have not been elucidated. Ethylene is required for the induction of βGLU I expression in cultured tobacco cells (Felix and Meins, 1987). High levels of ethylene-dependent transcription of βGLU I genes requires a cis-acting, ethylene-responsive element that binds ethylene-responsive binding proteins in nuclear extracts (Ohme-Takagi and Shinshi, 1995). Different regions of the Glb promoter are important for high-level, ethylene-dependent expression and for the down-regulation of expression by auxin and cytokinins (Vögeli-Lange et al., 1994b). We found that inhibition of βGLU I expression by ABA was not appreciably affected by combinations of auxin and cytokinin that down-regulate expression of βGLU I and CHN I. Taken together, these results suggest as a working hypothesis that ethylene, combinations of auxin and cytokinin, and ABA act by independent signaling pathways, possibly with different responsive elements in the βGLU I promoters as targets.
We suggest that ABA down-regulation of βGLU I could play a role in pathogenesis. A marked decrease in local ABA concentration occurs early in the incompatible reaction but not in the compatible reaction of soybean infected with the fungal pathogen Phytophthora megasperma (Cahill and Ward, 1989). Treatment of potato plants with ABA prior to infection with the fungi Phytophthora infestans or Cladosporium cucumberium suppresses the accumulation of phytoalexins and decreases significantly the resistance of the plants (Henfling et al., 1980). Similarly, treatment of tobacco plants with ABA increases the susceptibility of the plants to Peronospora tabacina (Salt et al., 1986). Therefore, ABA appears to increase the susceptibility of plants to fungus infection. Combinations of βGLU I and CHN I have potent antifungal activity in vitro (Mauch et al., 1988). Moreover, constitutive coexpression of βGLU I and CHN I genes has been shown to increase resistance to fungal infection in transgenic plants of several species (Zhu et al., 1994; Jongedijk et al., 1995; Masoud et al., 1996). Therefore, it is plausible that ABA could increase fungus susceptibility by down-regulating the induction of antifungal βGLU I.
There is also indirect evidence linking ABA and βGLU I with viral pathogenesis. Although the ABA content of leaves infected with TMV has not been measured, pretreatment with ABA significantly increases the resistance to TMV infection in tobacco plants showing the local-lesion response (Fraser et al., 1979; Fraser, 1982). Tobacco mutants deficient in βGLU I generated by antisense transformation also show increased resistance to TMV infection, which is inversely proportional to βGLU I content in a graded series of independent transformants (Beffa et al., 1996). Deposition of callose, which is a substrate for βGLU and is thought to act as a physical barrier to virus spread, was also increased in these deficient mutants. It has been suggested that βGLU I is required for callose degradation and, therefore, that decreased susceptibility to virus might result from increased callose deposition in response to infection. Deposition of callose is important for the regulation of molecular traffic through plasmodesmata (Lucas and Wolf, 1993). Our results raise the intriguing possibility that ABA down-regulation of βGLU I might also play a role in regulating cell-to-cell communication via plasmodesmata.
ACKNOWLEDGMENTS
We thank our colleagues Thomas Boller and Gerd Leubner-Metzger for their critical comments.
Abbreviations:
- ABA
cis-S(+)-ABA
- βGLU
β-1,3-glucanase
- βGLU I (or II or III)
class I (or II or III) β-1,3-glucanase
- CHN
chitinase
- CHN I (or II or III)
class I (or II or III) chitinase
- LS
Linsmaier and Skoog
- TMV
tobacco mosaic virus
Footnotes
This work was partially supported by the Swiss National Science Foundation (grant nos. 31-40883.94 and 5002-39818; Swiss Priority Program Biotechnology) and by the Foundation Herbette (E.R. and R.B.).
LITERATURE CITED
- Beffa RS, Hofer R-M, Thomas M, Meins F., Jr Decreased susceptibility to virus disease of β-1,3-glucanase-deficient plants generated by antisense transformation. Plant Cell. 1996;8:1001–1011. doi: 10.1105/tpc.8.6.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffa RS, Neuhaus J-M, Meins F., Jr Physiological compensation in antisense transformants: specific induction of an “ersatz” glucan endo-1,3-β-glucosidase in plants infected with necrotizing viruses. Proc Natl Acad Sci USA. 1993;90:8792–8796. doi: 10.1073/pnas.90.19.8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD, Black M (1994) Seed—Physiology of Development and Germination. Plenum Press, New York
- Boller T. Ethylene and the regulation of antifungal hydrolases in plants. Oxf Surv Plant Mol Cell Biol. 1988;5:145–174. [Google Scholar]
- Boller T, Gehri A, Mauch F, Vögeli U. Chitinase in bean leaves: induction by ethylene, purification, properties, and possible function. Planta. 1983;157:23–31. doi: 10.1007/BF00394536. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bucciaglia PA, Smith AG. Cloning and characterization of Tag1, a tobacco anther β-1,3-glucanase expressed during tetrad dissolution. Plant Mol Biol. 1994;24:903–914. doi: 10.1007/BF00014444. [DOI] [PubMed] [Google Scholar]
- Cahill DM, Ward EWB. Rapid localized changes in abscisic acid concentrations in soybean in interactions with Phytophthora megasperma f. sp. glycinea or after treatment with elicitors. Physiol Mol Plant Pathol. 1989;35:483–493. [Google Scholar]
- Chandler PM, Robertson M. Gene expression regulated by abscisic acid and its relation to stress tolerance. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:113–141. [Google Scholar]
- Chen R-D, Yu L-X, Greer AF, Cheriti H, Tabaeizadeh Z. Isolation of an osmotic stress- and abscisic acid-induced gene encoding an acidic endochitinase from Lycopersicon chilense. Mol Gen Genet. 1994;245:195–202. doi: 10.1007/BF00283267. [DOI] [PubMed] [Google Scholar]
- Cordero MJ, Raventos D, Segundo BS. Differential expression and induction of chitinase and β-1,3-glucanases in response to fungal infection during germination of maize seeds. Mol Plant-Microbe Interact. 1994;7:23–31. [Google Scholar]
- De Jong AJ, Cordewener J, Lo Schiavo F, Terzi M, Vandekerckhove J, van Kammen A, De Vries SC. A carrot somatic embryo mutant is rescued by chitinase. Plant Cell. 1992;4:425–433. doi: 10.1105/tpc.4.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Meins F., Jr Developmental and hormonal regulation of β-1,3-glucanase in tobacco. Planta. 1986;167:206–211. doi: 10.1007/BF00391416. [DOI] [PubMed] [Google Scholar]
- Felix G, Meins F., Jr Ethylene regulation of β-1,3-glucanase in tobacco. Planta. 1987;172:386–392. doi: 10.1007/BF00398668. [DOI] [PubMed] [Google Scholar]
- Fraser RSS. Are ‘pathogenesis-related’ proteins involved in acquired systemic resistance of tobacco plants to tobacco mosaic virus? J Gen Virol. 1982;58:305–313. [Google Scholar]
- Fraser RSS, Loughlin SAR, Whenham RJ. Acquired systemic susceptibility to infection by tobacco mosaic virus in Nicotiana glutinosa L. J Gen Virol. 1979;43:131–141. [Google Scholar]
- Gubler F, Jacobsen JV. Gibberellin-responsive elements in the promoter of a barley high-pI α-amylase gene. Plant Cell. 1992;4:1435–1441. doi: 10.1105/tpc.4.11.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CM, Nagy F, Meins F., Jr A 61-bp enhancer element of the tobacco β-1,3-glucanase B gene interacts with a regulated nuclear protein(s) Plant Mol Biol. 1993;21:121–131. doi: 10.1007/BF00039623. [DOI] [PubMed] [Google Scholar]
- Henfling JWDM, Bostock R, Kuc J. Effect of abscisic acid on rishitin and lubimin accumulation and resistance to Phytophthorainfestans and Cladosporiumcucumerinum in potato tuber tissue slices. Phytopathology. 1980;70:1074–1078. [Google Scholar]
- Jefferson RA, Kavanah TA, Bevan MW. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongedijk E, Tigelaar H, van Roekel JSC, Bres-Vloemans SA, Dekker I, van den Elzen PJM, Cornelissen BJC, Melchers LS. Synergetic activity of chitinases and β-1,3-glucanases enhances fungal resistance in transgenic tomato plants. Euphytica. 1995;85:173–180. [Google Scholar]
- Kauffmann S, Legrand M, Geoffroy P, Fritig B. Biological function of ‘pathogenesis-related’ proteins: four PR proteins of tobacco have β-1,3-glucanase activity. EMBO J. 1987;6:3209–3212. doi: 10.1002/j.1460-2075.1987.tb02637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe D, Hinz U, Meins F., Jr The effect of ethylene on the cell-type-specific and intracellular localization of β-1,3-glucanase and chitinase in tobacco leaves. Planta. 1990;182:43–51. doi: 10.1007/BF00239982. [DOI] [PubMed] [Google Scholar]
- Leubner-Metzger G, Fründt C, Meins F., Jr Effects of gibberellins, darkness and osmotica on endosperm rupture and class I β-1,3-glucanase induction in tobacco seed germination. Planta. 1996;199:282–288. [Google Scholar]
- Leubner-Metzger G, Fründt C, Vögeli-Lange R, Meins F., Jr Class I β-1,3-glucanases in the endosperm of tobacco during germination. Plant Physiol. 1995;109:751–759. doi: 10.1104/pp.109.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsmaier EM, Skoog F. Organic growth factor requirements of tobacco tissue cultures. Physiol Plant. 1965;18:100–127. [Google Scholar]
- Linthorst HJM. Pathogenesis-related proteins of plants. Crit Rev Plant Sci. 1991;10:123–150. [Google Scholar]
- Lotan T, Ori N, Fluhr R. Pathogenesis-related proteins are developmentally regulated in tobacco flowers. Plant Cell. 1989;1:881–887. doi: 10.1105/tpc.1.9.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas WJ, Wolf S. Plasmodesmata: the intercellular organelles of green plants. Trends Cell Biol. 1993;3:308–315. doi: 10.1016/0962-8924(93)90013-q. [DOI] [PubMed] [Google Scholar]
- Masoud SA, Zhu Q, Lamb C, Dixon RA. Constitutive expression of an inducible β-1,3-glucanase in alfalfa reduces disease severity caused by the oomycete pathogen Phytophthora megasperma f. sp medicaginis, but does not reduce disease severity of chitin-containing fungi. Transgenic Res. 1996;5:313–323. [Google Scholar]
- Mauch F, Mauch-Mani B, Boller T. Antifungal hydrolases in pea tissue. II. Inhibition of fungal growth by combinations of chitinase and β-1,3-glucanase. Plant Physiol. 1988;88:936–942. doi: 10.1104/pp.88.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meins F, Jr, Binns AN. Epigenetic variation of cultured somatic cells: evidence for gradual changes in the requirement for factors promoting cell division. Proc Natl Acad Sci USA. 1977;74:2928–2932. doi: 10.1073/pnas.74.7.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meins F, Jr, Lutz J. The induction of cytokinin habituation in primary pith explants of tobacco. Planta. 1980;149:402–407. doi: 10.1007/BF00571176. [DOI] [PubMed] [Google Scholar]
- Meins F Jr, Neuhaus J-M, Sperisen C, Ryals J (1992) The primary structure of plant pathogenesis-related glucanohydrolases and their genes. In T Boller, F Meins Jr, eds, Genes Involved in Plant Defense. Springer Verlag, Vienna, Austria, pp 245–282
- Mohnen D, Shinshi H, Felix G, Meins F., Jr Hormonal regulation of β-1,3-glucanase messenger RNA levels in cultured tobacco tissues. EMBO J. 1985;4:1631–1635. doi: 10.1002/j.1460-2075.1985.tb03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AE, Stone BA. Effect of senescence and hormone treatment on the activity of a β-1,3-glucan hydrolase in Nicotiana glutinosa leaves. Planta. 1972;104:93–109. doi: 10.1007/BF00386986. [DOI] [PubMed] [Google Scholar]
- Nagy F, Kay SA, Chua N-H. Analysis of gene expression in transgenic plants. In: Gelvin SB, Schilperoot RA, editors. Plant Molecular Biology Manual. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1988. p. B4. : 1–29. [Google Scholar]
- Neale AD, Wahleithner JA, Lund M, Bonnett HT, Kelly A, Meeks Wagner DR, Peacock WJ, Dennis ES. Chitinase, β-1,3-glucanase, osmotin, and extensin are expressed in tobacco explants during flower formation. Plant Cell. 1990;2:673–684. doi: 10.1105/tpc.2.7.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohme-Takagi M, Shinshi H. Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell. 1995;7:173–182. doi: 10.1105/tpc.7.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori N, Sessa G, Lotan T, Himmelhoch S, Fluhr R. A major stylar matrix polypeptide (sp41) is a member of the pathogenesis-related protein superclass. EMBO J. 1990;9:3429–3436. doi: 10.1002/j.1460-2075.1990.tb07550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne G, Ahl P, Moyer M, Harper A, Beck J, Meins F, Jr, Ryals J. Isolation of complementary DNA clones encoding pathogenesis-related proteins P and Q, two acidic chitinases from tobacco. Proc Natl Acad Sci USA. 1990a;87:98–102. doi: 10.1073/pnas.87.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne G, Ward E, Gaffney T, Ahl-Goy P, Moyer M, Harper A, Meins F, Jr, Ryals J. Evidence for a third structural class of β-1,3-glucanase in tobacco. Plant Mol Biol. 1990b;15:797–808. doi: 10.1007/BF00039420. [DOI] [PubMed] [Google Scholar]
- Roggen HP, Stanley RG. Cell wall hydrolysing enzymes in wall formation as measured by pollen-tube extension. Planta. 1969;84:295–303. doi: 10.1007/BF00396421. [DOI] [PubMed] [Google Scholar]
- Salt SD, Tuzum S, Kuc J. Effects of β-ionone and abscisic acid on the growth of tobacco and resistance to blue mold. Mimicry of effects of stem infection by Peronospora tabacina Adam. Physiol Mol Plant Pathol. 1986;28:287–297. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning. A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Shinshi H, Mohnen D, Meins F., Jr Regulation of plant pathogenesis-related enzyme: inhibition of chitinase and chitinase mRNA accumulation in cultured tobacco tissues by auxin and cytokinin. Proc Natl Acad Sci USA. 1987;64:89–93. doi: 10.1073/pnas.84.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons CR. The physiology and molecular biology of plant 1,3-β-d-glucanases and 1,3;1,4-β-d-glucanases. Crit Rev Plant Sci. 1994;13:325–387. [Google Scholar]
- Sperisen C (1993) Structure, evolution, and regulation of β-1,3-glucanase genes of tobacco. PhD dissertation. Swiss Federal Institute of Technology, Zürich, Switzerland
- Sperisen C, Ryals J, Meins F., Jr Comparison of cloned genes provides evidence for intergenomic exchange of DNA in the evolution of a tobacco glucan endo-1,3-β-glucosidase gene family. Proc Natl Acad Sci USA. 1991;88:1820–1824. doi: 10.1073/pnas.88.5.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sticher L, Hinz U, Meyer AD, Meins F., Jr Intracellular transport and processing of a tobacco vacuolar β-1,3-glucanase. Plant. 1992;188:559–565. doi: 10.1007/BF00197049. [DOI] [PubMed] [Google Scholar]
- Stone BA, Clarke AE. Chemistry and Biology of (1→3)-β-Glucans. La Trobe. Victoria, Australia: University Press; 1992. [Google Scholar]
- Thalmair M, Bauw G, Thiel S, Döhring T, Langebartels C, Sandermann H., Jr Ozone and ultraviolet B effects on the defense-related proteins β-1,3-glucanase and chitinase in tobacco. J Plant Physiol. 1996;148:222–228. [Google Scholar]
- van Buuren M, Neuhaus J-M, Shinshi H, Ryals J, Meins F., Jr The structure and regulation of homeologous tobacco endochitinase genes of Nicotiana sylvestris and N. tomentosiformis origin. Mol Gen Genet. 1992;232:460–469. doi: 10.1007/BF00266251. [DOI] [PubMed] [Google Scholar]
- Vögeli-Lange R, Fründt C, Hart CM, Beffa R, Nagy F, Meins F., Jr Evidence for a role of β-1,3-glucanase in dicot seed germination. Plant J. 1994a;5:273–278. [Google Scholar]
- Vögeli-Lange R, Fründt C, Hart CM, Nagy F, Meins F., Jr Developmental, hormonal, and pathogenesis-related regulation of the tobacco class I β-1,3-glucanase B promoter. Plant Mol Biol. 1994b;25:299–311. doi: 10.1007/BF00023245. [DOI] [PubMed] [Google Scholar]
- Ward E, Payne GB, Moyer MB, Williams SC, Dincher SS, Sharkey KC, Beck JJ, Taylor HT, Ahl-Goy P, Meins F, Jr and others. Differential regulation of β-1,3-glucanase messenger RNAs in response to pathogen infection. Plant Physiol. 1991;96:390–397. doi: 10.1104/pp.96.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterkeyn L. Sur l'existence d'un “stade callosique” présenté par la paroi cellulaire, au cours de la cytokinèse. CR Acad Sci Paris D. 1967;265:1792–1794. [Google Scholar]
- Wessels JGH, Sietsma JH. Fungal cell walls: a survey. In: Tanner W, Loewus FA, editors. Encyclopedia of Plant Physiology, Vol 13B: Plant Carbohydrates II. Berlin: Springer Verlag; 1981. pp. 352–394. [Google Scholar]
- Worrall D, Hird DL, Hodge R, Paul W, Draper J, Scott R. Premature dissolution of the microsporocyte callose wall causes male sterility in transgenic tobacco. Plant Cell. 1992;4:759–771. doi: 10.1105/tpc.4.7.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Maher EA, Masoud S, Dixon RA, Lamb CJ. Enhanced protection against fungal attack by constitutive co-expression of chitinase and glucanase genes in transgenic tobacco. Biotechnology. 1994;12:807–812. [Google Scholar]