Abstract
Filovirus infections can cause a severe and often fatal disease in humans and nonhuman primates, including great apes. Here, three anti-Ebola virus mouse/human chimeric mAbs (c13C6, h-13F6, and c6D8) were produced in Chinese hamster ovary and in whole plant (Nicotiana benthamiana) cells. In pilot experiments testing a mixture of the three mAbs (MB-003), we found that MB-003 produced in both manufacturing systems protected rhesus macaques from lethal challenge when administered 1 h postinfection. In a pivotal follow-up experiment, we found significant protection (P < 0.05) when MB-003 treatment began 24 or 48 h postinfection (four of six survived vs. zero of two controls). In all experiments, surviving animals that received MB-003 experienced little to no viremia and had few, if any, of the clinical symptoms observed in the controls. The results represent successful postexposure in vivo efficacy by a mAb mixture and suggest that this immunoprotectant should be further pursued as a postexposure and potential therapeutic for Ebola virus exposure.
Keywords: passive immunization, therapy
For more than 35 y, a therapy has been sought to treat the severe lethal disease caused by Ebola virus (EBOV; family Filoviridae), which are among the most virulent infectious agents known, causing acute and frequently fatal hemorrhagic fever in humans and nonhuman primates (NHP) (1, 2). Outbreaks in humans occur intermittently, causing localized high morbidity and mortality. Due to its infectiousness, the lack of approved diagnostics, and the rapidity of modern travel, the potential exists for any outbreak to become an international epidemic. Currently there are no licensed vaccines or treatments against EBOV infection. Candidate postexposure interventions in advanced development include siRNA and phosphorodiamidate morpholino oligomers (PMOs) antisense strategies, as well as postexposure immunization with a vesicular stomatitis virus (VSV)-based vaccine (3–5). All of these interventions have demonstrated reduced mortality in NHPs when delivered 0.5–1 h postinfection (p.i.), but clinical and sometimes severe disease is observed in survivors.
Although the majority of the 20 or more mAb products approved by the FDA are for noninfectious disease indications (6), passive immunization with antibodies has been an effective method to prevent a variety of viral, bacterial, fungal, and parasitic diseases that cause disease in humans and animals (7, 8). However, the use of antibody therapy for EBOV has been fraught with conflicting data as to the utility of this class of intervention. Although convalescent-phase serum has been used in small numbers for sporadic outbreaks of EBOV and other filovirus infections (9), it is unclear if these treatments were beneficial. Early proof-of-concept efforts with equine and ovid hyperimmune serum protected baboons from a low challenge (<30 LD50 and <1 LD50, respectively) when treatment was initiated less than 1 h p.i (10–12). However, later efforts with macaque models of EBOV disease using a more robust challenge were unsuccessful (13). Some successes with antibodies in rodents (14–16) have been described, but these results are tempered by the lack of translation to the more robust and lethal macaque models of EBOV infection (17). For example, a potent neutralizing human IgG1 mAb, KZ52, protected guinea pigs but provided no protection in macaques (18, 19). Recently, studies have demonstrated a role for polyclonal IgG in protection in the macaque model of disease; transfer of concentrated IgG with a high neutralizing titer purified from convalescent-phase EBOV-exposed rhesus macaques demonstrated protection in naïve animals that received the IgG up to 48 h p.i (20). However, the use of purified convalescent macaque serum as a medical countermeasure is likely cost-prohibitive and would have significant logistical and regulatory hurdles. As an approach to develop a mAb-based medical countermeasure, Marzi et al. (18) demonstrated that two neutralizing mouse/human chimeric mAbs against EBOV could provide limited protection in rhesus macaques (one of three animals survived) when dosing was initiated 24 h before challenge (1,000 pfu ∼1,000 LD50). More recently, Qiu et al. (21) found a mixture of neutralizing murine mAbs could protect cynomolgus macaques when given 1 (four of four survived) or 2 d p.i. (two of four survived).
Development of the mAb-based EBOV immunoprotectant MB-003 was built upon prior work characterizing three mouse mAbs (13C6, 13F6, and 6D8) directed against three distinct, nonoverlapping EBOV glycoprotein (GP) epitopes (14). Only one of these mAbs (13C6) binds to secreted GP (sGP), which has been speculated to act as a decoy for protective antibodies (22). Two of these mAbs (13C6 and 6D8) neutralize virus in the presence of complement, and one has no neutralizing activity (13F6). These mAbs were shown to individually protect against lethal challenge prophylactically in mouse models of EBOV infection and have a therapeutic window of at least 48 h after viral exposure (14). To develop a product that would be appropriate for human use, the murine mAbs were deimmunized (23) and/or chimerized with human constant regions, yielding c13C6, h-13F6, and c6D8. To evaluate a more cost-effective and scalable alternative to production in Chinese hamster ovary (CHO) cells, the mAbs were produced in a Nicotiana benthamiana-based rapid antibody manufacturing platform (RAMP) using magnICON (ICON Genetics) deconstructed viral vectors (24). The RAMP system allows rapid, scalable production of mAbs in less than a month, and has been used to produce mAbs under cGMP conditions (25). Via the use of a transgenic strain of N. benthamiana in which plant-specific glycosyltransferases (α1,3 fucosyltransferase and β1,2 xylosyltransferase) are inhibited by RNAi (26), the RAMP-derived mAbs have homogenous mammalian glycans. In the mouse-adapted Ebola model, the RAMP mAbs provided protection superior to CHO-derived mAbs, likely due to the increased antibody-dependent cellular cytotoxicity (ADCC) activity conferred by the N-glycans lacking core fucose present on the fragment crystallizable (Fc) region (23).
Here, we report the efficacy of CHO and RAMP-derived MB-003 mixture (MB-003CHO, MB-003RAMP, respectively) using the lethal EBOV NHP model. The rhesus macaque model was chosen because symptom onset more closely parallels human disease progression compared with the cynomolgus model (27). This proof-of-concept study presents data demonstrating that the plant-produced MB-003RAMP is efficacious in preventing lethal disease in EBOV-infected macaques when administered 24 or 48 h after virus challenge.
Results
Antibody Analysis.
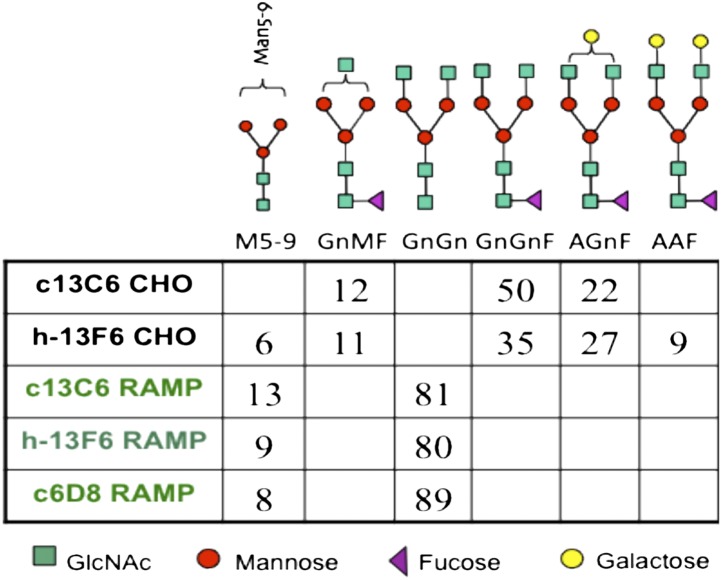
Analysis of the CHO-derived mAbs indicated core fucosylated nongalactosylated (GnGnF) and monogalactosylated (AGnF) N-glycan structures were the major glycoforms (Fig. 1). In contrast, no major core fucosylated structures were detected in the RAMP-derived mAbs (Fig. 1). These RAMP mAbs carried a single major biantennary N-glycan with terminal GlcNAc on each branch—namely, GnGn—corresponding to a fucose-free form of one of the major glycoforms found in the CHO-produced mAbs (GnGnF). EBOV glycoprotein antigen-binding ELISAs and mouse efficacy testing performed as part of the release testing (i.e., potency assays) for the mAbs demonstrated binding capability indistinguishable between the CHO- and RAMP-derived mAbs.
Fig. 1.
N-glycan profiles of different MB-003 mAb glycoforms as determined by 2-AA glycan analysis. Numbers represent the different glycospecies in percentages. Minor glycoforms (below 5%) are not indicated. N-glycan nomenclature is according to www.proglycan.com.
Clinical Observations and Outcomes.
In the first pilot study (Table 1), macaques were challenged i.m. with 100 pfu EBOV, and treatment (n = 2) was initiated 1 h p.i. with MB-003CHO (50 mg⋅kg−1⋅mAb−1). Animals received an additional dose on days 4 and 8. MB-003CHO-treated animals displayed no evidence of infection, and no virus was detected in serum by RT-PCR. In contrast, the two control animals treated with PBS or irrelevant control mAb (Synagis; MedImmune) displayed symptoms of infection and subsequently died (days 7 and 9, respectively).
Table 1.
Clinical events on days 1–28 post-EBOV challenge
| Treatment | Finding | |
| Study 1 | ||
| 1A (S) | 1 h p.i. CHO | No findings |
| 2A (S) | 1 h p.i. CHO | No findings |
| 3A (NS, day 9) | 1 h p.i. mAb control | Diarrhea/lack of bowel movements (days 5–9)* |
| 4A (NS, day 7) | 1 h p.i. PBS control | Unresponsiveness (day 6–7), widespread petechial rash (day 6–7), AST ↑↑↑, ALT ↑↑↑, BUN ↑↑, TBIL ↑↑, GGT ↑↑, thrombocytopenia |
| Study 2 | ||
| 1B (S) | 1 h p.i. CHO | Thrombocytopenia |
| 2B (NS, day 12) | 1 h p.i. CHO | Increasing unresponsiveness to prostrate (days 9–12), lack of bowel movements, thrombocytopenia, GLU ↓, AST ↑↑↑, ALT ↑↑↑, BUN ↑, TBIL ↑↑, GGT ↑ |
| 3B (S) | 1 h p.i. RAMP | Thrombocytopenia |
| 4B (S) | 1 h p.i. RAMP | Mild depression (day 10), thrombocytopenia |
| 5B (S) | 1 h p.i. RAMP | Thrombocytopenia |
| 6B (S) | 1 h p.i. PBS control | Mild depression (days 10, 12–13), thrombocytopenia, AST ↑ |
| Study 3 | ||
| 1C (S) | 24 h p.i. RAMP | Mild depression (days 7–9) |
| 2C (S) | 24 h p.i. RAMP | Mild depression (days 6–9) |
| 3C (NS, day 11) | 24 h p.i. RAMP | Depression (days 6–10), unresponsiveness (days 9–10), anorexia (day 9), bleeding (day 9), thrombocytopenia, GLU ↓, AST ↑↑↑, ALT ↑↑↑ |
| 4C (NS, day 7) | 24 h p.i. mAb control | Depression (day 6), unresponsiveness (day 6), significant petechial rash (day 6), thrombocytopenia, GLU ↓, AST ↑↑↑, ALT ↑↑↑ |
| 5C (NS, day 16) | 48 h p.i. RAMP | Depression (days 6–16), increasing unresponsiveness (days 6–16), diarrhea (days 10–13), lack of bowel movements (days 12–16 intermittent), dehydration (days 12–16), petechial rash (moderate days 8–14, fading days 15–16), seizures (16) |
| 6C (S) | 48 h p.i. RAMP | Mild depression (days 6–9) |
| 7C (S) | 48 h p.i. RAMP | Mild depression (days 6–9), diarrhea (days 17–19, 23–24, 27) |
| 8C (NS, day 7) | 48 h p.i. PBS control | Depression (day 6), petechial rash (day 6), increasing unresponsiveness (day 6), lack of bowel movements (day 6), thrombocytopenia, GLU ↓, AST ↑↑↑, ALT ↑↑↑ |
Thrombocytopenia is defined as ≥35% decrease in platelets. Petechial rash is defined as 10–40% of body surface area; significant petechial rash is defined as >40% of body surface area. ↑, two- to threefold increase; ↑↑, four- to fivefold increase; ↑↑↑, >fivefold increase; ↓, two- to threefold decrease. BUN, blood urea nitrogen; GGT, γ-glutamyl transpeptidase GLU, glucose; NS, nonsurvivor; S, survivor; TBIL, total bilirubin.
*Euthanized by Veterinary Medicine Division staff (viremic via plaque assay).
In a follow-up pilot study, the challenge was increased to 1,000 pfu, and treatment was again initiated 1 h p.i. (with additional dosing on day 4 and 8). Macaques received either MB-003CHO (50 mg⋅kg−1⋅mAb−1) or MB-003RAMP (16.7 mg⋅kg−1⋅mAb−1), and the control animal received PBS. The difference in dosing between CHO- and RAMP-derived mAbs was based on murine studies showing a threefold improvement in potency of the RAMP-derived mAbs compared with the CHO-derived mAbs (23). One of two macaques treated with MB-003CHO and three of three treated with MB-003RAMP (P < 0.02 vs. historical controls) survived challenge (Table 1) and had no detectable virus by plaque assay and RT-PCR. One CHO-treated animal was euthanized on day 12 when the clinical score surpassed euthanasia criteria. The time to death was within the range seen historically with the stock used for challenge, and the pathology report concluded that findings appeared consistent with filoviral infection. This animal displayed decreased platelets and glucose levels and increased aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels. The PBS control animal became clinically ill (Table 1) but recovered and displayed a transient drop in platelets and a transient rise in AST. This control animal and the nonsurviving MB-003CHO–treated primate both showed significant levels of virus from serum as determined by RT-PCR.
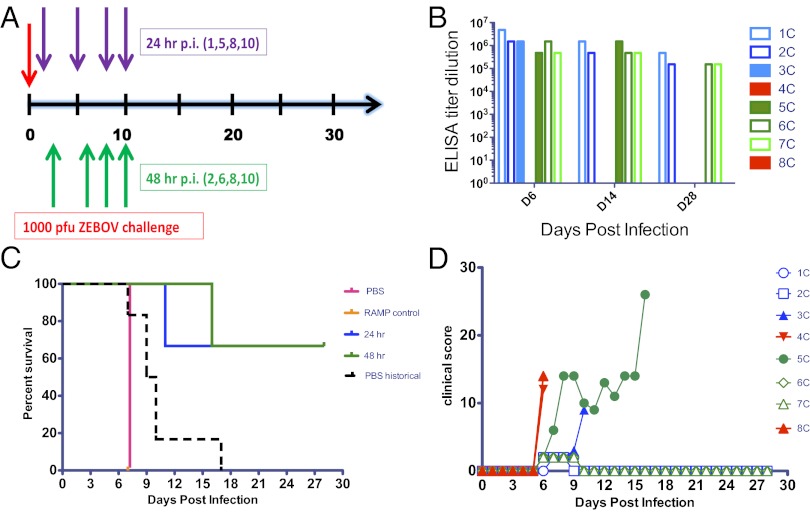
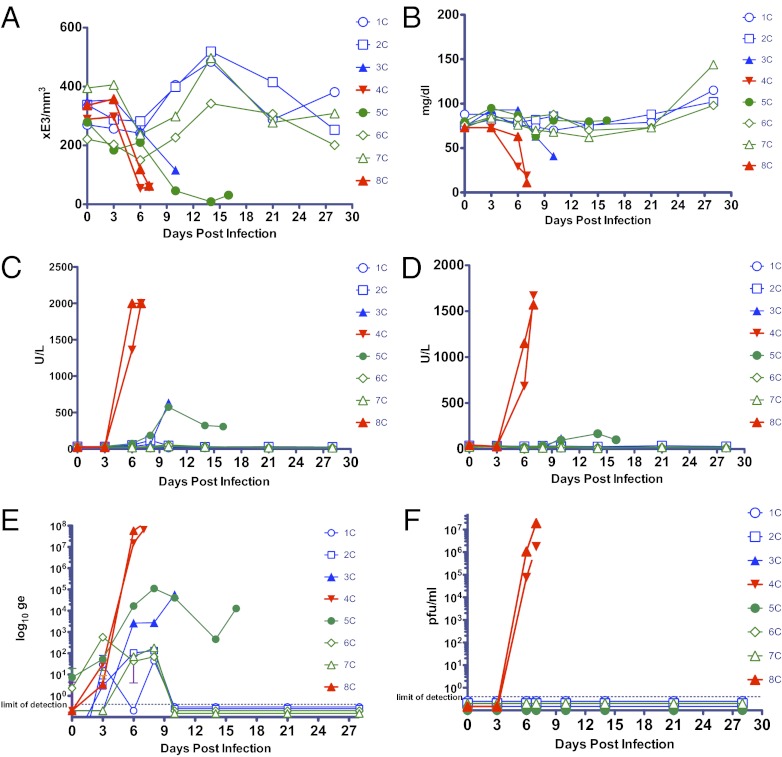
Testing in the pivotal study was confined to RAMP-derived mAbs due to their superior efficacy to CHO-derived mAbs in murine studies (23) and the suggested superiority in Study 2 (e.g., 100% protection vs. 50% with threefold less MB-003). Initiation of treatment with the MB-003RAMP (16.7 mg⋅kg−1⋅mAb−1) 24 or 48 h p.i. (1,000 pfu ∼1,000 LD50) was tested using a different viral stock (Fig. 2A). Animals received three additional MB-003RAMP doses (days 5, 8, and 10 for the 24-h p.i. group and days 6, 8, and 10 for the 48-h p.i. group). Four of the six animals treated (two from each group) survived challenge (P < 0.05 for both groups vs. historical controls challenged with this viral stock and P < 0.05 for the 48-h group against the two internal controls; Fig. 2C). Further, these animals displayed no signs of illness and no significant changes in platelet count, glucose, or liver enzyme levels (Fig. 3 A–D). These survivors had virus detected in serum by RT-PCR, but at levels 100,000-fold less than controls (Fig. 3E). One 24-h p.i.-treated animal was found dead on day 11, and one 48-h p.i.-treated animal was euthanized on day 16 when the clinical score surpassed euthanasia criteria (Fig. 2D). These two animals displayed signs of illness (Table 1) and had blood chemistry changes, but at reduced scale from controls. Viral levels in serum detected via RT-PCR in these animals were 1,000-fold less than controls (Fig. 3E). Both control animals (one received an irrelevant RAMP-derived mAb 24 h p.i., and the other received PBS 48 h p.i.) succumbed to infection on day 7. These two animals had dramatic drops in platelet and glucose levels, substantial increases in enzyme levels, and high viral titers determined by RT-PCR and viral culture (Fig. 3). Pathology findings for all four nonsurviving animals were consistent with filovirus infection.
Fig. 2.
Initiation of treatment of rhesus macaques with MB-003 at 24 or 48 h postinfection provides protection against EBOV. Delayed treatment with MB-003RAMP (16.67 mg⋅kg−1⋅mAb−1) was given 24 h (purple arrows) and 48 h (green arrows) after being challenged i.m. with 1,000 pfu of EBOV. (A) After challenge (red arrow), treatments were given on days 1, 5, 8, and 10 for the 24-h group and the irrelevant anti-HIV RAMP mAb control; the 48-h group and PBS control were treated on days 2, 6, 8, and 10. (B) Dosing was verified by recombinant GP binding ELISA. (C) A survival curve was generated with experimental groups, corresponding controls, and historical controls from the last 2 y challenged i.m. with stock 22433. Animals were observed daily (D) and scored for the duration of the study postchallenge. The sudden spike in observational score for primate 5C was due to a seizure, and the animal was euthanized shortly thereafter. Open symbols represent animals that survived, and filled symbols represent animals that succumbed to infection. Blue symbols indicate treatment initiation 24 h p.i. and green symbols 48 h p.i.; red indicates controls.
Fig. 3.
Clinical analysis and viral titers for the duration of study. Changes observed on days 0, 3, 6, 10, 14, 21, and 28 (additional day of euthanasia measurement on day 16 for primate 5C; additional day 8 chemistry analysis for all primates) for (A) platelet counts; (B) glucose; (C) AST; and (D) ALT. (E) RT-PCR–derived genomic equivalents (ge) and (F) viral titer as calculated by agarose plaque assay. All samples were analyzed via RT-PCR and plaque assay. Error bars (too small of an error to be seen for most points) in E and F represent SD (n = 3). Open symbols represent animals that survived, and filled symbols represent animals that succumbed to infection. Blue symbols indicate treatment initiation 24 h p.i. and green symbols 48 h p.i.; red indicates controls.
Discussion
There is an urgent need for a cost-effective postexposure therapeutic to treat infection and halt transmission during EBOV outbreaks, as well as for use in the event of a bioterror threat. The importance of antibodies in the adaptive immune response during vaccination is well recognized, and the use of antibodies as therapeutics against infectious disease is building credibility as well (6, 28, 29). It is only recently, however, that passive immunization for EBOV—with macaque polyclonal IgG and with mouse mAbs—has been demonstrated (20, 21). The results presented here further extend these findings to a mixture of mAbs appropriate for human administration. The evaluation of protection using MB-003 in the macaque model shows a system in which both the pathogenesis of disease and immune responses of the animal host imitate the human condition. The mixture of three mAbs in a postexposure treatment regimen against EBOV was designed to mimic a potential needle-stick scenario in a laboratory setting.
Although involving small numbers, the results from the first two pilot studies were informative. In the first study, MB-003CHO protected the two treated animals from a lethal 100 pfu challenge. These animals were completely protected from morbidity, in contrast to the two controls that displayed clinical illness and succumbed to infection. In the second experiment, MB-003 from the CHO and RAMP system were compared, using a more rigorous challenge of 1,000 pfu. Results from this experiment are confounded by the survival of the control, which has occurred one other time in the last 2 y with this viral stock (of 15 animals total; given the likely genetic diversity of these wild-caught macaques, it is not entirely surprising that controls occasionally can survive, as do humans, perhaps due to differences in CD4+, CD8+, or IFN-γ responses) (30). Nevertheless, the control did show symptoms of infection and viremia. The protection conferred by MB-003RAMP (three of three protected; P < 0.02 compared with historical controls), administered at one-third of the dose of MB-003CHO–treated animals (one of two protected), is consistent with our findings in mice that the RAMP-derived mAbs have superior potency (22). Because the only difference between the CHO and RAMP mAbs is their glycosylation, this would suggest a significant role for glycan-dependent Fc receptor-mediated effector functions in the protection conferred by these mAbs. Indeed, the absence of core fucose on IgG1 is known to increase binding of mAb to FcγRIII, resulting in a dramatic improvement in ADCC activity compared with antibody with core fucose. These results in total suggest that ADCC plays a critical role in the protective efficacy conferred by the MB-003 mAbs. It is interesting to speculate whether the incomplete or absence of protection previously observed with polyclonal and monoclonal antibodies of high neutralizing activity (17–19, 31) may have been due to relatively reduced ADCC activity conferred by their glycosylation—CHO cells, and mammalian cells in general, produce only a small percentage of IgG lacking core fucose.
In our final experiment, four of six animals treated 1 or 2 d p.i. with MB-003RAMP survived challenge with a historically 100% lethal viral stock (n = 6), whereas both controls died. The two treated animals that succumbed to challenge experienced either hindered or delayed course of disease. Because c13C6 and c6D8 neutralize EBOV in the presence of complement (14), the inability to detect virus in serum by plaque assay in these two animals (Fig. 3F), even when significant levels of viral RNA were detected by RT-PCR (Fig. 3E), suggests these animals did not succumb to escape mutants. Further, EBOV GP-specific ELISA showed that the animals had high levels of antibody (Fig. 2B) through the entire study, indicating that mAb was not consumed by viral replication as reported by Marzi et al. (18). The four treated animals that survived challenge experienced little to no viremia and had few, if any, of the clinical symptoms observed in the two controls. With a caveat that direct comparisons between studies performed in different laboratories are difficult, this is in contrast to the course of disease seen in survivors treated with other EBOV therapeutic candidates in advanced development (siRNA, PMOs, and VSV) in which surviving animals experience varying levels of morbidity (3–5). Further, MB-003RAMP provided this level of protection out to 48 h p.i. in the majority of treated animals.
Several factors may explain the incomplete (67%) protection observed by the RAMP-derived mAbs. Although equally effective individually in the mouse model, the three mAbs in MB-003 have not been tested individually in macaques. Elimination of an ineffective mAb from the mixture and/or higher dosing could improve efficacy. Alternatively, because ADCC is an important mechanism of action for these mAbs (23), polymorphisms of the ADCC-mediating FcγRIIIa receptor (28, 32) within the macaque population used in the studies could have affected mAb potency. A polymorphism in approximately half of humans (32) has been shown to affect ADCC and tumor-cell killing by the FDA-approved mAb, rituximab (33). Although less well-characterized, three FcγRIIIa polymorphisms have been reported in macaques (34, 35), and in one study, 33% (n = 9) of macaques had a FcγRIIIa polymorphism (35). Finally, there may have been an as-yet-uncharacterized variability within the study population that affected the host response to infection.
In this study we used a well-characterized EBOV source (Zaire Kikwit). With improved sequencing tools, the role of EBOV isolate sequence differences has recently become topical (36). For instance, the sequence of EBOV used in this study appears to contain an additional uracil residue in the GP gene-editing site. If this is a true mutation (the site causes stuttering of polymerases and may therefore cause sequencing artifacts) (37), this mutation may reduce the amount of sGP processed during an infection (36, 37). Although the function of sGP is unknown, sGP has been hypothesized to act as a decoy for protective antibodies. One of the three MB-003 mAbs does bind to sGP (13C6), and if this mAb alone is providing the protective efficacy observed in the studies described here, the mAb may have reduced efficacy against a strain without this mutation. However, 13C6 is highly protective in a mouse model that uses a mouse adapted strain (Zaire Mayinga) that lacks this mutation, which would argue against this concern. Regardless, testing of MB-003 against strains other than Zaire Kikwit is planned.
These proof-of-concept studies were designed to reflect postexposure prophylaxis circumstances potentially seen in human cases, and show the potential for improvement upon the 1 h p.i. treatment window demonstrated with other EBOV products (siRNA, PMOs, and VSV) currently in advanced development. Additional studies will be performed with larger groups to determine if MB-003RAMP has efficacy as a therapeutic (i.e., the treatment window extends to the onset of diagnosis). Future experiments will also be aimed at determining which of the mAbs or combination of mAbs used in the mixture offers optimal protection, and identifying the best treatment regimen. Stability studies are ongoing, and the RAMP-derived mAbs, like mAbs from mammalian cell culture, are expected to have a good stability profile, an important trait for drugs that may be included in the Strategic National Stockpile.
Methods
Production of MB-003 mAbs in CHO.
Stable CHO cell lines were generated (38) expressing h-13F6, c13C6, and c6D8 mAbs and cultured in CD OptiCHO Medium (Invitrogen) supplemented daily with CHO Feed Bioreactor Supplement (Sigma). The CHO culture was grown in suspension using a Wave Bioreactor 20/50 EHT System (GE Healthcare) equipped with a WAVEPOD (GE Healthcare). The glucose level was manually monitored daily and adjusted with sterile 45% glucose solution (Mediatech). Conditioned medium was harvested and clarified via centrifugation, then filtered (0.2 μm) before purification using a 100-mL MabSelect SuRe (GE Healthcare) Protein A column. The column was washed with 1× PBS running buffer and mAb eluted with acetic acid. Eluate was neutralized to pH 7 and diafiltered against the formulation buffer [PBS (pH 7) for h-13F6 and 50 mM potassium citrate, 200 mM glycine, 8% (wt/vol) trehalose (pH 5.5) for c13C6 and c6D8] using the Minim Tangential Flow Filtration System (Pall). The mAb solution was then polished with Sartobind Q (Sartorius), sterile-filtered, distributed, and stored at −80 °C until used. All purified mAbs were fully assembled as determined by SDS/PAGE and had less than 8% aggregate as determined by HPLC size-exclusion chromatography (SEC). An Ebola GP antigen-binding ELISA (39) was used to confirm binding activity in comparison with reference standards prepared from previously manufactured mAbs that had been shown to be efficacious in the mouse EBOV model.
Production of MB-003 mAbs in ΔXTFT N. benthamiana.
For transient expression of the MB-003 mAbs in plants, we used the “magnifection” procedure (24) with minor modifications as described previously (23). Briefly, plants grown for 24–26 d in an enclosed growth room at 22–24 °C were used for vacuum infiltration. Equal volumes of overnight-grown Agrobacterium cultures were mixed in infiltration buffer [10 mM Mes (pH 5.5) and 10 mM MgSO4], resulting in a 1:1,000 dilution for each individual culture. Using a 300-L custom-built (Kentucky Bioprocessing) vacuum chamber, the aerial parts of entire plants were inverted into the bacterial/buffer solution and a vacuum of 22 inches of mercury was applied for 2 min. At 7 d postinfiltration, leaf tissue was extracted with a Corenco double-stack disintegrator using a 0.5:1 buffer-to-plant tissue ratio [extraction buffer: 100 mM Tris, 40 mM ascorbic acid, 1 mM EDTA (pH 8.5)]. The extract was adjusted to pH 8.0 with 10 M NaOH. Extract was clarified using a plate-frame filter press (ErtelAlsop) with 1.0-μm pads. The antibody was captured from the filtrate using a MabSelect SuRe (GE Healthcare) Protein A column. The column was equilibrated and washed with Tris running buffer and eluted with acetic acid. The resulting eluate was neutralized using 1 M Tris. The mAb solution was then further purified via Q filtration (Mustang Q membrane; Pall). The final polishing column for c13C6 and c6D8 was a CHT, Type I 40-μm (Bio-Rad) column. The column was equilibrated and washed with phosphate running buffer and eluted with running buffer containing NaCl. The final polishing column for h-13F6 was a MEP HyperCel column (Pall), equilibrated and washed with Hepes buffer, washed a second time at a slightly acidic pH to remove product related impurities, and eluted under acidic conditions. The eluate was neutralized to pH 7.0. The final eluates for each antibody were concentrated and diafiltered against their respective formulations [c6D8: 50 mM sodium phosphate, 100 mM NaCl, 200 mM arginine, and 4% (wt/vol) mannitol; h-13F6 and c13C6: 50 mM sodium phosphate, 200 mM glycine, 8% (wt/vol) mannitol, and 0.005% polysorbate 20] using tangential flow ultrafiltration with 30-kDa molecular weight cutoff polyethersulfone membranes. All three antibodies were sterile filtered, aseptically filled into glass vials with serum stoppers, sealed, and stored at −80 °C. mAbs were fully assembled as determined by SDS/PAGE and had less than 5% aggregate as determined by HPLC-SEC. An Ebola GP antigen-binding ELISA was used to confirm binding activity in comparison with reference standards.
N-Glycan Analysis.
N-linked glycans were released by digestion with N-glycosidase F (PNGase F) and subsequent derivatization of the free glycan with anthranilic acid (2-AA). The 2-AA–derivatized oligosaccharide was separated from excess reagent by normal-phase HPLC. The column was calibrated with 2-AA–labeled glucose homopolymers and glycan standards. Test sample and 2-AA–labeled glycan standards were detected fluorometrically. Glycoforms were assigned either by comparing their glucose unit (GU) values with those of 2-AA–labeled glycan standards or by comparing with theoretical GU values (40). Confirmation of glycan structure was performed using liquid chromatography–MS.
Virus Stocks.
EBOV (Zaire Kikwit strain) stocks were developed at the US Army Medical Research Institute of Infectious Diseases (USAMRIID) using virus originally isolated from an infected patient during the 1995 outbreak and passaged in Vero E6 cells. Stock material used in the first two studies was three passages from the original isolate (the first two of which were performed at the Centers for Disease Control); stock material used in the third study was four passages from the original isolate. This stock has been deemed the national stock for preclinical studies for advanced products. The stock was made by the Department of Defense Critical Reagents Program under the Joint Program Executive Office for Chemical and Biological Defense. The virus and future viruses generated under this program have been developed under the Filovirus Animal Non-Clinical Group, a multiple-agency group including the Food and Drug Administration to prepare for validated/regulated clinical studies. Statistical comparisons between the stocks used here found no difference in the mean times to death (P = 0.7854) or in the survival curves of all historic controls (P = 0.7873).
NHP Challenge and Care.
Adult male and female rhesus macaques (RHM) were caged individually. RHMs were placed in training jackets (Lomir Biomedical Inc.) for acclimatization at least 4 d before surgery. Surgeries to insert central venous catheters (Groshong 7F; Bard) were performed, and adequate recovery time was given before transfer into BSL4 containment. After catheter placement, custom jackets were used to house and protect the lines (Lomir Biomedical Inc.). These lines were flushed with PBS (BD) and locked with 3 mL heparin (BD) at least once every other day to maintain catheter functionality. Animals were attached via a mounted swivel system (Lomir Biomedical Inc.) and acclimatized to containment ∼7 d before challenge. Animals were given monkey chow, primate treats, fruits, and vegetables throughout the course of the study. Animals were observed at least once daily to monitor overall health.
Before challenge, RHMs were anesthetized via i.m. injection of Telazol (0.03 mL/kg) and given a brief physical, at which time baseline weight and temperature were established. Animals were challenged via i.m. route with a target dose of 1,000 pfu/mL diluted from stock virus in DMEM; the actual dose of 690 pfu/mL was confirmed via agarose plaque assay. Animals were placed back into cages and observed until they had regained reasonable mobility.
Verification of Target Dose and Viremia.
Challenge target dose was verified via agarose-based plaque assay. Dilution points were serially diluted 10-fold in EMEM and adsorbed onto Vero E6 cell monolayer in six-well plates. These plates were incubated 1 h at 37 °C/5% CO2 with rocking approximately every 15 min, then immobilized with 2 mL of a 1:1 medium [2× Eagle's Basal Medium with Earle's salts (EBME), 10% (vol/vol) FBS, 1% antibiotics] and 1% agarose (Lonza) mixture. Plates were then incubated for 7 d at 37 °C/5% CO2. After incubation, a neutral red stain medium (2× EBME, 10% FBS, neutral red) was blended 1:1 with 1% agarose, and 2 mL was placed into each well. After an additional 24 h of incubation at 37 °C/5% CO2, plates were counted and viremia titers were calculated. Viremia analyses on serum samples and postnecropsy-processed tissues were performed using the same method.
Treatment Preparation.
Treatments were comprised of a three-mAb mixture. Each mAb was equally represented in the treatment mixture. EU/mg values for each mAb were averaged together and were less than 0.5 EU/mg. Treatment for each group was set at 50 mg⋅kg−1⋅mAb−1 of CHO-derived mAb mixture and 16.7 mg⋅kg−1⋅mAb−1 of RAMP N. benthamiana-derived mAb mixture. Treatment for each specific RHM was determined by the animal’s weight before challenge.
RHM Treatments.
The initial treatment for all groups in all studies was a triroute format, involving an i.m. injection at the site of challenge, an i.p. injection, and a continuous i.v. infusion using 60-mL syringes (BD) and syringe pumps (Lomir Biomedical Inc.). The i.m. and i.p. dosing remained consistent in experimental groups, whereas maximum rate of i.v. infusion was calculated based on endotoxin levels of treatment and animal weight. The infusion rates did not exceed safe volume requirements as determined by the United States Army Medical Research Institute of Infectious Diseases Institutional Animal Care and Use Committee (IACUC) and remained within Food and Drug Administration limits for human use (<5 EU⋅kg−1⋅h−1). Animals were periodically monitored during i.v. treatment and for at least 5 min after placement of a new treatment syringe. After the initial triroute treatment, subsequent treatments were given only by i.v. infusion on predetermined days. RHMs in the two pilot studies were treated on days 0 (1 h p.i.), 4, and 8. The third study included RHMs in 24-h (n = 3) and 48-h p.i. (n = 3) treatment groups, treated on days 1/2, 5/6, 8, and 10, respectively. The irrelevant mAb control (n = 1) was paired with the 24-h group, and the PBS control (n = 1) was paired with the 48-h group in terms of treatment schedule.
Animal Monitoring and Sample Collection.
RHMs were monitored at least once a day for changes in health and diet, as well as notable deviations in behavior. Due to catheterization for the duration of the study, animals were not anesthetized; consequently, weight and temperature data points were not collected. Blood was collected on days 0, 3, 6, 10, 14, 21, and 28 via the external catheter line for complete blood count and chemistry analyses. Blood was also collected on days of euthanasia, time points where animals succumbed to challenge (if possible), and at IACUC-approved time points to determine if optional treatments were required.
Complete blood count analysis was performed using the Coulter Act 10 (Beckman Coulter) on samples collected in EDTA plasma vacuette tubes (Greiner Bio-One). Blood was collected in 2-mL serum vacuette tubes (Greiner Bio-One) and allowed to clot for 30 min before use. These samples were spun for 15 min at 500 × g, and the resulting serum was then used in Piccolo 13 discs for chemistry analysis. Additional serum and plasma samples were distributed and frozen at −80 °C for further analysis.
Experiments were conducted under BSL-4 containment conditions; approval for experiments and animal manipulations was given by the USAMRIID IACUC. Animal work was completed by Association for Assessment and Accreditation of Laboratory Animal Care certified staff and under NIH guidelines (41).
Necropsy and Tissue Processing.
Necropsies were performed on all RHMs. Tissues collected were from liver, kidney, spleen, adrenal, pancreas, and inguinal lymph node.
Tissues were processed using Miltenyi Biotec M tubes and GentleMacs apparatus. All tissues were weighed, and a 10% homogenate (wt/vol) was created in appropriate volumes of EMEM. The homogenates were stored at −80 °C for analysis as needed.
ELISA.
ELISAs were performed using a recombinant EBOV glycoprotein (National Cancer Institute), and plasma samples serially diluted at half-log increments. Goat anti-human IgG (Heavy + Light) (KPL) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) (Millipore) were used as secondary and substrate, respectively. Assays were read at 405 nm absorbance (SpectraMax M5; Molecular Devices). Cutoff values were calculated as 1.5× the average naïve serum reading, plus 3× the SD.
RNA Isolation and RT-PCR.
A 0.25-mL of sample was placed with 0.75 mL of TRIzol (Invitrogen) for tissue homogenates and TRIzol LS (Invitrogen) for serum. RNeasy kits (Qiagen) were used for RNA extraction. One-step quantitative real-time RT-PCR reactions were done on a LightCycler 480 (Roche) in 20-μL vol with 5 μL of purified RNA and the SuperScript III One-Step RT-PCR System (Invitrogen). Primers (forward 5′-CGGACCTGGTTTGGTTGTG-3′; reverse 5′-GCTGCAGTGTCGCATCTGA-3′) and TaqMan probe (6-carboxyfluorescein-5′-CCCTTGCCACAATCT-minor groove binder nonfluorescent quencher-3′) (Applied Biosystems) specific for the Ebola Zaire glycoprotein gene were used. Program conditions were reverse transcription at 50 °C for 20 min, and initial denaturation at 95 °C for 5 min; this was followed by 45 cycles of denaturation at 95 °C for 5 s, and annealing, synthesis, and signal acquisition at 60 °C for 20 s; and concluded with final cooling at 40 °C for 30 s. Measurement of viral gene expression was based on a viral RNA standard.
Statistics.
Survival curves were analyzed with the log-rank Mantel–Cox test using Prism software (GraphPad).
Acknowledgments
We thank Dr. Yuri Gleba for providing access to the magnICON expression system; Dr. Herta Steinkellner for access to the ΔXT/FT N. benthamiana; and the Veterinarian and Pathology Division staff at USAMRIID for assistance with this study. This work was supported by National Institute of Allergy and Infectious Diseases Grants AI61270 and AI 72915 and Department of Defense Grant DAMD 17-02-2-0015, and partially supported by Defense Threat Reduction Agency Grant 4.10007-08-RD-B.
Footnotes
Conflict of interest statement: K.J.W. and L.Z. are owners of Mapp Biopharmaceutical, Inc.
This article is a PNAS Direct Submission.
References
- 1.Feldmann H, Jones S, Klenk HD, Schnittler HJ. Ebola virus: From discovery to vaccine. Nat Rev Immunol. 2003;3(8):677–685. doi: 10.1038/nri1154. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez A, et al. Filoviridae: Marburg and Ebola viruses. In: Knipe DM, Howley PM, editors. Fields Virology. 4th Ed. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 3.Warfield KL, et al. Gene-specific countermeasures against Ebola virus based on antisense phosphorodiamidate morpholino oligomers. PLoS Pathog. 2006;2(1):e1. doi: 10.1371/journal.ppat.0020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldmann H, et al. Effective post-exposure treatment of Ebola infection. PLoS Pathog. 2007;3(1):e2. doi: 10.1371/journal.ppat.0030002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geisbert TW, et al. Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: A proof-of-concept study. Lancet. 2010;375(9729):1896–1905. doi: 10.1016/S0140-6736(10)60357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang XR, et al. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat Rev Drug Discov. 2011;10(2):101–111. doi: 10.1038/nrd3365. [DOI] [PubMed] [Google Scholar]
- 7.Casadevall A, Scharff MD. Return to the past: The case for antibody-based therapies in infectious diseases. Clin Infect Dis. 1995;21(1):150–161. doi: 10.1093/clinids/21.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeitlin L, Cone RA, Moench TR, Whaley KJ. Preventing infectious disease with passive immunization. Microbes Infect. 2000;2(6):701–708. doi: 10.1016/s1286-4579(00)00355-5. [DOI] [PubMed] [Google Scholar]
- 9.Mupapa K, et al. International Scientific and Technical Committee Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. J Infect Dis. 1999;179(Suppl 1):S18–S23. doi: 10.1086/514298. [DOI] [PubMed] [Google Scholar]
- 10.Borisevich IV, et al. Development and study of the properties of immunoglobulin against Ebola fever. Vopr Virusol. 1995;40(6):270–273. Russian. [PubMed] [Google Scholar]
- 11.Kudoyarova-Zubavichene NM, Sergeyev NN, Chepurnov AA, Netesov SV. Preparation and use of hyperimmune serum for prophylaxis and therapy of Ebola virus infections. J Infect Dis. 1999;179(Suppl 1):S218–S223. doi: 10.1086/514294. [DOI] [PubMed] [Google Scholar]
- 12.Markin VAMV, Mikhaĭlov VV, Krasnianskiĭ VP, Borisevich IV, Firsova IV. [Developing principles for emergency prevention and treatment of Ebola fever] Vopr Virusol. 1997;42(1):31–34. Russian. [PubMed] [Google Scholar]
- 13.Jahrling PBGT, et al. Evaluation of immune globulin and recombinant interferon-alpha2b for treatment of experimental Ebola virus infections. J Infect Dis. 1999;179(Suppl 1):S224–S234. doi: 10.1086/514310. [DOI] [PubMed] [Google Scholar]
- 14.Wilson JA, et al. Epitopes involved in antibody-mediated protection from Ebola virus. Science. 2000;287(5458):1664–1666. doi: 10.1126/science.287.5458.1664. [DOI] [PubMed] [Google Scholar]
- 15.Parren PWHI, Geisbert TW, Maruyama T, Jahrling PB, Burton DR. Pre- and postexposure prophylaxis of Ebola virus infection in an animal model by passive transfer of a neutralizing human antibody. J Virol. 2002;76(12):6408–6412. doi: 10.1128/JVI.76.12.6408-6412.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parren PW, Burton DR. The antiviral activity of antibodies in vitro and in vivo. Adv Immunol. 2001;77:195–262. doi: 10.1016/S0065-2776(01)77018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jahrling PB, Geisbert JB, Swearengen JR, Larsen T, Geisbert TW. Ebola hemorrhagic fever: Evaluation of passive immunotherapy in nonhuman primates. J Infect Dis. 2007;196(Suppl 2):S400–S403. doi: 10.1086/520587. [DOI] [PubMed] [Google Scholar]
- 18.Marzi A, et al. Protective efficacy of neutralizing monoclonal antibodies in a nonhuman primate model of Ebola hemorrhagic fever. PLoS ONE. 2012;7(4):e36192. doi: 10.1371/journal.pone.0036192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oswald WB, et al. Neutralizing antibody fails to impact the course of Ebola virus infection in monkeys. PLoS Pathog. 2007;3(1):e9. doi: 10.1371/journal.ppat.0030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dye JM, et al. Postexposure antibody prophylaxis protects nonhuman primates from filovirus disease. Proc Natl Acad Sci USA. 2012;109(13):5034–5039. doi: 10.1073/pnas.1200409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu X, et al. Successful treatment of Ebola virus-infected cynomolgus macaques with monoclonal antibodies. Sci Transl Med. 2012;4(138):138ra81. doi: 10.1126/scitranslmed.3003876. [DOI] [PubMed] [Google Scholar]
- 22.Ito H, Watanabe S, Takada A, Kawaoka Y. Ebola virus glycoprotein: Proteolytic processing, acylation, cell tropism, and detection of neutralizing antibodies. J Virol. 2001;75(3):1576–1580. doi: 10.1128/JVI.75.3.1576-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeitlin L, et al. Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc Natl Acad Sci USA. 2011;108(51):20690–20694. doi: 10.1073/pnas.1108360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giritch A, et al. Rapid high-yield expression of full-size IgG antibodies in plants coinfected with noncompeting viral vectors. Proc Natl Acad Sci USA. 2006;103(40):14701–14706. doi: 10.1073/pnas.0606631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pogue GP, et al. Production of pharmaceutical-grade recombinant aprotinin and a monoclonal antibody product using plant-based transient expression systems. Plant Biotechnol J. 2010;8(5):638–654. doi: 10.1111/j.1467-7652.2009.00495.x. [DOI] [PubMed] [Google Scholar]
- 26.Strasser R, et al. Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol J. 2008;6(4):392–402. doi: 10.1111/j.1467-7652.2008.00330.x. [DOI] [PubMed] [Google Scholar]
- 27.Bente D, Gren J, Strong JE, Feldmann H. Disease modeling for Ebola and Marburg viruses. Dis Model Mech. 2009;2(1-2):12–17. doi: 10.1242/dmm.000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holl V, Peressin M, Moog C. Antibody-mediated Fcγ receptor-based mechanisms of HIV inhibition: Recent findings and new vaccination strategies. Viruses. 2009;1(3):1265–1294. doi: 10.3390/v1031265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bubnoff A. Antibodies: Beyond neutralization. IAVI Rep. 2010;14(1):8–12. [PubMed] [Google Scholar]
- 30.Warfield KL, et al. Induction of humoral and CD8+ T cell responses are required for protection against lethal Ebola virus infection. J Immunol. 2005;175(2):1184–1191. doi: 10.4049/jimmunol.175.2.1184. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan NJ, et al. CD8+ cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nat Med. 2011;17(9):1128–1131. doi: 10.1038/nm.2447. [DOI] [PubMed] [Google Scholar]
- 32.Koene HR, et al. FcgammaRIIIa-158V/F polymorphism influences the binding of IgG by natural k cell FcgammaRIIIa, independently of the FcgammaRIIIa-48L/R/H phenotype. Blood. 1997;90(3):1109–1114. [PubMed] [Google Scholar]
- 33.Veeramani S, et al. Rituximab infusion induces NK activation in lymphoma patients with the high-affinity CD16 polymorphism. Blood. 2011;118(12):3347–3349. doi: 10.1182/blood-2011-05-351411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finstad SL, Zhang G. Influence of Fc gamma-receptor polymorphisms on efficacy of antibody-mediated lymphocyte depletion in rhesus macaques. J Med Primatol. 2010;39(4):279–280. (abstr) [Google Scholar]
- 35.Nguyen DC, Scinicariello F, Attanasio R. Characterization and allelic polymorphisms of rhesus macaque (Macaca mulatta) IgG Fc receptor genes. Immunogenetics. 2011;63(6):351–362. doi: 10.1007/s00251-011-0514-z. [DOI] [PubMed] [Google Scholar]
- 36.Kuhn JH, et al. Evaluation of perceived threat differences posed by filovirus variants. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science. 2011;9(4):361–371. doi: 10.1089/bsp.2011.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez A, Trappier SG, Mahy BW, Peters CJ, Nichol ST. The virion glycoproteins of Ebola viruses are encoded in two reading frames and are expressed through transcriptional editing. Proc Natl Acad Sci USA. 1996;93(8):3602–3607. doi: 10.1073/pnas.93.8.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burton DR, et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266(5187):1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan NJ, et al. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature. 2003;424(6949):681–684. doi: 10.1038/nature01876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guile GR, Rudd PM, Wing DR, Prime SB, Dwek RA. A rapid high-resolution high-performance liquid chromatographic method for separating glycan mixtures and analyzing oligosaccharide profiles. Anal Biochem. 1996;240(2):210–226. doi: 10.1006/abio.1996.0351. [DOI] [PubMed] [Google Scholar]
- 41.Committee On Care and Use of Laboratory Animals . Guide for the Care and Use of Laboratory Animals. Bethesda: Natl Inst Health; 1985. DHHS Pub No (NIH) 85–23. [Google Scholar]