Abstract
Asymmetric cell division (ACD) is believed to be a physiological event that occurs during development and tissue homeostasis in a large variety of organisms. ACD produces two unequal daughter cells, one of which resembles a multipotent stem and/or progenitor cell, whereas the other has potential for differentiation. Although recent studies have shown that the balance between self-renewal and differentiation potentials is precisely controlled and that alterations in the balance may lead to tumorigenesis in Drosophila neuroblasts, it is largely unknown whether human cancer cells directly show ACD in an evolutionarily conserved manner. Here, we show that the conserved polarity/spindle protein NuMA is preferentially localized to one side of the cell cortex during cell division, generating unequal inheritance of fate-altering molecules in human neuroblastoma cell lines. We also show that the cells with a single copy of MYCN showed significantly higher percentages of ACD than those with MYCN amplification. Moreover, suppression of MYCN in MYCN-amplified cells caused ACD, whereas expression of MYCN in MYCN-nonamplified cells enhanced symmetric cell division. Furthermore, we demonstrate that centrosome inheritance follows a definite rule in ACD: The daughter centrosome with younger mother centriole is inherited to the daughter cell with NuMA preferentially localized to the cell cortex, whereas the mother centrosome with the older mother centriole migrates to the other daughter cell. Thus, the mechanisms of cell division of ACD or symmetric cell division and centrosome inheritance are recapitulated in human cancer cells, and these findings may facilitate studies on cancer stem cells.
Keywords: mitosis, CD133
Neuroblastoma is one of the most common childhood solid tumors and has a broad spectrum of clinical behavior: Some cases have a good prognosis with minimal therapy, whereas others have a very poor prognosis despite aggressive therapy (1, 2). Cases are also classified into three groups: low-, intermediate-, and high-risk groups, based on the clinical and biological characteristics (1, 2). This incredible heterogeneity defied explanation until molecular genetic and biochemical analyses of tumors began to shed light on these disparate clinical behaviors. Of many genetic and biochemical features of neuroblastomas, MYCN oncogene amplification has been correlated with an aggressive phenotype and a poor outcome (1, 2). Recent studies have shown that MYCN shows not only oncogenic activity but also plays a central role in self-renewal of normal neural stem and precursor cells (3, 4).
Neuroblastoma arises from the cells that normally make up an embryonic structure called the neural crest (5). The neural crest cells consist of multipotent and migratory cell populations that give rise to diverse cell lineages including Schwann cells, melanocytes, craniofacial cartilage and bone, smooth muscle, peripheral and enteric neurons, and glia (5). Thus, neural crest cells serve as multipotent stem cells that differentiate into mature neural tissues. It is now suspected that the multipotent neural crest cells might contribute to neuroblastoma tumorigenesis due to aberrant MYCN expression (5).
Asymmetric cell division (ACD) is a physiological process during development and tissue homeostasis in a large variety of model organisms such as Drosophila, Caenorhabditis elegans, and mouse brain and skin epidermis (6, 7). In addition, ACD has been reported in human hematopoietic stem and progenitor cells and lung cancer cells (8, 9). A stem cell will divide asymmetrically, with one of its daughters retaining self-renewal potential, whereas the other proceeds to differentiate into transit-amplifying cells. To date, ACD has been examined in the systems mentioned above, and these ACD studies have provided evidence that the molecules involved are highly conserved among invertebrates and vertebrates (6, 7). Although some adult stem cells divide asymmetrically in normal homeostasis, they retain the capacity to divide symmetrically to restore stem-cell pools depleted by injury or disease, as has been observed in the nervous and hematopoietic systems (10). Moreover, it is known that the balance between stem cell self-renewal and differentiation is precisely controlled and an imbalance leads to tumorigenesis in Drosophila neuroblasts (11–13). Therefore, we investigated the behavior of ACD and symmetric cell division (SCD) in human neuroblastoma cells.
Results
ACD Preferentially Occurs in Human Neuroblastoma Cells with a Normal MYCN Copy, but Not in Cells with MYCN Amplification.
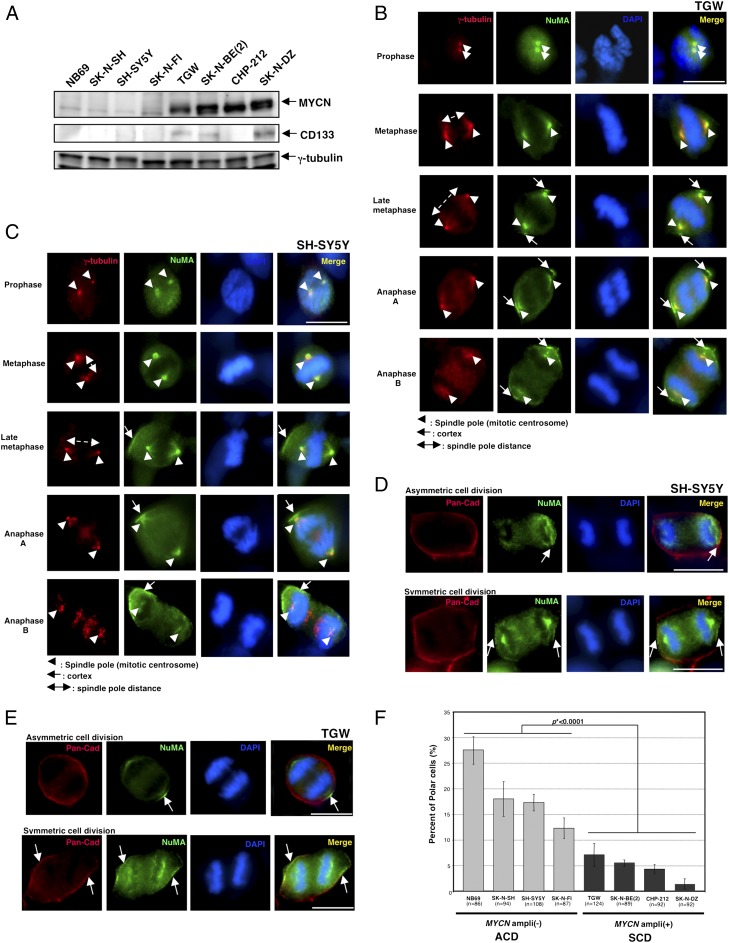
We first addressed whether NuMA (Nuclear Mitotic Apparatus protein) (14–19), one of the conserved polar proteins, is localized to the cell cortex as well as spindle poles during cell division by using HeLa cells as a control (16). Immunostaining experiments showed that NuMA is localized to the nucleus during interphase, as reported previously (15), and to spindle poles throughout mitosis (Fig. S1). In addition, we found that NuMA is also localized to both sides of the cell cortex in late metaphase and this localization signal showed a high-intensity peak during anaphase (Fig. S1). Centrosomes were also stained with anti–γ-tubulin antibody to avoid false results caused by uneven dyeing. Thus, this result showed that HeLa cells displayed symmetrical polar cell division and that cells showing asymmetric division were very rare when NuMA staining was used as a cell cortex marker (0.7%, n = 301). We next selected several human neuroblastoma cell lines with or without MYCN gene amplification. The MYCN gene status was confirmed in each cell line by fluorescence in situ hybridization (FISH) (Fig. S2). We continuously examined the expression levels of MYCN protein in the neuroblastoma cell lines by immunoblotting and immunostaining (Fig. 1A and Fig. S3). Both results showed that, whereas MYCN expression levels were very low in the cell lines with a normal MYCN copy [NB69, SK-N-SH, SH-SY5Y (a subclone of SK-N-SH), (20) and SK-N-FI], MYCN expression levels were high but slightly different among MYCN-amplified cell lines [TGW, SK-N-BE (2), CHP-212, and SK-N-DZ (Fig. 1A and Fig. S3)]. β-Catenin staining showed that, whereas the tight junctions were completely formed in cells with a single copy of MYCN (SH-SY5Y), those were partly [TGW and SK-N-BE (2)] or completely (SK-N-DZ) disrupted in cells with MYCN amplification (Fig. S3). In addition, expression of CD133, a putative neural stem cell marker (21), was positive in all MYCN-amplified cell lines except for CHP-212 (Fig. 1A). However, CD133 expression was negative in cell lines with a normal MYCN copy (Fig. 1A). For the CHP-212 cell line, we may consider that, whereas CHP212 with the double minutes form of MYCN amplification is derived from the primary site (22), the other MYCN-amplified cell lines with the homogeneously staining region form of MYCN amplification are derived from bone marrow metastasis (www.atcc.org). By using these cell lines, we tested whether these neuroblastoma cell lines showed asymmetric distribution of NuMA to the cell cortex. In all cell lines with MYCN amplification, the NuMA crescent was localized to both cell cortexes during mitosis (Fig. 1B and Fig. S4). However, in the cell lines with a normal MYCN copy number, asymmetric distribution of NuMA to one side of the cell cortex was observed during the late mitotic stages (Fig. 1C and Fig. S4). In addition, we also examined immunostaining analysis using anti-pan cadherin antibody as a cell membrane marker to avoid false images caused by uneven dyeing and found asymmetric distribution of NuMA, as expected (Fig. 1 D and E and Fig. S4). We additionally carried out similar experiments by using several single clones isolated from parental cell lines with (TGW) or without (SH-SY5Y) MYCN amplification because a specific population of cells within the cultured cells could retain the capacity to undergo ACD. As a result, we found that each clone showed no significant difference in the incidence of asymmetric distribution of NuMA in mitosis compared with parental cell lines (Fig. S5). Thus, MYCN-nonamplified cell lines showed asymmetric cell distribution of NuMA far more frequently than MYCN-amplified cell lines (P < 0.0001) (Fig. 1F).
Fig. 1.
Asymmetric cell division (ACD) occurs in human neuroblastoma cells with a normal MYCN copy, but not in the cells with MYCN amplification. (A) Immunoblot of MYCN and CD133 in human neuroblastoma cells. Both MYCN and CD133 expressions were detected in the MYCN-amplified cells, whereas the cells with a normal MYCN copy showed no expression of MYCN and CD133. Immunoblot of γ-tubulin served as a loading control. (B) Representative images of symmetric distribution of NuMA during the late stage of mitosis in TGW cells. γ-Tubulin is red, NuMA is green, and DNA is blue. Arrowheads show spindle poles. Arrows show the distribution of NuMA on the cell cortex. Double arrow shows a spindle pole distance. (C) Representative images of asymmetric distribution of NuMA during the late stage of mitosis in the SH-SY5Y cells. Centrosome is red, NuMA is green, and DNA is blue. Arrowheads show a spindle pole. Arrows show the distribution of NuMA on the cell cortex. Double arrow shows spindle pole distance. (D) Representative images of ACD at anaphase in the SH-SY5Y cells. Pan-cadherin (Pan-Cad) is red, NuMA is green, and DNA is blue. Arrows show the distribution of NuMA on the cell cortex. (E) Representative images of ACD and symmetric cell division (SCD) at anaphase in TGW cells. Pan-cadherin (Pan-Cad) is red, NuMA is green, and DNA is blue. Arrows show the cell cortex distribution of NuMA. (F) Quantification of cells with ACD in human neuroblastoma cells during the late metaphase and anaphase. Error bars represent SEM from three experiments, P < 0.0001. (Scale bars, 10 μm.)
Centrosome Inheritance in the ACD of Human Neuroblastoma Cells.
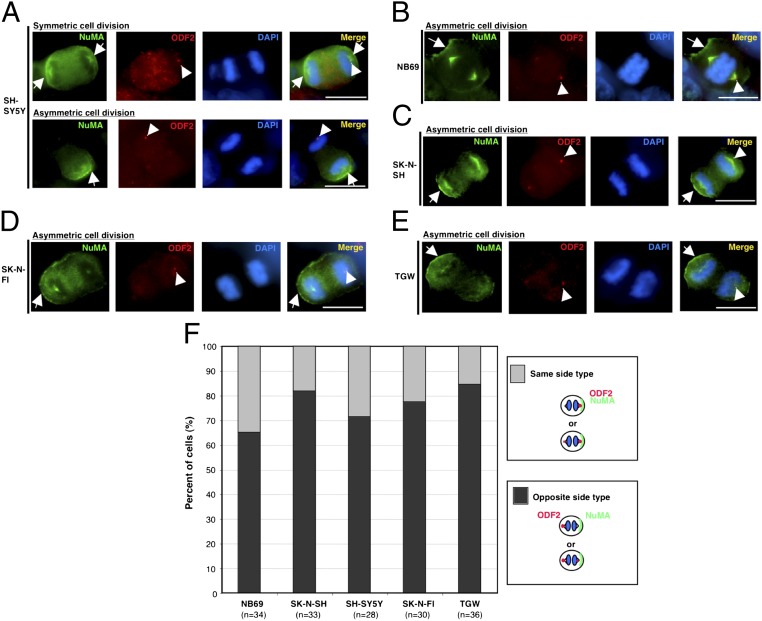
There is some controversy about the patterns of centrosome inheritance in the process of ACD because different results have been reported among studies using different model systems. In the G1 phase during the cell cycle, a centrosome contains one mother centriole and one daughter centriole. After centriole duplication, three generations of centrioles are present: an older mother, a younger mother, and two new daughters. The centrosome with the older mother centriole is termed the mother centrosome. In the case of Drosophila melanogaster male germ line stem cells, the mother centrosome stays at a stem cell that is anchored to the niche, whereas the daughter centrosome migrates to the cells at the opposite side with differentiation potential (23). However, in the case of Drosophila melanogaster neuroblasts, the mother centrosome does not stay at the daughter cell with self-renewal capacity, but migrates to the daughter cell with differentiation potential (24, 25). To address this issue, we conducted immunostaining studies of the cells with (TGW) or without (NB69, SK-N-SH, SH-SY5Y, and SK-N-FI) MYCN amplification using an antibody for the mother centrosome marker, ODF2/cenexin (26). ODF2/cenexin is acquired by young mother centrioles in G2/M transition and during mitosis, and the level of ODF2/cenexin remains higher in the centrosome with old mother centriole than in the one with young mother centriole (Fig. S6) (26). Interestingly, our results showed that, whereas the mother centrosome migrated to the daughter cell without a NuMA crescent, the daughter centrosome stayed at the other daughter cell with a NuMA crescent in a larger population of ACD cells (Fig. 2 A–F). In addition, it is known that NuMA crescent cells stayed as self-renewal neuroblasts, whereas the others had more differentiation potential in Drosophila neuroblast system (17, 18). Therefore, by analogy, our findings may also suggest that NuMA crescent cells have more self-renewal potential than the other cells, as reported previously (14).
Fig. 2.
Centrosome inheritance in ACD of human neuroblastoma cells. (A) Representative images of symmetric (Upper) and asymmetric (Lower) cell division in the SH-SY5Y cells. ODF2/cenexin is red, NuMA is green, and DNA is blue. Arrowheads show the mother centrosome because, during mitosis, one old mother centriole (at the right in the upper panel and the left in the lower panel) stained more brightly for ODF2/cenexin than the other (young mother centriole). In all images, NuMA and ODF2 were in the same focal plane. (B) Representative images of ACD in the NB69 cells. Arrowheads show a centrosome. (C) Representative images of the ACD in SK-N-SH cells. Arrowheads show a mother centrosome. (D) Representative images of ACD in the SK-N-FI cells. Arrowheads show a mother centrosome. (E) Representative images of ACD in the TGW cells. Arrowheads show a mother centrosome. (F) Quantification of centrosome inheritance during ACD in “the opposite side type” and “the same side type” in human neuroblastoma cells. Data represent average ratios from three experiments. (Scale bars, 10 μm.)
Knockdown of MYCN Expression Causes ACD in Human Neuroblastoma Cells with MYCN Amplification.
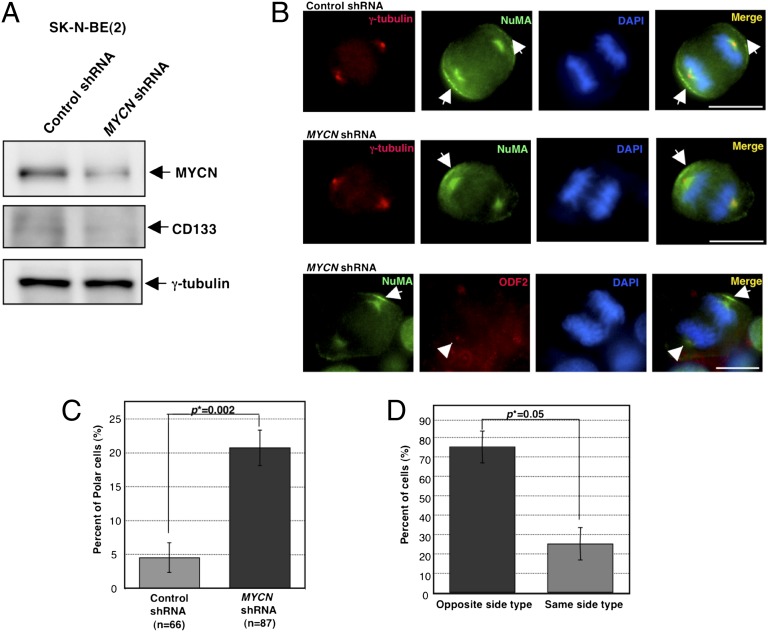
As mentioned above, it is suggested that the absence of MYCN protein expression decreases the self-renewal population and maintains a balance between stem cells and differentiated progeny via ACD. Conversely, in the MYCN-amplified cells, ACD was hardly detectable because of the self-renewal ability given by MYCN. These findings lead to the idea that MYCN may be a key regulator that controls the balance of whether cells display ACD or SCD. To test this idea, MYCN expression was knocked down with short-hairpin RNA (shRNA) in MYCN-amplified cells [SK-N-BE (2) and SK-N-DZ (Fig. S7)]. After 72 h of shRNA transfection, the down-regulation of MYCN was confirmed by immunoblotting (Fig. 3 A and Fig. S7A), and the status of cell division was investigated by immunostaining (Fig. 3 B and Fig. S7B). Our findings showed that the numbers of cells with the ACD phenotype significantly increased in both the MYCN–knocked-down cell lines compared with that in the control cells (Fig. 3 C and Fig. S7C). Moreover, the pattern of centrosome inheritance was also conserved in these cells: The mother centrosome migrated to the daughter cells without NuMA crescent (Fig. 3 B and D and Fig. S7 B and D). Importantly, CD133 expression was also down-regulated in both MYCN-amplified cells when MYCN expression was knocked down (Fig. 3A and Fig. S7A).
Fig. 3.
The knockdown of MYCN expression causes ACD in human neuroblastoma cells with MYCN amplification. (A) Immunoblot of MYCN and CD133 in SK-N-BE (2) cells transfected with control or MYCN shRNA. Immunoblot of γ-tubulin served as a loading control. (B) Representative images of SCD in SK-N-BE (2) transfected with control shRNA (Top) and ACD in SK-N-BE (2) transfected with MYCN shRNA (Middle). γ-Tubulin is red, NuMA is green, and DNA is blue. Arrows show the distribution of NuMA on the cell cortex. Representative images of centrosome inheritance during SCD in SK-N-BE (2) transfected with MYCN shRNA (Bottom). ODF2/cenexin is red, NuMA is green, and DNA is blue. An arrowhead shows a mother centrosome. Arrows show cell cortex distribution of NuMA. (C) Quantification of cells with ACD during the late metaphase and anaphase in SK-N-BE (2) transfected with control or MYCN shRNA. Error bars represent SEM from three experiments, P = 0.002. (D) Quantification of centrosome inheritance during the ACD in “the opposite side type” vs. “the same side type” in SK-N-BE (2) transfected with MYCN shRNA. Error bars represent SEM from two experiments, P = 0.05. (Scale bars, 10 μm.)
We additionally carried out similar experiments by using a MYC inhibitor, 10058-F4, which was originally identified as a small molecular compound and interferes with the dimerization of MYC-Max (27). Subsequently, it was reported that 10058-F4 decreased both levels of both MYC mRNA (45%) and MYC protein (50%) expression to the endogenous levels (28). Therefore, we exposed 10058-F4 to TGW cells and conducted immunoblotting and immunostaining experiments. As expected, 10058-F4 down-regulated MYCN expression in TGW cells (Fig. S8A). Moreover, the ratio of cells with the ACD phenotype significantly increased in 10058-F4-treated cells and became similar to that of cells with the ACD phenotype in the MYCN knocked-down cells (Fig. S8 B and C).
Overexpression of MYCN Suppresses ACD and Enhances SCD in Human Neuroblastoma Cells with a Normal MYCN Copy.
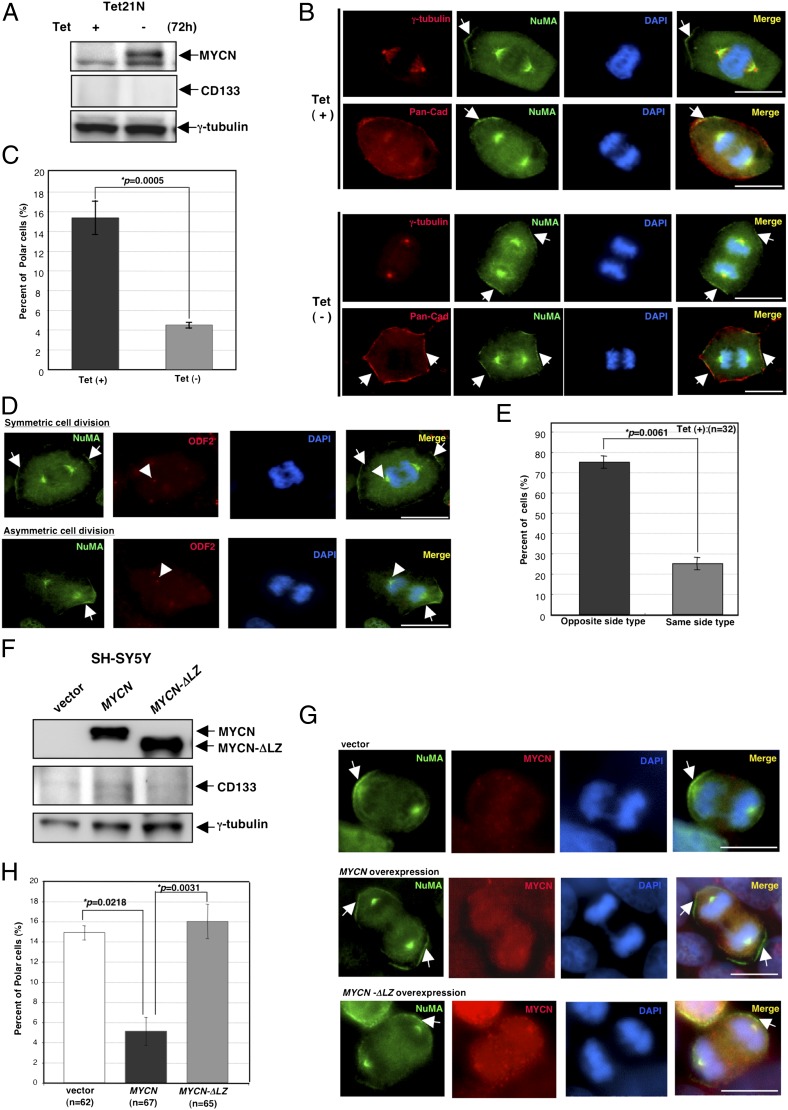
We next tried to induce MYCN expression by using the tetracycline-repressible MYCN expression cell line, which is termed Tet21N (29). The Tet21N cell line is derived from a neuroblastoma cell line, SH-EP (the substrate-adherent type), which is a subclone of SK-N-SH (the neuroblastic type) with a normal MYCN copy (5, 20). The Tet21N cells showed the ACD phenotype when MYCN expression was not induced (Fig. 4 A and B). At 72 h after induction of MYCN expression, which was confirmed by immunoblotting (Fig. 4A), the cell division status was analyzed by immunostaining (Fig. 4B). As expected, the induction of MYCN expression resulted in the suppression of the ACD phenotype and enhanced the self-renewal potential (Fig. 4 B and C). In addition, the manner of centrosome inheritance was also conserved in the cells when MYCN expression was not induced (Fig. 4 D and E).
Fig. 4.
Overexpression of MYCN suppresses ACD and enhances SCD in human neuroblastoma cells with a normal MYCN copy. (A) Immunoblot of MYCN and CD133 in Tet21N cells with or without tetracycline (1 μg/mL) treatment (Tet) for 72 h. Immunoblot of γ-tubulin served as a loading control. (B) Representative images of ACD in Tet21N cells treated with tetracycline (Tet+), and SCD in Tet21N cells without tetracycline treatment (Tet−). γ-Tubulin or pan-cadherin is red, NuMA is green, and DNA is blue. Arrows show the distribution of NuMA on the cell cortex. (C) Quantification of cells with ACD during the late metaphase and anaphase in Tet21N cells with (Tet+) or without tetracycline (Tet−). Error bars represent SEM from three experiments, P = 0.0005. (D) Representative images of SCD (Upper) and ACD (Lower) in Tet21N cells treated with tetracycline (Tet+). NuMA is green, ODF2/cenexin is red, and DNA is blue. Arrows show the distribution of NuMA on the cell cortex. An arrowhead shows a mother centrosome. (E) Quantification of centrosome inheritance during ACD in “the opposite side type” vs. “the same side type” in Tet21N cells treated with tetracycline. Error bars represent SEM from two experiments. (F) Immunoblot of MYCN and CD133 in SH-SY5Y cells transfected with a control vector (vector), pCMV-MYCN, or pCMV-MYCN-ΔLZ vector. Immunoblot of γ-tubulin served as a loading control. (G) Representative images of the ACD in SH-SY5Y transfected with the control vector (vector), those of SCD in SH-SY5Y transfected with the MYCN vector (MYCN), and those of ACD in SH-SY5Y transfected with the MYCN-ΔLZ vector (MYCN-ΔLZ). NuMA is green, MYCN is red, and DNA is blue. Arrows show the distribution of NuMA on the cell cortex. (H) Quantification of cells with ACD during late metaphase and anaphase in SH-SY5Y transfected with a control vector (vector), MYCN vector (MYCN), or MYCN-ΔLZ vector (MYCN-ΔLZ). Error bars represent SEM from three experiments, P = 0.0218 and P = 0.0031, respectively. (Scale bars, 10 μm.)
The major function of MYCN is known to be transcription, which regulates the expression and repression of a large variety of downstream target genes (30). Finally, we examined whether the transcription of MYCN is responsible for the ACD or SCD phenotype. We tried to induce overexpression of MYCN (wild-type) or MYCN-ΔLZ vector, which is a mutant with a deletion of the leucine zipper domain (31) and cannot interact with Max, resulting in transcription deficiency in the cells with a normal MYCN copy (SH-SY5Y: the neuroblastic type). After 72 h of transfection with the MYCN or MYCN-ΔLZ expression vector, immunoblotting confirmed the overexpression of MYCN or MYCN-ΔLZ in the cells (Fig. 4F), and the status of the cell division was analyzed by immunostaining (Fig. 4G). When MYCN was overexpressed in the SH-SY5Y cells, CD133 expression was up-regulated (Fig. 4F). This result is not surprising because the SH-SY5Y cells are known to show the neuroblastic phenotype (5). In contrast, CD133 expression was not up-regulated in the cells transfected with the MYCN-ΔLZ expression vector (Fig. 4F) or MYCN-induced Tet21N cells (Fig. 4A). The different expression status of CD133 may be due to the different phenotypes represented by the two cell lines (neuroblastic or substrate-adherent), although they were derived from the same parental cell line, SK-N-SH. In addition, when MYCN was overexpressed, the cells showing the ACD phenotype were significantly decreased (Fig. 4 G and H). However, even when MYCN-ΔLZ was overexpressed, the ratios of the cells showing the ACD phenotype were similar between the control and MYCN-ΔLZ–transfected cells (Fig. 4 G and H). These results strongly suggested that MYCN controls the balance of cell fate in terms of whether cells display ACD or SCD via its transcriptional function.
Discussion
In the present study, we found that human neuroblastoma cell lines without MYCN expression showed the ACD phenotype. Recent studies showed that human neuroblastoma cells contain tumor-initiating cells whose phenotype resembles that of cancer stem cells, including features such as self-renewal, induction of multilineage cell differentiation, and high drug efflux capacity (32, 33). Therefore, the present finding may add the ACD phenotype to the features of cancer-initiating/stem cells that are present in the neuroblastoma cell lines with a normal copy of MYCN.
Our findings showed that whereas MYCN overexpression suppressed ACD and enhanced SCD, the down-regulation of MYCN caused ACD and suppressed SCD in human neuroblastoma cells. These results are reasonable because the overexpression of MYCN may overproduce cancer-initiating/stem cells with high frequency through SCD, leading to the emergence of accumulating cancer stem cells (10). The MYC gene family is a key transcriptional factor for producing the induced-pluripotent stem cells (34), and the present study using the wild-type or deletion-mutant type of MYCN vector showed that the transcriptional activity of MYCN is involved in the enhanced accumulation of the self-renewal population through SCD. Neuroblastoma with MYCN amplification is known to show aggressive behavior, and the present findings that MYCN overproduces CD133-positive cells through SCD may explain one of the mechanisms behind the aggressiveness and poor outcome of MYCN-amplified tumors.
We speculate that MYCN is one of the components that enhance the SCD phenotype, and that there may be additional positive regulators; one such candidate is Bmi-1, which is overexpressed in MYCN-amplified cells (35–37) and required for the self-renewal of stem cells in the peripheral and central nervous system (38). In addition, β-catenin staining in the present study shows that whereas the tight junctions were completely formed in cells with a single copy of MYCN (SH-SY5Y), those were partly [TGW and SK-N-BE (2)] or completely (SK-N-DZ) disrupted in cells with MYCN amplification (Fig. S3). The complete tight junctions and the high frequency of ACD were found in the cells with a normal copy of MYCN, indicating that such cells might receive an extrinsic clue for ACD through cell–cell contact (39).
We showed that the ACD ratios ranged from 1.7 to 30% in the neuroblastoma cell lines with or without MYCN amplification. ACD is a very smart strategy because it maintains appropriate numbers of self-renewal and differentiated cells with a single division; however, a disadvantage of this strategy is that it leaves stem cells unable to expand in number (10). The ACD ratio of 30% may be the upper limit for the neuroblastoma cell lines without MYCN expression because the cell lines will lose their self-renewal population if more than 30% of the cells undergo ACD. Interestingly, the human CD34+ CD133+ hematopoietic stem and progenitor cells showed ∼20% ACD (8). The percentages of ACD are similar to those of ACD identified in the present study of neuroblastoma cell lines.
We showed that a MYC inhibitor, 10058-F4, suppressed MYCN expression, CD133 expression, and SCD and caused ACD in a neuroblastoma cell line. These findings suggest that certain therapeutic agents could affect the balance between ACD and SCD in tumor-initiating cell populations.
In the case of ACD, there is controversy over the pattern of centrosome inheritance because different results were reported among various model systems: the mother centrosome remained in the daughter cells with self-renewal potential in Drosophila male germ line stem cells (23) and in developing mouse brain neocortex cells (40), whereas the mother centrosome migrated into the daughter cells with differentiation potential in Drosophila neuroblasts (24, 25). Because NuMA (Mud: NuMA ortholog in Drosophila) is known to bind to apical polar proteins to ensure the apical–basal orientation of the mitotic spindle in Drosophila neuroblasts and mouse dermal epidermis cells (14, 18, 19), our findings suggest that the pattern of centrosome migration in neuroblastoma was consistent with that of centrosome migration reported in Drosophila neuroblasts. We would explain these conflicting findings by the following possibility. ODF2/cenexin, which was used as an old mother centrosome marker in our experiment, is known to be a component of appendages in the mother centriole. Because ODF2/cenexin has differentiation activity such as formation of primary cilia (41), multipotent stem cells might not tolerate the mother centrosome because of its differentiation potential. These issues should be addressed in the near future.
In summary, we showed here that it is possible to analyze asymmetric cell division in human cancer by using cultured neuroblastoma cells. The manners of cell division of ACD or SCD and centrosome inheritance conserved in Drosophila neuroblasts are recapitulated in human cancer cells. Our findings may accelerate studies on the molecular mechanism of asymmetric cell division in human stem cells.
Materials and Methods
Detailed materials and methods are provided in SI Materials and Methods.
Cell Lines and Transfections.
All cell lines were maintained in complete medium DMEM, supplemented with 10% (vol/vol) FBS, penicillin [100 units/mL (vol/vol)] and streptomycin [100 μg/mL (vol/vol)] in an atmosphere containing 5% CO2 at 37 °C.
The shRNAs and vector DNAs were transfected using lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Indirect Immunofluorescence.
Indirect Immunofluorescences were performed as previously described (42).
Immunoblot Analyses.
Immunoblot analyses were performed as previously described (42).
Supplementary Material
Acknowledgments
We thank Dr. M. Schwab for providing Tet21N cells, Dr. T. Kamijo for providing anti–N-Myc antibody, and K. Ono and H. Odagawa for technical assistance. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan and The Kawano Masanori Memorial Foundation for the Promotion of Pediatrics, Japan.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1205525109/-/DCSupplemental.
References
- 1.Brodeur GM. Neuroblastoma: Biological insights into a clinical enigma. Nat Rev Cancer. 2003;3(3):203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 2.Schwab M, Westermann F, Hero B, Berthold F. Neuroblastoma: Biology and molecular and chromosomal pathology. Lancet Oncol. 2003;4(8):472–480. doi: 10.1016/s1470-2045(03)01166-5. [DOI] [PubMed] [Google Scholar]
- 3.Knoepfler PS, Cheng PF, Eisenman RN. N-myc is essential during neurogenesis for the rapid expansion of progenitor cell populations and the inhibition of neuronal differentiation. Genes Dev. 2002;16(20):2699–2712. doi: 10.1101/gad.1021202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotterman R, Knoepfler PS. N-Myc regulates expression of pluripotency genes in neuroblastoma including lif, klf2, klf4, and lin28b. PLoS ONE. 2009;4(6):e5799. doi: 10.1371/journal.pone.0005799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross RA, Spengler BA. Human neuroblastoma stem cells. Semin Cancer Biol. 2007;17(3):241–247. doi: 10.1016/j.semcancer.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Knoblich JA. Asymmetric cell division: Recent developments and their implications for tumour biology. Nat Rev Mol Cell Biol. 2010;11(12):849–860. doi: 10.1038/nrm3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morin X, Bellaïche Y. Mitotic spindle orientation in asymmetric and symmetric cell divisions during animal development. Dev Cell. 2011;21(1):102–119. doi: 10.1016/j.devcel.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Beckmann J, Scheitza S, Wernet P, Fischer JC, Giebel B. Asymmetric cell division within the human hematopoietic stem and progenitor cell compartment: Identification of asymmetrically segregating proteins. Blood. 2007;109(12):5494–5501. doi: 10.1182/blood-2006-11-055921. [DOI] [PubMed] [Google Scholar]
- 9.Pine SR, Ryan BM, Varticovski L, Robles AI, Harris CC. Microenvironmental modulation of asymmetric cell division in human lung cancer cells. Proc Natl Acad Sci USA. 2010;107(5):2195–2200. doi: 10.1073/pnas.0909390107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441(7097):1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- 11.Caussinus E, Gonzalez C. Induction of tumor growth by altered stem-cell asymmetric division in Drosophila melanogaster. Nat Genet. 2005;37(10):1125–1129. doi: 10.1038/ng1632. [DOI] [PubMed] [Google Scholar]
- 12.Clevers H. Stem cells, asymmetric division and cancer. Nat Genet. 2005;37(10):1027–1028. doi: 10.1038/ng1005-1027. [DOI] [PubMed] [Google Scholar]
- 13.Neumüller RA, et al. Genome-wide analysis of self-renewal in Drosophila neural stem cells by transgenic RNAi. Cell Stem Cell. 2011;8(5):580–593. doi: 10.1016/j.stem.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437(7056):275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radulescu AE, Cleveland DW. NuMA after 30 years: The matrix revisited. Trends Cell Biol. 2010;20(4):214–222. doi: 10.1016/j.tcb.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodard GE, et al. Ric-8A and Gi alpha recruit LGN, NuMA, and dynein to the cell cortex to help orient the mitotic spindle. Mol Cell Biol. 2010;30(14):3519–3530. doi: 10.1128/MCB.00394-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izumi Y, Ohta N, Hisata K, Raabe T, Matsuzaki F. Drosophila Pins-binding protein Mud regulates spindle-polarity coupling and centrosome organization. Nat Cell Biol. 2006;8(6):586–593. doi: 10.1038/ncb1409. [DOI] [PubMed] [Google Scholar]
- 18.Siller KH, Cabernard C, Doe CQ. The NuMA-related Mud protein binds Pins and regulates spindle orientation in Drosophila neuroblasts. Nat Cell Biol. 2006;8(6):594–600. doi: 10.1038/ncb1412. [DOI] [PubMed] [Google Scholar]
- 19.Bowman SK, Neumüller RA, Novatchkova M, Du Q, Knoblich JA. The Drosophila NuMA Homolog Mud regulates spindle orientation in asymmetric cell division. Dev Cell. 2006;10(6):731–742. doi: 10.1016/j.devcel.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Ross RA, Spengler BA, Biedler JL. Coordinate morphological and biochemical interconversion of human neuroblastoma cells. J Natl Cancer Inst. 1983;71(4):741–747. [PubMed] [Google Scholar]
- 21.Singh SK, Clarke ID, Hide T, Dirks PB. Cancer stem cells in nervous system tumors. Oncogene. 2004;23(43):7267–7273. doi: 10.1038/sj.onc.1207946. [DOI] [PubMed] [Google Scholar]
- 22.Granchi D, Baglìo SR, Amato I, Giunti A, Baldini N. Paracrine inhibition of osteoblast differentiation induced by neuroblastoma cells. Int J Cancer. 2008;123(7):1526–1535. doi: 10.1002/ijc.23654. [DOI] [PubMed] [Google Scholar]
- 23.Yamashita YM, Mahowald AP, Perlin JR, Fuller MT. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science. 2007;315(5811):518–521. doi: 10.1126/science.1134910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conduit PT, Raff JW. Cnn dynamics drive centrosome size asymmetry to ensure daughter centriole retention in Drosophila neuroblasts. Curr Biol. 2010;20(24):2187–2192. doi: 10.1016/j.cub.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 25.Januschke J, Llamazares S, Reina J, Gonzalez C. Drosophila neuroblasts retain the daughter centrosome. Nat Commun. 2011;2:243. doi: 10.1038/ncomms1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson CT, Stearns T. Centriole age underlies asynchronous primary cilium growth in mammalian cells. Curr Biol. 2009;19(17):1498–1502. doi: 10.1016/j.cub.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin X, Giap C, Lazo JS, Prochownik EV. Low molecular weight inhibitors of Myc-Max interaction and function. Oncogene. 2003;22(40):6151–6159. doi: 10.1038/sj.onc.1206641. [DOI] [PubMed] [Google Scholar]
- 28.Sampson VB, et al. MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Res. 2007;67(20):9762–9770. doi: 10.1158/0008-5472.CAN-07-2462. [DOI] [PubMed] [Google Scholar]
- 29.Lutz W, et al. Conditional expression of N-myc in human neuroblastoma cells increases expression of alpha-prothymosin and ornithine decarboxylase and accelerates progression into S-phase early after mitogenic stimulation of quiescent cells. Oncogene. 1996;13(4):803–812. [PubMed] [Google Scholar]
- 30.Laurenti E, Wilson A, Trumpp A. Myc’s other life: Stem cells and beyond. Curr Opin Cell Biol. 2009;21(6):844–854. doi: 10.1016/j.ceb.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Nakagawa M, Takizawa N, Narita M, Ichisaka T, Yamanaka S. Promotion of direct reprogramming by transformation-deficient Myc. Proc Natl Acad Sci USA. 2010;107(32):14152–14157. doi: 10.1073/pnas.1009374107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirschmann-Jax C, et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA. 2004;101(39):14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahller YY, et al. Neuroblastoma cell lines contain pluripotent tumor initiating cells that are susceptible to a targeted oncolytic virus. PLoS ONE. 2009;4(1):e4235. doi: 10.1371/journal.pone.0004235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, et al. A transcriptional roadmap to the induction of pluripotency in somatic cells. Stem Cell Rev. 2010;6(2):282–296. doi: 10.1007/s12015-010-9137-2. [DOI] [PubMed] [Google Scholar]
- 35.Cui H, et al. Bmi-1 is essential for the tumorigenicity of neuroblastoma cells. Am J Pathol. 2007;170(4):1370–1378. doi: 10.2353/ajpath.2007.060754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ochiai H, et al. Bmi1 is a MYCN target gene that regulates tumorigenesis through repression of KIF1Bbeta and TSLC1 in neuroblastoma. Oncogene. 2010;29(18):2681–2690. doi: 10.1038/onc.2010.22. [DOI] [PubMed] [Google Scholar]
- 37.Huang R, et al. MYCN and MYC regulate tumor proliferation and tumorigenesis directly through BMI1 in human neuroblastomas. FASEB J. 2011;25(12):4138–4149. doi: 10.1096/fj.11-185033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molofsky AV, et al. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425(6961):962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siegrist SE, Doe CQ. Extrinsic cues orient the cell division axis in Drosophila embryonic neuroblasts. Development. 2006;133(3):529–536. doi: 10.1242/dev.02211. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, et al. Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature. 2009;461(7266):947–955. doi: 10.1038/nature08435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishikawa H, Kubo A, Tsukita S, Tsukita S. Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nat Cell Biol. 2005;7(5):517–524. doi: 10.1038/ncb1251. [DOI] [PubMed] [Google Scholar]
- 42.Izumi H, et al. BubR1 localizes to centrosomes and suppresses centrosome amplification via regulating Plk1 activity in interphase cells. Oncogene. 2009;28:2806–2820. doi: 10.1038/onc.2009.141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.