Seeing “nitrification” and “Archaea” in the title of the paper in PNAS by Alonso-Sáez et al. (1) will not surprise anyone following the story about the role of these microbes in an important pathway of the nitrogen cycle. What will puzzle, if not surprise, everyone is the other key word, “urea.” That nitrogenous compound never comes up in discussions of nitrification and Archaea, and even other organic nitrogen forms figure into the story only indirectly. Thus, it is a surprise to see the evidence from Alonso-Sáez et al. (1) indicating that a group of Archaea uses urea to fuel a key step in nitrification in the Arctic Ocean and Antarctic seas. Their study suggests a previously undescribed, shorter pathway in the nitrogen cycle and may help to explain the relatively high abundance of Archaea in some ecosystems.
Once thought to be relegated to extreme environments, Archaea are now known to be everywhere. They are particularly common in the deep ocean, where Archaea rival bacteria in abundance, although both prokaryotic groups are about 100-fold less abundant than in surface waters (2). Originally classified as being in the Crenarchaeota phylum, many of these oceanic Archaea have been put into a new phylum, the Thaumarchaeota, because of differences with thermophilic relatives. Hints about the biogeochemical role of these Archaea first came from the Sargasso Sea metagenomic study of Venter et al. (3), which was soon followed up by several surveys of the abundance of a key functional gene in marine as well as terrestrial systems (4). The defining feature of these microbes seemed to be nailed down by studies of Nitrosopumilus maritimus, an archaeon originally isolated from a saltwater aquarium (5). This isolate remains the only one representing the marine Thaumarchaeota, although genomes of a few Archaea in consortia have been deduced by metagenomic sequencing.
The pure culture work, metagenomic data, and biogeochemical studies all indicated that marine Thaumarchaeota are ammonia oxidizers, mediating the first, rate-limiting step in nitrification. The energy gained from ammonia oxidation is used to fuel reduction of carbon dioxide, making these microbes chemoautotrophs or to use their full name, chemoautolithotrophs (Fig. 1A). Previous to these discoveries over the past 10 y, chemoautotrophic ammonia oxidation was thought to be carried out mainly by Betaproteobacteria in a reaction first suggested by Pasteur in 1861 (6). The recent findings helped to explain the success of Archaea in the deep ocean and other environments. Still, they did not change our ideas about nitrification. Ammonia oxidation seemed to be the same as in Pasteur’s time, just carried out by a different group of microbes.
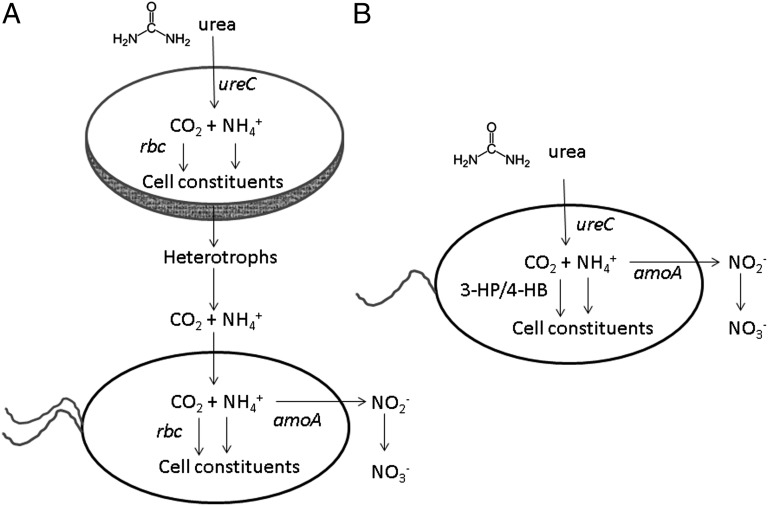
Fig. 1.
Pathways linking urea and nitrification. (A) Current pathway, featuring an autotroph, such as an alga, that takes up and degrades urea using urease (ureC). The resulting CO2 is fixed by ribulose bisphosphate carboxylase oxygenase (rbc) in the Calvin–Benson–Bassham (CBB) cycle. The resulting ammonium is taken up by a chemolithotrophic bacterium that oxidizes ammonia to nitrite using ammonia monooxygenase (amoA). The second step in nitrification, the oxidation of nitrite to nitrate, is carried out by other microbes. (B) Shorter pathway suggested Alonso-Sáez et al. (1). Instead of one microbe degrading urea and another oxidizing ammonia, the authors suggest that some marine Archaea combine the reactions. Rather than the CBB cycle, these Archaea use the 3-hydroxypropionate-4-hydroxybutyrate pathway (3-HP/4-HB) for inorganic carbon fixation.
Alonso-Sáez et al. (1) knew all this as they began their studies in the Beaufort Sea, north of Canada. They also knew of previous work showing a fairly high abundance of Archaea even in surface waters of polar seas, especially in winter. It was no surprise then when they found Archaea to be abundant in the Beaufort Sea and Antarctic seas. Using a type of FISH, a microscopic approach to identify microbes without cultivation, Alonso-Sáez et al. (1) discovered that Archaea made up 6% of total microbial abundance in the Beaufort Sea in January 2008. It was more of a surprise to see these Archaea increase in abundance to 18% by March of the same year. These investigators then ran into their first puzzle. They did microautoradiography assays to examine whether these Archaea are chemoautotrophs, and thus use 14C-CO2, or are heterotrophs, and thus take up 3H-leucine. To the investigators’ chagrin, the Beaufort Sea Archaea took up neither compound, even though bacteria were active in taking up both, especially leucine. Some of the Archaea undoubtedly were inactive, but many had to be doing something to account for the threefold increase in abundance in the Beaufort Sea.
Metagenomics provided a possible answer. Alonso-Sáez et al. (1) analyzed sequence data collected in March from 65 m deep in the water column, where nitrite concentrations, and possibly nitrification, were highest. They found ammonia oxidation genes (amoA) as well as 16S rRNA genes most similar to genes from N. maritimus and other marine Thaumarchaeota. The abundance of the two genes suggested that most of the Archaea in these waters are ammonia oxidizers. More revealing, they found several genes (ureA, ureB, and ureC) for urease (urea amidohydrolase), the enzyme catalyzing the degradation of urea. The urease genes were most closely related to genes from “Candidatus Cenarchaeum symbiosum,” a symbiotic archaeon thought to use urea excreted as a waste product by its sponge host. What is more, using quantitative PCR, Alonso-Sáez et al. (1) found that the ratio of ureC to archaeal 16S rRNA genes was low in Arctic surface waters (0.2) but close to one for deep waters. It was even higher for the circumpolar deep waters of Antarctica. Ratios of one and higher suggested that a large fraction of Thaumarchaeota in these waters degrade urea. Finally, based on simple calculations with a few estimates of concentrations and turnover, Alonso-Sáez et al. (1) suggest that fluxes of urea, but not of ammonium, were high enough to support the net archaeal growth they observed from January to March in the Beaufort Sea.
These data led Alonso-Sáez et al. (1) to hypothesize that polar marine Thaumarchaeota take up and degrade urea, oxidize the resulting ammonia to nitrite, and fix the carbon into biomass by a chemoautotrophic pathway (Fig. 1B). They found that polar marine Thaumarchaeota have genes for the 3-hydroxypropionate-4-hydroxybutyrate pathway used by N. maritimus and other chemoautotrophic Archaea for carbon fixation (7). The authors’ model explains how 14CO2 uptake could be low yet net growth high in the Beaufort Sea. Because of low energetic yield, many ammonia molecules have to be oxidized to support the fixation of one carbon (the authors assume a nitrogen/carbon ratio of about 22). Consequently, urea could supply all the carbon needed for chemoautotrophic growth, resulting in little 14CO2
Alonso-Sáez et al. build a persuasive case for urea-dependent growth by chemoautotrophic Thaumarchaeota in polar seas.
use in microautoradiography assays. The model explains all the data and suggestsa shortcut between urea and nitrate, the end product of nitrification.
Alonso-Sáez et al. (1) build a persuasive case for urea-dependent growth by chemoautotrophic Thaumarchaeota in polar seas. Recent soil studies provide indirect support for the authors’ model (8, 9). However, the case for a urea-nitrification connection would be really bolstered by data on actual activity rather than just genetic potential. A key experiment would be to use microautoradiography coupled with FISH to show assimilation of 14C from 14C-urea by Thaumarchaeota communities in marine waters. Finally, many microbiologists will be convinced only by cultivating a microbe in the laboratory that can grow on urea, using the ammonia and CO2 resulting from urea degradation as the sole energy and carbon sources. An ammonia-oxidizing archeaon isolated from soil can grow on urea as an energy source (9).
Even if Alonso-Sáez et al. (1) have the answer for polar waters, more work is needed, of course, to see how far afield their results can take us. Urea may be less important in other environments, because fluxes appear to be unusually high in the Arctic Sea, as reviewed by Alonso-Sáez et al. (1). What about other nitrogenous compounds? Although the pool size of dissolved organic nitrogen is much larger than that of either urea or ammonium, the known ammonia oxidizers, including N. maritimus, are obligate chemolithotrophs unable to use organic nitrogen (4, 5). Regardless, urea degradation says little about the capacity to use other nitrogenous compounds, because urea is more inorganic than organic as viewed by microbes (10); the carbon compound released by urease, carbamate, cannot be used to support heterotrophic growth because it spontaneously decomposes to carbonate. The biggest unknown is whether urea-fueled nitrification occurs in the deep oceans of lower latitudes, where most marine Thaumarchaeota live. Answering these questions is key to understanding a critical part of the nitrogen cycle and a fascinating group of microbes.
Footnotes
The author declares no conflict of interest.
See companion article on page 17989.
References
- 1.Alonso-Sáez L, et al. Role for urea in nitrification by polar marine Archaea. Proc Natl Acad Sci USA. 2012;109:17989–17994. doi: 10.1073/pnas.1201914109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karner MB, DeLong EF, Karl DM. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature. 2001;409(6819):507–510. doi: 10.1038/35054051. [DOI] [PubMed] [Google Scholar]
- 3.Venter JC, et al. Environmental genome shotgun sequencing of the Sargasso Sea. Science. 2004;304(5667):66–74. doi: 10.1126/science.1093857. [DOI] [PubMed] [Google Scholar]
- 4.Zehr JP, Kudela RM. Nitrogen cycle of the open ocean: From genes to ecosystems. Annu Rev Mar Sci. 2011;3(1):197–225. doi: 10.1146/annurev-marine-120709-142819. [DOI] [PubMed] [Google Scholar]
- 5.Könneke M, et al. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature. 2005;437(7058):543–546. doi: 10.1038/nature03911. [DOI] [PubMed] [Google Scholar]
- 6.Dworkin M. Sergei Winogradsky: A founder of modern microbiology and the first microbial ecologist. FEMS Microbiol Rev. 2012;36(2):364–379. doi: 10.1111/j.1574-6976.2011.00299.x. [DOI] [PubMed] [Google Scholar]
- 7.Hanson TE, Alber BE, Tabita FR. Phototrophic CO2 fixation: Recent insights into ancient metabolisms. In: Burnap RL, Vermaas W, editors. Functional Genomics and Evolution of Photosynthetic Systems, Advances in Photosynthesis and Respiration. Dordrecht, The Netherlands: Springer; 2012. pp. 225–251. [Google Scholar]
- 8.Lu L, et al. Nitrification of archaeal ammonia oxidizers in acid soils is supported by hydrolysis of urea. ISME J. 2012;6(10):1978–1984. doi: 10.1038/ismej.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tourna M, et al. Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc Natl Acad Sci USA. 2011;108(20):8420–8425. doi: 10.1073/pnas.1013488108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solomon CM, Collier JL, Berg GM, Glibert PM. Role of urea in microbial metabolism in aquatic systems: A biochemical and molecular review. Aquat Microb Ecol. 2010;59(1):67–88. [Google Scholar]