Abstract
Chronic intermittent access to alcohol leads to the escalation of alcohol intake, similar to binge drinking in humans. Converging lines of evidence suggest that impairment of medial prefrontal cortex (mPFC) cognitive function and overactivation of the central nucleus of the amygdala (CeA) are key factors that lead to excessive drinking in dependence. However, the role of the mPFC and CeA in the escalation of alcohol intake in rats with a history of binge drinking without dependence is currently unknown. To address this issue, we examined FBJ murine osteosarcoma viral oncogene homolog (Fos) expression in the mPFC, CeA, hippocampus, and nucleus accumbens and evaluated working memory and anxiety-like behavior in rats given continuous (24 h/d for 7 d/wk) or intermittent (3 d/wk) access to alcohol (20% vol/vol) using a two-bottle choice paradigm. The results showed that abstinence from alcohol in rats with a history of escalation of alcohol intake specifically recruited GABA and corticotropin-releasing factor (CRF) neurons in the mPFC and produced working memory impairments associated with excessive alcohol drinking during acute (24–72 h) but not protracted (16 –68 d) abstinence. Moreover, abstinence from alcohol was associated with a functional disconnection of the mPFC and CeA but not mPFC and nucleus accumbens. These results show that recruitment of a subset of GABA and CRF neurons in the mPFC during withdrawal and disconnection of the PFC–CeA pathway may be critical for impaired executive control over motivated behavior, suggesting that dysregulation of mPFC interneurons may be an early index of neuroadaptation in alcohol dependence.
Keywords: addiction, alcoholism, stress, compulsivity, connectivity
Alcoholism is a chronic relapsing disorder associated with compulsive drinking, loss of control over intake, and emergence of a negative emotional state during abstinence from the drug (1). Although no known animal model of addiction fully emulates the condition in humans, some models are better suited for the investigation of specific elements of the addiction process in a clinically relevant manner. Recently, an animal model of alcohol binge drinking with good face and predictive validity for what may be considered a transition to alcoholism has been reintroduced (2, 3). Rats given extended (24 h/d) and intermittent (every other day) choice access to ethanol escalate their intake of alcohol over the course of 2–4 wk in a binge-like pattern (2–6), and alcohol drinking using this paradigm was reduced by two drugs approved by the US Food and Drug Administration for the treatment of alcoholism (i.e., naltrexone and acamprosate) (2). Moreover, escalation of alcohol drinking using this model is associated with decreased dopamine levels in the nucleus accumbens after 24 h of abstinence (7), decreased endocannabinoid signaling in the dorsolateral striatum (8), and activation of FBJ murine osteosarcoma viral oncogene homolog B (∆FosB) in the nucleus accumbens core, dorsolateral striatum, and orbitofrontal cortex.
Converging lines of evidence from human and animal studies suggest that impairment of medial prefrontal cortex (mPFC) cognitive function related to impaired executive control over motivated behavior and overactivation of the brain stress system in the central nucleus of the amygdala (CeA) are key factors in the transition from goal-directed to compulsive alcohol drinking in alcoholism (9). However, the respective role of the mPFC and CeA in the escalation of alcohol intake in rats with binge drinking without dependence is currently unknown, and the anatomical and functional consequences of a history of escalation of alcohol intake remain to be investigated.
In the present study, we tested the hypothesis that abstinence and renewed access to alcohol dysregulate the mPFC and CeA to produce long-term cognitive impairment and increased anxiety-like behavior in rats with a history of escalation of alcohol intake, and dysfunction of these brain regions predicts alcohol drinking when access to alcohol is renewed. To test this hypothesis, we combined the model of escalation of alcohol intake [intermittent access to 20% (vol/vol) alcohol] with measurements of mPFC, CeA, nucleus accumbens core and shell, and hippocampus neuronal reactivity, reflected by Fos expression, 24 h into abstinence and after 2 h of renewed access to alcohol. The mPFC is composed of two main populations of neurons (10): pyramidal glutamatergic neurons that send long excitatory projections to the basal ganglia and amygdala and GABA interneurons that send local GABAergic (GAD67) inhibitory projections to pyramidal neurons and scattered corticotropin-releasing factor (CRF) interneurons, with function that is currently unknown (11). To determine the mechanism of action of the recruitment of the mPFC, we measured the degree of colocalization of Fos with GAD67, a marker of GABAergic interneurons, and CRF, a marker of CRFergic interneurons, in the mPFC. To evaluate the functional integrity of the mPFC and CeA, respectively, we tested working memory performance in a Y-maze and the delayed nonmatching-to-sample task (DNMS) and anxiety-like behavior in the elevated plus maze. Overall, the results showed that alcohol withdrawal activated a specific population of GABAergic and CRFergic neurons in the mPFC, associated with a specific functional disconnection between the mPFC and the CeA that predicted impaired working memory function and increased binge drinking during acute abstinence. Dysregulation of the mPFC and impairment of executive control over motivated behavior may be an early index of neuroadaptation that leads to excessive binge drinking in rats.
Results
Intermittent Access to Alcohol Produces an Escalation of Alcohol Intake Associated with Increased Blood Alcohol Levels.
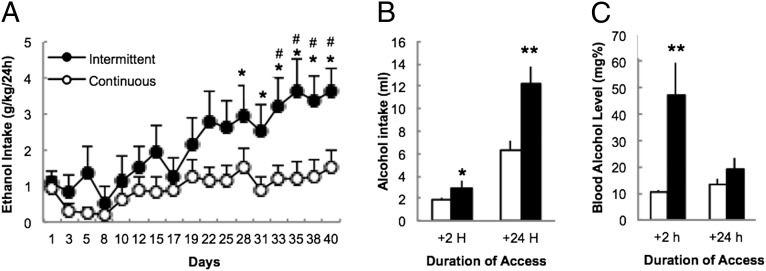
Rats given intermittent (3 d/wk; n = 12) access to alcohol increased their intake over the course of 6 wk (F1,16 = 3.1, P < 0.00001), whereas rats given continuous access (24 h/d for 7 d/wk; n = 12) maintained stable intake (Fig. 1A). Escalation of intake was observed after 2 (t22 = 2.1, P = 0.05) and 24 h (t22 = 3.2, P = 0.003) of access (Fig. 1B), which was associated with elevated blood alcohol levels (BALs) after 2 h (t21 = 2.9, P = 0.009) (Fig. 1C).
Fig. 1.
Intermittent access to alcohol induces an escalation of alcohol intake and increased blood alcohol levels. (A) Alcohol intake in rats given intermittent or continuous access to 20% alcohol. *P < 0.05 vs. day 1; #P < 0.005 vs. continuous. (B) Average alcohol intake (milliliters) after 2 and 24 h of access to alcohol. *P < 0.05; **P < 0.005. (C) Blood alcohol levels after 2 and 24 h of access to alcohol. The data are expressed as mean ± SEM.
Abstinence-Induced Activation of the mPFC Predicts Excessive Alcohol Intake and Is Associated with Working Memory Impairment.
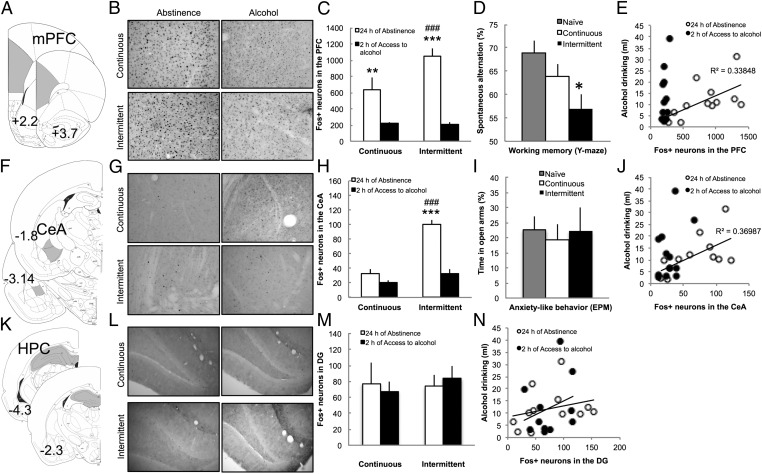
To test the hypothesis that excessive alcohol intake is associated with dysfunction of the mPFC, we evaluated spontaneous alternation in a Y-maze after 24 h of abstinence and measured the number of Fos-positive (Fos+) neurons in the mPFC (Fig. 2A) after 24 h of abstinence and after 2 h of access to alcohol after 24 h of abstinence. No difference was observed between the cingulate + dorsal prelimbic (dPFC) and ventral prelimbic + infralimbic (vPFC), and therefore, it was combined as the mPFC. The intermittent group exhibited increased Fos+ neurons during abstinence in the mPFC compared with the continuous access group (F1,20 = 5.0, P = 0.03) (Fig. 2 B and C and SI Results have statistical details). Renewed access to alcohol reduced the number of Fos+ neurons in the continuous access group and normalized the level of Fos+ neurons in the intermittent access group to a level similar to rats with continuous access (Fig. 2 B and C). Rats with intermittent access to alcohol exhibited lower spontaneous alternation in the Y-maze compared with naive and continuous access rats (F2,35 = 4.6, P = 0.017) (Fig. 2D). A positive correlation was observed between the number of Fos+ neurons recruited in the mPFC during abstinence and the level of alcohol intake after 24 h of abstinence (R2 = 0.34, P < 0.05) (Fig. 2E). After renewed access to alcohol for 2 h, when Fos+ activity was normalized, the number of Fos+ neurons no longer predicted alcohol intake (R2 = 0.17, P = 0.17) (Fig. 2E).
Fig. 2.
Abstinence-induced increases in Fos+ neurons in the mPFC predict alcohol intake and are associated with working memory impairment. (A) Anatomical schema of the regions of the mPFC comprising the anterior cingulate, prelimbic, and infralimbic cortices. (B) Representative 40-μm coronal sections of the mPFC that show Fos+ neurons after 24 h of abstinence (Left) and 2 h of access to alcohol (Right) in rats with continuous (Upper) or intermittent (Lower) access to alcohol. (C) Average number of Fos+ neurons in the mPFC. ###P < 0.001 vs. continuous; **P < 0.05; ***P < 0.001 vs. 2 h of access to alcohol. (D) Spontaneous alternation in a Y-maze after 24 h of forced abstinence in naive, continuous, and intermittent access rats. Notice that 50% corresponds to chance. *P < 0.01 vs. naive. (E) Correlation between number of Fos+ neurons in the mPFC after 24 h of abstinence (white circle) or 2 h of access to alcohol (black circle) and alcohol drinking after 24 h of abstinence. (F) Anatomical schema of the regions of the CeA. (G) Representative 40-μm coronal sections of the mPFC that show Fos+ neurons after 24 h of abstinence (Left) and 2 h of access to alcohol (Right) in rats with continuous (Upper) or intermittent (Lower) access to alcohol. (H) Average number of Fos+ neurons in the CeA. ###P < 0.001 vs. continuous; ***P < 0.001 vs. 2 h of access to alcohol. (I) Percentage of time spent in the open arms of the elevated plus maze in naive, continuous, and intermittent access rats. (J) Correlation between the number of Fos+ neurons in the CeA after 24 h of abstinence (white circle) or 2 h of access to alcohol (black circle) and alcohol drinking after 24 h of abstinence. (K) Anatomical schema of the regions of the hippocampus (HPC), including the dentate gyrus, CA1, and CA3. (L) Representative 40-μm coronal sections of the dentate gyrus that show Fos+ neurons after 24 h of abstinence (Left) and 2 h of access to alcohol (Right) in rats with continuous (Upper) or intermittent (Lower) access to alcohol. (M) Average number of Fos+ neurons in the dentate gyrus. (N) Correlation between number of Fos+ neurons in the dentate gyrus after 24 h of abstinence (white circle) or 2 h of access to alcohol (black circle) and alcohol drinking after 24 h of abstinence. The data are expressed as mean ± SEM.
Abstinence-Induced Activation of Fos in the CeA Is Not Associated with Anxiety-Like Behavior.
To test the hypothesis that excessive alcohol intake is associated with dysfunction of the CeA, we evaluated anxiety-like behavior in the elevated plus maze after 24 h of abstinence and measured the number of Fos+ neurons in the CeA (Fig. 2F). The intermittent access group exhibited increased Fos+ neurons during abstinence in the CeA compared with the continuous access group (F1,20 = 17.2, P = 0.0001) (Fig. 2 G and H). Renewed access to alcohol reduced and normalized the number of Fos+ neurons in the intermittent access group to a level similar to rats with continuous access (Fig. 2 G and H). Rats with intermittent access to alcohol did not show increased anxiety-like behavior, reflected by a similar time spent in the open arms of the elevated plus maze (F2,35 = 0.1, P = 0.91) (Fig. 2I). There was no effect on the distance traveled in the open arms or proportion of entries into the open arms. A positive correlation was found between the number of Fos+ neurons recruited in the CeA during abstinence and the level of alcohol intake after 24 h of abstinence (R2 = 0.37, P < 0.05) (Fig. 2J). After renewed access to alcohol for 2 h, when Fos+ activity was normalized, the number of Fos+ neurons no longer predicted excessive alcohol intake (R2 = 0.16, P = 0.20) (Fig. 2J).
Effect of Alcohol Drinking on Fos Activation in the Hippocampus and Nucleus Accumbens.
To test the specificity of the effects observed in the mPFC and CeA, we measured the number of Fos+ neurons in the hippocampus (dentate gyrus, CA1, and CA3) and nucleus accumbens core (NAc core) and shell (NAc shell). The number of Fos+ neurons in the dentate gyrus (Fig. 2M), CA1, and CA3 was similar across conditions (details in SI Results). The number of Fos+ neurons in the NAc core and shell increased during abstinence, but again, no difference was observed between continuous and intermittent rats (details in SI Results). Moreover, the number of Fos+ neurons in the dentate gyrus (Fig. 2N) or NAc was not correlated with alcohol drinking.
Abstinence from Intermittent Access to Alcohol Is Associated with a Functional Disconnection of the PFC and CeA.
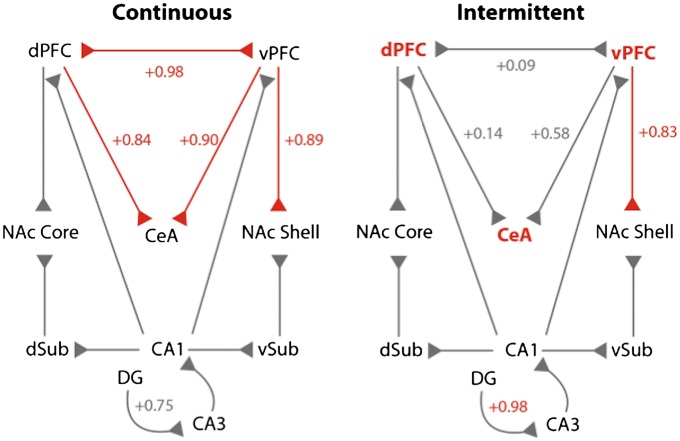
To determine the neural pathways that are dysregulated during abstinence from alcohol binge drinking, we measured the level of correlation of Fos+ neurons between anatomically connected structures (Fig. 3 and Fig. S1). When given access to alcohol, the number of Fos+ neurons in a given structure was not correlated with any other structures (all P > 0.05). In contrast, during abstinence, strong positive correlations were observed in rats with continuous access between the dPFC–vPFC, dPFC–CeA, vPFC–CeA, and vPFC–NAc shell (all P < 0.05) (Fig. 3, red connections) but not between the dPFC–NAc core, hippocampus–PFC, or hippocampus–NAc core/shell (all P > 0.05) (Fig. 3, gray connections). During abstinence, rats with intermittent access maintained a strong correlation in the vPFC–NAc shell (P < 0.05), but the correlations between the dPFC–vPFC, dPFC–CeA, and vPFC–CeA were lost (all P > 0.05).
Fig. 3.
Abstinence from alcohol in rats with intermittent access is associated with a functional disconnection of the PFC and CeA. Correlational analysis of Fos+ cell number between anatomically connected structures. The connections with significant correlation (P < 0.05) are represented in red with the respective R value of the Pearson correlation coefficient. Nonsignificant correlations are in gray. Notice that, for comparison purposes, nonsignificant R values of connections that are significant in the other condition are also represented in gray, despite being nonsignificant.
Intermittent Access to Alcohol Increases Alcohol Intake and Cognitive Impairment During Acute but Not Protracted Abstinence.
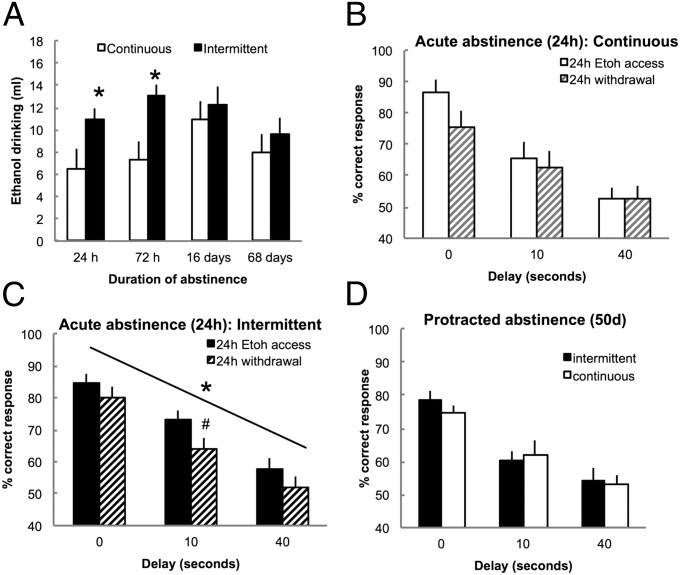
To further evaluate the time course of the effects on working memory and increased motivation to drink alcohol during abstinence, we evaluated alcohol drinking and working memory during acute and protracted abstinence using the DNMS task in a separate cohort of rats. Rats were given intermittent (n = 12) and continuous (n = 12) access to alcohol for a total of 5 mo. After stabilization of alcohol drinking, the rats were subjected to 24 h, 72 h, 16 d, or 68 d of abstinence, and alcohol drinking was measured for 24 h. After each period of abstinence, the rats were baselined for 2 wk before testing a different duration. Rats with intermittent access exhibited increased intake after 24 and 72 h but not after 16 and 68 d of abstinence compared with rats with continuous access (F2,44 = 3.4, P = 0.042) (Fig. 4A). After the evaluation of alcohol drinking, the rats remained abstinent for 68 d while they were trained in the DNMS task. After stabilization of performance, the rats were tested for performance with 0-, 10-, and 40-s delays immediately after 24 h of access to alcohol or 24 h of abstinence. No difference was observed in performance during training, and both groups reached a similar level of correct responding at the 0-s delay and each phase of training (Materials and Methods has details on training phases). However, when tested during acute abstinence, the within-subjects analysis revealed that rats with intermittent access exhibited a decrease in the percentage of correct responses at all delays and particularly, the intermediate delay of 10 s compared with rats tested after 24 h of access to alcohol (F1,11 = 10.0, P = 0.009) (Fig. 4C). In contrast, rats with continuous access did not show a decrease in correct response during acute abstinence (F1,9 = 2.5, P = 0.15) (Fig. 4B). When tested during protracted abstinence (i.e., 50 d), the intermittent and continuous access groups showed similar performance at all delays (F2,40 = 0.18, P = 0.84) (Fig. 4D).
Fig. 4.
Intermittent access increases alcohol drinking and impairs working memory during acute but not protracted abstinence. (A) Alcohol drinking after 24 h, 72 h, 16 d, and 68 d of abstinence. *P < 0.05 vs. continuous. (B) Percentage of correct responses after 24 h of access to alcohol or 24 h of withdrawal in rats with continuous access to alcohol. (C) Percentage of correct responses after 24 h of access to alcohol or 24 h of withdrawal in rats with intermittent access to alcohol. #P < 0.05 (paired) vs. 24 h alcohol access. (D) Percentage of correct responses in the DNMS task at 0-, 10-, and 40-s delays after 50 d of abstinence in rats with a history of intermittent and continuous access to alcohol. The data are expressed as mean ± SEM.
Abstinence from Intermittent Access to Alcohol Specifically Recruits a Subpopulation of GABAergic and CRFergic Neurons in the mPFC.
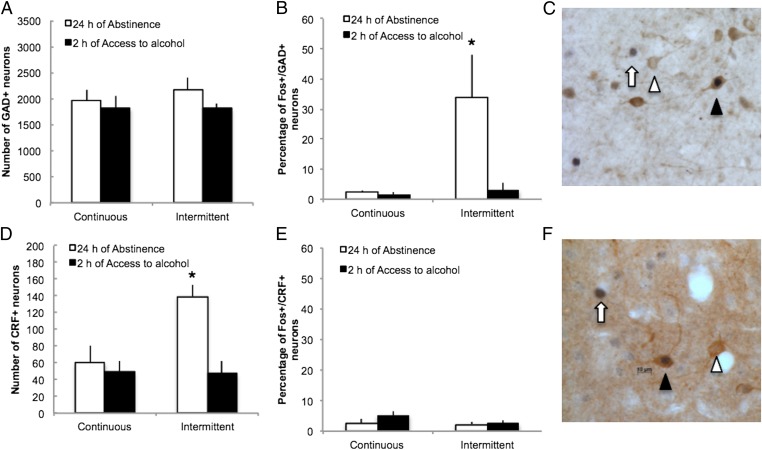
To determine the mechanism of action of the recruitment of the mPFC, we measured the degree of colocalization of Fos with GAD67, a marker of GABAergic interneurons in the mPFC, and CRF, a marker of CRFergic neurons. The vast majority of Fos+ neurons in the mPFC in rats with continuous access to alcohol was GAD67-negative (abstinence: 98 ± 2%; alcohol: 97 ± 1%; P > 0.05) or CRF-negative (abstinence: 97 ± 1%; alcohol: 95 ± 1%; P > 0.05), strongly suggesting that abstinence from alcohol in rats with continuous access to alcohol mainly recruits non-GABAergic cells and non-CRFergic cells, presumably glutamatergic pyramidal neurons. However, in rats abstinent from intermittent access to alcohol, a large proportion of Fos+ neurons was GAD67+ (34 ± 15% vs. 4 ± 3%, P < 0.05) (Fig. 5B), suggesting that abstinence from alcohol in rats with intermittent access is associated with specific recruitment of local GABAergic interneurons 24 h into withdrawal. This specific activation of GABAergic neurons was not associated with an increase in the number of GABAergic neurons in the mPFC (Fig. 5A). In contrast, abstinence from intermittent access to alcohol specifically increased the number of CRF neurons (F1,19 = 7.5, P < 0.05) (Fig. 5D) without specific Fos activation of CRFergic neurons (Fig. 5E).
Fig. 5.
Abstinence from alcohol in rats with intermittent access specifically recruits a subpopulation of GABAergic and CRFergic interneurons in the mPFC. (A and D) Number of GABA (A) and CRF (D) neurons in the mPFC. (B and E) Percentage of Fos+ neurons that are GAD67+ (B) or CRF+ (E) in the mPFC in rats with continuous and intermittent access to alcohol. (C and F) Representative 40-μm coronal section of the mPFC that shows Fos+/GAD67+ (C) or Fos+/CRF+ (F) neurons (black arrowhead), Fos−/GAD67+ (C) or Fos−/CRF+ (F) neurons (white arrowhead), and Fos+/GAD67− (C) or Fos+/CRF− (F) neurons (white arrow). *P < 0.05 vs. continuous. The data are expressed as mean ± SEM.
Discussion
The goal of the present study was to test the hypotheses that abstinence and renewed access to alcohol dysregulate the mPFC and CeA to produce long-term cognitive impairment and increased anxiety-like behavior in rats with a history of escalation of alcohol intake and that dysfunction in these brain regions predicts alcohol drinking when access to alcohol is renewed. This report shows that abstinence from alcohol in rats with a history of escalation of alcohol intake recruited a subpopulation of GABA and CRF neurons in the mPFC associated with specific functional disconnection of the PFC and CeA and produced working memory impairments associated with excessive alcohol drinking during acute (24–72 h) but not protracted (16–68 d) abstinence. Moreover, abstinence-induced increases in Fos+ neurons in the mPFC predicted excessive alcohol intake when access to alcohol was reinstated, and renewed access to alcohol normalized Fos expression and improved working memory performance in rats with intermittent access to alcohol.
Intermittent access to alcohol led to an escalation of alcohol intake, similar to the levels of intake associated with binge drinking in humans, when access to alcohol was reinstated. This effect was not observed when the rats had continuous access to alcohol. These data confirm previous results obtained with different strains of rats (2). Note that BALs averaged less than 80 mg% but that measurements were performed 2 h after the initiation of drinking, well after the peak of BALs (30 min) (2) to match the time point used for behavioral and anatomical studies. Our results suggest that this state of abstinence is not associated with increased anxiety-like behavior but rather, impairment of prefrontal executive control mechanisms, reflected in the present study by impaired working memory.
The number of Fos+ neurons in the mPFC was correlated with alcohol intake after abstinence. Previous studies showed that acute and passive administration of alcohol (1.5–3 g/kg, i.p.) increased Fos immunoreactivity in the mPFC and CeA, whereas tolerance to these effects was observed after chronic administration (12–18). In dependent rats, alcohol withdrawal increases the number of Fos+ neurons in the CeA and all subregions of the mPFC (19–21). Using a relevant animal model of binge-like excessive alcohol intake, the present study showed that withdrawal recruited the mPFC, CeA, and NAc but not hippocampus and that renewed access to alcohol actually decreased and normalized the Fos response in these regions, showing that voluntary alcohol intake in binge-drinking rats leads to neuroadaptations that are different from acute/passive administration. Other factors that may explain the different results compared with previous studies are the BALs, duration of exposure to alcohol, and stress related to the injection of alcohol in other models.
Spontaneous alternation in the Y-maze and performance in the DNMS task depend on the integrity of the mPFC and mainly reflect working memory processes (22, 23). Numerous targeted lesion studies in rats and monkeys have shown that working memory performance depends on the mPFC (24), and alcohol dependence produces long-term impairment in PFC function in humans (25, 26). The present results showed that rats with a history of escalation of alcohol intake in a binge-drinking model exhibited impairments in mPFC function during withdrawal, showing that mPFC dysfunction may appear early during the transition from alcohol abuse to alcohol dependence; also, these results suggest that repeated binge and withdrawal episodes in young adults may sensitize the mPFC to additional dysfunction and possibly facilitate the transition to alcohol dependence.
Our results suggest that the mPFC was more sensitive to the effect of abstinence than the CeA based on the greater number of Fos+ neurons in the mPFC compared with the CeA and the fact that there was a significant increase in the number of Fos+ neurons in the mPFC in the continuous group that was not observed in the CeA. Comparisons between structures need to be considered with caution because of the different neuronal populations and potentially different Fos reactivity. Nonetheless, we observed working memory impairment, with no changes in anxiety-like behavior, in rats with intermittent access to alcohol, supporting the hypothesis that behavior related to mPFC function was more impaired than behavior related to CeA function. Although there were low levels of Fos+ neurons in the CeA with no increase in anxiety-like behavior, excluding the possible involvement of the CeA in alcohol binge drinking is not possible when considering (i) the selective recruitment of Fos+ neurons in the CeA in intermittent rats during abstinence, (ii) the correlation between CeA Fos+ neurons and binge drinking, and (iii) the specific functional disconnection between the mPFC and CeA (Fig. 5). One possible explanation is that alcohol binge drinking first dysregulates the mPFC, leading to cognitive impairment and a loss of mPFC control over the CeA (i.e., a functional disconnection), and that continued binge drinking, with a repeated and more severe state of withdrawal, then recruits the CeA associated with anxiety-like behavior, similar to observations in alcohol-dependent rats (9). Additional studies that use the Daun02 technique (27) may help identify the specific contribution of Fos-activated neurons in the PFC and CeA to cognitive impairment and excessive alcohol drinking.
Notably, the present study found no differences in the number of Fos+, GAD67+, or CRF+ neurons induced by withdrawal between the subregions of the mPFC [i.e., dPFC (anterior cingulate cortex + dorsal prelimbic) vs. vPFC (ventral prelimbic + infralimbic cortices)]. Considering that these subregions have different anatomical connections to the amygdala and basal ganglia, the results suggest that dysfunction of the mPFC might impact the control of both the CeA and NAc, key regions involved in the emergence of negative affective states, impulsivity, and compulsivity, respectively. The correlational analysis between structures showed that, despite the global dysregulation of the dPFC and vPFC, abstinence from alcohol binge drinking was associated with a specific disconnection of the dPFC/vPFC with the CeA but not the dPFC–NAc core or vPFC NAc shell. Moreover, we did not observe any differences between continuous and intermittent rats in the number of Fos+ neurons in the hippocampus (dentate gyrus, CA1, and CA3). These results show the specificity of the effects observed in the CeA and mPFC and suggest that dysregulation of the hippocampus does not play a major role in the emergence of the excessive binge drinking and cognitive impairments observed during abstinence in this model.
The recruitment of the mPFC in rats with intermittent access to alcohol was strongly and selectively related to the recruitment of GAD67+ neurons and up-regulation of CRF interneurons, suggesting that withdrawal from alcohol specifically activates GABAergic and CRFergic interneurons, possibly leading to decreased activation of glutamatergic neurons in the mPFC. Indeed, GABAergic interneurons project to the somata of pyramidal neurons, and the GABAergic control of glutamatergic neuron activity depends on the activation of dopamine D1 and D2 receptors in the mPFC (28). Moreover, activation of GABAergic interneurons or GABAA receptors in the mPFC inhibits pyramidal glutamatergic neurons that project to subcortical nuclei and impair working memory (29). The recruitment of the subpopulation of GABAergic neurons in the mPFC during abstinence in rats with intermittent access to alcohol may lead to inhibition of pyramidal neurons and general hypofunction of the mPFC, ultimately leading to deficits that are similar to the deficits observed after inactivation or lesion of the mPFC (30, 31) or in rats with extended access to cocaine (32). The effect of activation of CRF interneurons on GABAergic and glutamatergic neurons is unknown. Cortical CRF neurons have been sparsely studied, and the functional role of mPFC CRF neurons is unknown. Our results show that the number of mPFC CRF neurons is increased during withdrawal from intermittent access to alcohol, but they did not show specific Fos activation 24 h into withdrawal, which is in contrast to what was observed with GABA neurons. Considering the facilitating role of CRF on GABAergic signaling in other structures (33, 34), it is likely that the up-regulation of cortical CRF neurons contributes to the selective activation of GABAergic neurons in rats with intermittent access.
In summary, the present results show that the recruitment of a specific subset of GABAergic and CRFergic interneurons in the mPFC during withdrawal, associated with functional disconnection of the mPFC and CeA, may contribute to impaired executive control over motivated behavior and suggest that the dysregulation of the mPFC may be an early index of neuroadaptation that leads to cognitive impairments and excessive binge drinking.
Materials and Methods
Animals.
Male Wistar rats (250–300 g) were used for all of the experiments. The rats were maintained on a 12 h/12 h light/dark cycle with ad libitum access to food and water. For the cognitive task, the rats were food-restricted and trained at 90% of their free-feeding body weight. All of the procedures were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by The Scripps Research Institute Institutional Animal Care and Use Committee. SI Materials and Methods has details on all of the methods.
Alcohol Self-Administration.
This procedure was similar to the procedure in the work by Simms et al. (2). Briefly, the rats were given either continuous (24 h/d for 7 d/wk) or intermittent (24 h/d for 3 d/wk) access to alcohol (20% vol/vol) using a two-bottle choice procedure (ethanol vs. water) for 5 mo. Tail BALs were obtained using an oxygen-rate alcohol analyzer (Analox Instruments).
Spontaneous Alternation and Anxiety-Like Behavior.
After 5 mo of access to alcohol, 36 rats (12 continuous, 12 intermittent, and 12 naïve rats) were tested after 24 h of abstinence for spontaneous alternation in a Y-maze and after 2 d for anxiety-like behavior in an elevated plus maze. Working memory performance was evaluated with a Y-maze (10 min) as described in the work by Gué et al. (23). Anxiety-like behavior was evaluated in a standard elevated plus maze using a videotracking system (Clever Sys).
Delayed Nonmatching-to-Sample Task.
An additional cohort of 24 rats was given access to alcohol for 5 mo as described above. The rats were then deprived of alcohol, and 4 d later, they were trained on the DNMS task using operant chambers (Med Associates) in daily sessions (40 min or 50 trials) for 5 wk until stabilization of performance (i.e., 70% correct response at the 0-s delay) was achieved using 0-, 10-, and 40-s delays. After the final phase of testing during protracted abstinence, the rats were given access to alcohol and tested two times per week after 24 h of abstinence or after 2 h access to alcohol.
Immunohistochemistry.
The rats were anesthetized with chloral hydrate and perfused with saline followed by 4% paraformaldehyde/0.15 M phosphate buffer, pH 7.4. The brains were harvested, cryoprotected, and stained for Fos (rabbit polyclonal, 1:5,000; SCBT), GAD67 (mouse monoclonal, 1:50,000; Millipore), and CRF (1:500; SCBT) using standard methods.
Microscopy.
The quantitative analysis to obtain unbiased estimates of the total number of Fos+, CRF+, and GAD67+ cell bodies was performed on a Zeiss Axiophot Microscope equipped with MicroBrightField Stereo Investigator software and a Q Imaging Retiga 2000R color digital camera. Three sections per region (systemic 1/8 random sampling) were analyzed bilaterally per animal by an investigator blind to the different groups. Cells were identified as neurons based on standard morphology, and only neurons with a focused nucleus within the nonforbidden regions of the counting frame were counted.
Statistics.
The results were analyzed using ANOVA (Statistica) after a normality and equal variance test. Newman–Keuls posthoc tests and Pearson correlations were used when necessary, and nonparametric analyses confirmed all of the results and are available in SI Materials and Methods. The data are expressed as mean ± SEM.
Supplementary Material
Acknowledgments
We thank Robert Lintz, Maury Cole, and Molly Brennan for their technical assistance and Michael Arends for his editorial assistance. This work is publication number 21476 from The Scripps Research Institute. This work was supported by National Institutes of Health Grants AA006420, AA020608, and AA008459 from the National Institute on Alcohol Abuse and Alcoholism and the Pearson Center for Alcoholism and Addiction Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1116523109/-/DCSupplemental.
References
- 1.Koob GF, et al. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27(8):739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Simms JA, et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32(10):1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacology (Berl) 1973;29(3):203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen CK, et al. A novel delta opioid receptor antagonist, SoRI-9409, produces a selective and long-lasting decrease in ethanol consumption in heavy-drinking rats. Biol Psychiatry. 2008;64(11):974–981. doi: 10.1016/j.biopsych.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simms JA, Bito-Onon JJ, Chatterjee S, Bartlett SE. Long-Evans rats acquire operant self-administration of 20% ethanol without sucrose fading. Neuropsychopharmacology. 2010;35(7):1453–1463. doi: 10.1038/npp.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steensland P, et al. The neurokinin 1 receptor antagonist, ezlopitant, reduces appetitive responding for sucrose and ethanol. PLoS One. 2010;5(9):e12527. doi: 10.1371/journal.pone.0012527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barak S, Carnicella S, Yowell QV, Ron D. Glial cell line-derived neurotrophic factor reverses alcohol-induced allostasis of the mesolimbic dopaminergic system: Implications for alcohol reward and seeking. J Neurosci. 2011;31(27):9885–9894. doi: 10.1523/JNEUROSCI.1750-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adermark L, Jonsson S, Ericson M, Söderpalm B. Intermittent ethanol consumption depresses endocannabinoid-signaling in the dorsolateral striatum of rat. Neuropharmacology. 2011;61(7):1160–1165. doi: 10.1016/j.neuropharm.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59(1):11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters A, Jones EG. Classification of cortical neurons. In: Peters A, Jones EG, editors. Cerebral Cortex, Volume 1, Cellular Components of the Cerebral Cortex. Vol 1. New York: Plenum; 1984. pp. 107–121. [Google Scholar]
- 11.Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: An immunohistochemical study. Neuroendocrinology. 1983;36(3):165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 12.Ryabinin AE, Criado JR, Henriksen SJ, Bloom FE, Wilson MC. Differential sensitivity of c-Fos expression in hippocampus and other brain regions to moderate and low doses of alcohol. Mol Psychiatry. 1997;2(1):32–43. doi: 10.1038/sj.mp.4000206. [DOI] [PubMed] [Google Scholar]
- 13.Hansson AC, Rimondini R, Neznanova O, Sommer WH, Heilig M. Neuroplasticity in brain reward circuitry following a history of ethanol dependence. Eur J Neurosci. 2008;27(8):1912–1922. doi: 10.1111/j.1460-9568.2008.06159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bachtell RK, et al. The Edinger-Westphal-lateral septum urocortin pathway and its relationship to alcohol consumption. J Neurosci. 2003;23(6):2477–2487. doi: 10.1523/JNEUROSCI.23-06-02477.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang SL, Patel NA, Romero AA. Activation and desensitization of Fos immunoreactivity in the rat brain following ethanol administration. Brain Res. 1995;679(1):89–98. doi: 10.1016/0006-8993(95)00210-h. [DOI] [PubMed] [Google Scholar]
- 16.Ryabinin AE, Galvan-Rosas A, Bachtell RK, Risinger FO. High alcohol/sucrose consumption during dark circadian phase in C57BL/6J mice: Involvement of hippocampus, lateral septum and urocortin-positive cells of the Edinger-Westphal nucleus. Psychopharmacology (Berl) 2003;165(3):296–305. doi: 10.1007/s00213-002-1284-y. [DOI] [PubMed] [Google Scholar]
- 17.Weitemier AZ, Woerner A, Bäckström P, Hyytiä P, Ryabinin AE. Expression of c-Fos in Alko alcohol rats responding for ethanol in an operant paradigm. Alcohol Clin Exp Res. 2001;25(5):704–710. [PubMed] [Google Scholar]
- 18.Sharpe AL, Tsivkovskaia NO, Ryabinin AE. Ataxia and c-Fos expression in mice drinking ethanol in a limited access session. Alcohol Clin Exp Res. 2005;29(8):1419–1426. doi: 10.1097/01.alc.0000174746.64499.83. [DOI] [PubMed] [Google Scholar]
- 19.Borlikova GG, Le Merrer J, Stephens DN. Previous experience of ethanol withdrawal increases withdrawal-induced c-fos expression in limbic areas, but not withdrawal-induced anxiety and prevents withdrawal-induced elevations in plasma corticosterone. Psychopharmacology (Berl) 2006;185(2):188–200. doi: 10.1007/s00213-005-0301-3. [DOI] [PubMed] [Google Scholar]
- 20.Knapp DJ, Duncan GE, Crews FT, Breese GR. Induction of Fos-like proteins and ultrasonic vocalizations during ethanol withdrawal: Further evidence for withdrawal-induced anxiety. Alcohol Clin Exp Res. 1998;22(2):481–493. [PubMed] [Google Scholar]
- 21.Olive MF, et al. Reduced ethanol withdrawal severity and altered withdrawal-induced c-fos expression in various brain regions of mice lacking protein kinase C-epsilon. Neuroscience. 2001;103(1):171–179. doi: 10.1016/s0306-4522(00)00566-2. [DOI] [PubMed] [Google Scholar]
- 22.Sarter M, Bodewitz G, Stephens DN. Attenuation of scopolamine-induced impairment or spontaneous alternation behaviour by antagonist but not inverse agonist and agonist beta-carbolines. Psychopharmacology (Berl) 1988;94(4):491–495. doi: 10.1007/BF00212843. [DOI] [PubMed] [Google Scholar]
- 23.Gué M, et al. Sex differences in learning deficits induced by prenatal stress in juvenile rats. Behav Brain Res. 2004;150(1–2):149–157. doi: 10.1016/S0166-4328(03)00250-X. [DOI] [PubMed] [Google Scholar]
- 24.Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: Comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol. 2006;73(1):19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: A review of the literature. Alcohol Alcohol. 2001;36(5):357–368. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- 26.Stephens DN, Duka T. Review. Cognitive and emotional consequences of binge drinking: Role of amygdala and prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3169–3179. doi: 10.1098/rstb.2008.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fanous S, et al. Role of orbitofrontal cortex neuronal ensembles in the expression of incubation of heroin craving. J Neurosci. 2012;32(34):11600–11609. doi: 10.1523/JNEUROSCI.1914-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001;21(10):3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sawaguchi T, Iba M. Prefrontal cortical representation of visuospatial working memory in monkeys examined by local inactivation with muscimol. J Neurophysiol. 2001;86(4):2041–2053. doi: 10.1152/jn.2001.86.4.2041. [DOI] [PubMed] [Google Scholar]
- 30.Mishkin M. Perseveration of central sets after frontal lesions in man. In: Warren JM, Akert K, editors. The Frontal Granular Cortex and Behavior. New York: McGraw-Hill; 1964. pp. 219–294. [Google Scholar]
- 31.Pribram KH. A further experimental analysis of the behavioral deficit that follows injury to the primate frontal cortex. Exp Neurol. 1961;3:432–466. doi: 10.1016/0014-4886(61)90021-8. [DOI] [PubMed] [Google Scholar]
- 32.George O, Mandyam CD, Wee S, Koob GF. Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology. 2008;33(10):2474–2482. doi: 10.1038/sj.npp.1301626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberto M, et al. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67(9):831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kash TL, Winder DG. Neuropeptide Y and corticotropin-releasing factor bi-directionally modulate inhibitory synaptic transmission in the bed nucleus of the stria terminalis. Neuropharmacology. 2006;51(5):1013–1022. doi: 10.1016/j.neuropharm.2006.06.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.