Abstract
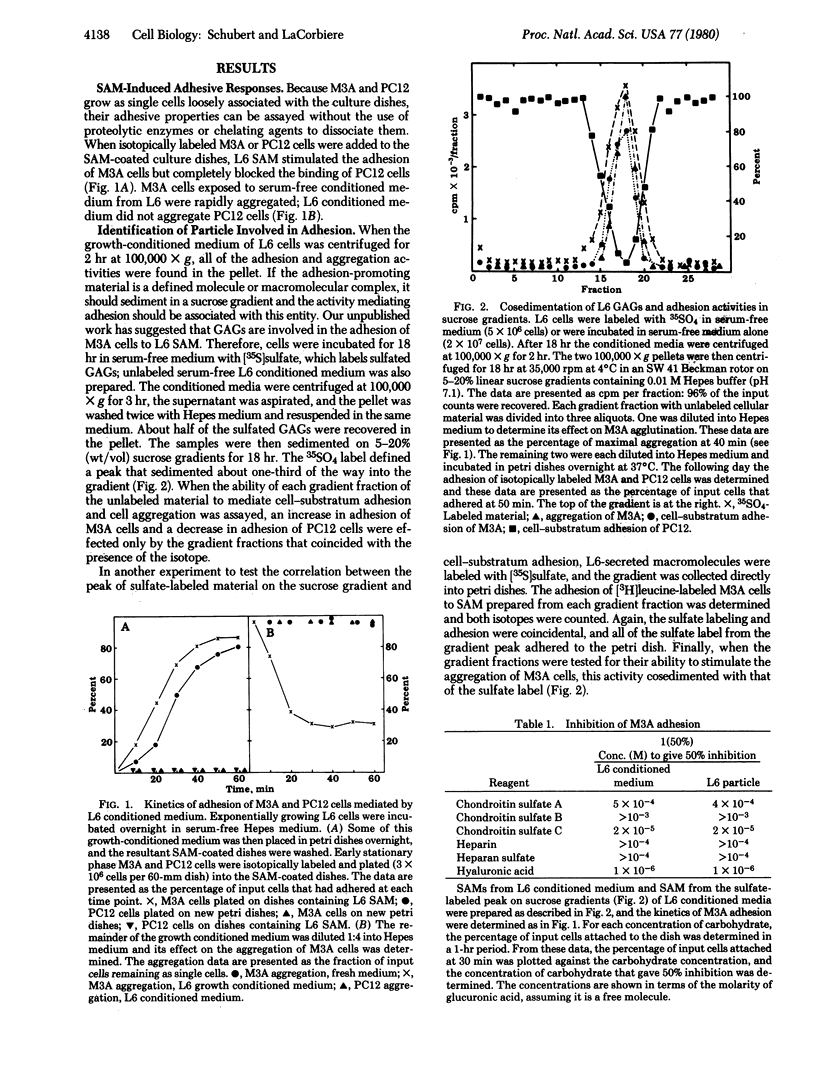
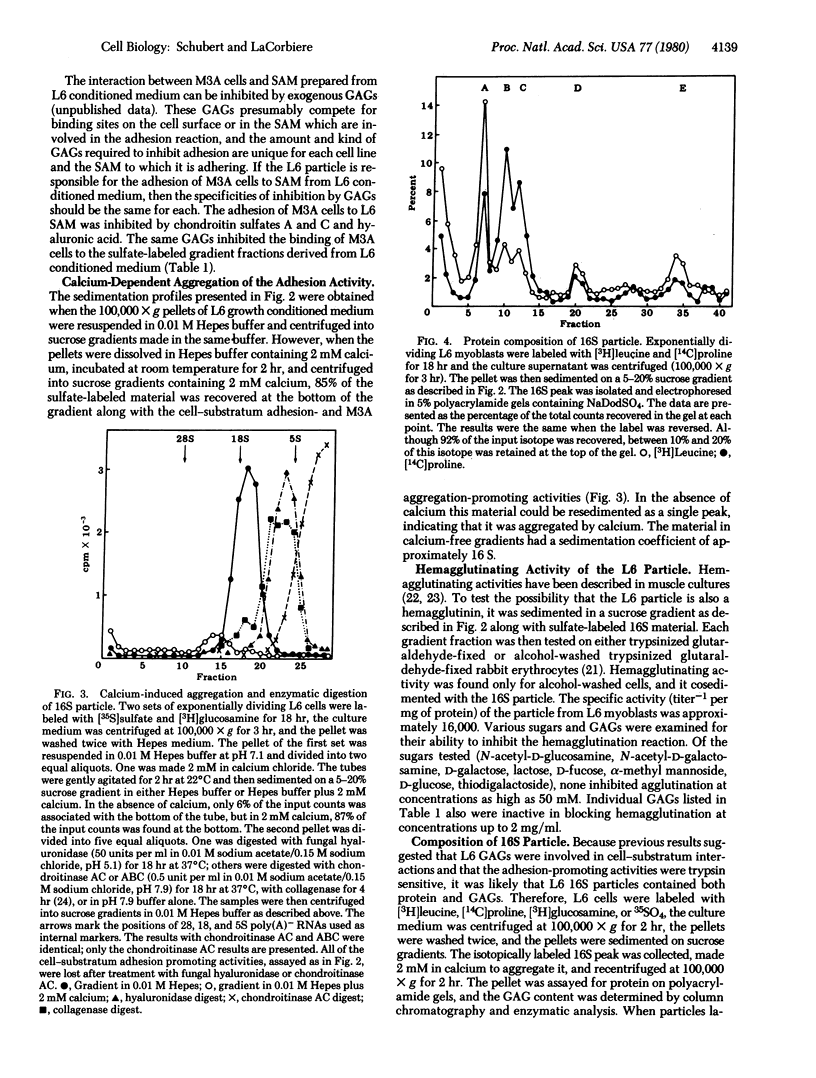
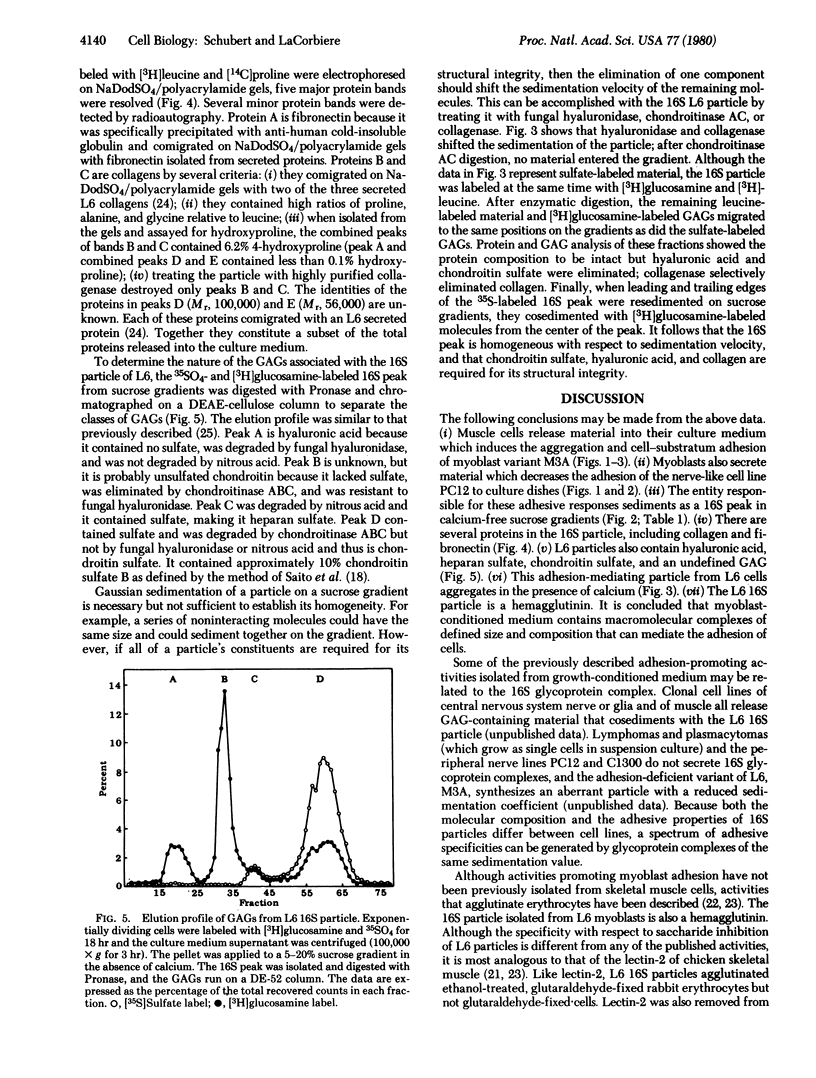
Myogenic cells release into their culture medium a glycoprotein complex that mediates cellular adhesion. In the absence of calcium this complex has a sedimentation value of 16S; it aggregates in the presence of calcium. The 16S material both agglutinates and increases the rate of cell-substratum adhesion of a myoblast variant and inhibits the adhesion of a nerve-like cell line to culture dishes. It is also a hemagglutinin. The 16S particle is composed of glycosaminoglycans and several proteins, including fibronectin and collagen.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnhart B. J., Cox S. H., Kraemer P. M. Detachment variants of Chinese hamster cells. Hyaluronic acid as a modulator of cell detachment. Exp Cell Res. 1979 Mar 15;119(2):327–332. doi: 10.1016/0014-4827(79)90360-4. [DOI] [PubMed] [Google Scholar]
- Baum B. J., McDonald J. A., Crystal R. G. Metabolic fate of the major cell surface protein of normal human fibroblasts. Biochem Biophys Res Commun. 1977 Nov 7;79(1):8–15. doi: 10.1016/0006-291x(77)90053-5. [DOI] [PubMed] [Google Scholar]
- Ceri H., Shadle P. J., Kobiler D., Barondes S. H. Extracellular lectin and its glycosaminoglycan inhibitor in chick muscle cultures. J Supramol Struct. 1979;11(1):61–67. doi: 10.1002/jss.400110107. [DOI] [PubMed] [Google Scholar]
- Culp L. A. Substrate-attached glycoproteins mediating adhesion of normal and virus-transformed mouse fibroblasts. J Cell Biol. 1974 Oct;63(1):71–83. doi: 10.1083/jcb.63.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doljanski F., Kapeller M. Cell surface shedding--the phenomenon and its possible significance. J Theor Biol. 1976 Oct 21;62(2):253–270. doi: 10.1016/0022-5193(76)90119-3. [DOI] [PubMed] [Google Scholar]
- Gail M. H., Boone C. W., Thompson C. S. A calcium requirement for fibroblast motility and prolifertion. Exp Cell Res. 1973 Jun;79(2):386–390. doi: 10.1016/0014-4827(73)90458-8. [DOI] [PubMed] [Google Scholar]
- Henkart M. P., Nelson P. G. Evidence for an intracellular calcium store releasable by surface stimuli ifibroblasts (L cells). J Gen Physiol. 1979 May;73(5):655–673. doi: 10.1085/jgp.73.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao W. W., Berg R. A., Prockop D. J. Kinetics for the secretion of procollagen by freshly isolated tendon cells. J Biol Chem. 1977 Dec 10;252(23):8391–8397. [PubMed] [Google Scholar]
- Kobiler D., Barondes S. H. Lectin from embryonic chick muscle that interacts with glycosaminoglycans. FEBS Lett. 1979 May 15;101(2):257–261. doi: 10.1016/0014-5793(79)81020-0. [DOI] [PubMed] [Google Scholar]
- Kraemer P. M., Barnhart B. J. Elevated cell-surface hyaluronate in substrate-attached cells. Exp Cell Res. 1978 Jun;114(1):153–157. doi: 10.1016/0014-4827(78)90047-2. [DOI] [PubMed] [Google Scholar]
- McClay D. R., Moscona A. A. Purification of the specific cell-aggregating factor from embryonic neural retina cells. Exp Cell Res. 1974 Aug;87(2):438–443. doi: 10.1016/0014-4827(74)90514-x. [DOI] [PubMed] [Google Scholar]
- McGuire E. J. Intercellular adhesive selectivity. II. Properties of embryonic chick liver cell-cell adhesion. J Cell Biol. 1976 Jan;68(1):90–100. doi: 10.1083/jcb.68.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millis A. J., Hoyle M. Fibroblast-conditioned medium contains cell surface proteins required for cell attachment and spreading. Nature. 1978 Feb 16;271(5646):668–669. doi: 10.1038/271668a0. [DOI] [PubMed] [Google Scholar]
- Moore E. G. Cell to substratum adhesion-promoting activity released by normal and virus-transformed cells in culture. J Cell Biol. 1976 Sep;70(3):634–647. doi: 10.1083/jcb.70.3.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrink B., Kuhlenschmidt M. S., Roseman S. Adhesive specificity of juvenile rat and chicken liver cells and membranes. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1077–1081. doi: 10.1073/pnas.74.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins M. E., Ji T. H., Hynes R. O. Cross-linking of fibronectin to sulfated proteoglycans at the cell surface. Cell. 1979 Apr;16(4):941–952. doi: 10.1016/0092-8674(79)90109-0. [DOI] [PubMed] [Google Scholar]
- Podleski T. R., Greenberg I., Nichols S. C. Studies on lectin activity during myogenesis. Exp Cell Res. 1979 Sep;122(2):305–316. doi: 10.1016/0014-4827(79)90307-0. [DOI] [PubMed] [Google Scholar]
- Pouysségur J. M., Pastan I. Mutants of Balb/c 3T3 fibroblasts defective in adhesiveness to substratum: evidence for alteration in cell surface proteins. Proc Natl Acad Sci U S A. 1976 Feb;73(2):544–548. doi: 10.1073/pnas.73.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J., Culp L. A. Preliminary characterization of the proteoglycans in the substrate adhesion sites of normal and virus-transformed murine cells. Biochemistry. 1979 Dec 11;18(25):5621–5629. doi: 10.1021/bi00592a016. [DOI] [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Schubert D. Proteins secreted by clonal cell lines. Changes in metabolism with culture growth. Exp Cell Res. 1976 Oct 15;102(2):329–340. doi: 10.1016/0014-4827(76)90048-3. [DOI] [PubMed] [Google Scholar]
- Schubert D., Tarikas H., Humphreys S., Heinemann S., Patrick J. Protein synthesis and secretion in a myogenic cell line. Dev Biol. 1973 Jul;33(1):18–37. doi: 10.1016/0012-1606(73)90161-9. [DOI] [PubMed] [Google Scholar]
- Schubert D., Whitlock C. Alteration of cellular adhesion by nerve growth factor. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4055–4058. doi: 10.1073/pnas.74.9.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarikas H., Schubert D. Regulation of adenylate kinase and creatine kinase activities in myogenic cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2377–2381. doi: 10.1073/pnas.71.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery J. P., Brackenbury R., Rutishauser U., Edelman G. M. Adhesion among neural cells of the chick embryo. II. Purification and characterization of a cell adhesion molecule from neural retina. J Biol Chem. 1977 Oct 10;252(19):6841–6845. [PubMed] [Google Scholar]
- Tupper J. T., Del Rosso M., Hazelton B., Zorgniotti F. Serum-stimulated changes in calcium transport and distribution in mouse 3T3 cells and their modification by dibutyryl cyclic AMP. J Cell Physiol. 1978 Apr;95(1):71–84. doi: 10.1002/jcp.1040950110. [DOI] [PubMed] [Google Scholar]
- Underhill C. B., Keller J. M. Density-dependent changes in the amount of sulfated glycosaminoglycans associated with mouse 3T3 cells. J Cell Physiol. 1976 Sep;89(1):53–63. doi: 10.1002/jcp.1040890106. [DOI] [PubMed] [Google Scholar]
- VOGT M., DULBECCO R. Steps in the neoplastic transformation of hamster embryo cells by polyoma virus. Proc Natl Acad Sci U S A. 1963 Feb 15;49:171–179. doi: 10.1073/pnas.49.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipple A. P., Millis A. J. Human lymphoblastoid cell variants defective in cell-cell adhesion. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2838–2842. doi: 10.1073/pnas.76.6.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]



