Abstract
Octamer-binding protein 4 (OCT4) is a key player in pluripotent embryonic stem (ES) cells and is essential for the generation of induced pluripotent stem cells. Recently, several reports indicated the spontaneous recovery of pluripotency in cultured adult human testis-derived cells. This was evidenced also by the detection of OCT4 using antibodies. However, the soundness of some data was recently put into question. During our attempts to derive pluripotent cells from the common marmoset monkey (Callithrix jacchus) testis, we obtained inconsistent data which prompted us to analyze deeper the characteristics of three independent OCT4 antibodies that were used in numerous published studies that received greatest attention. All antibodies detected OCT4 by immunofluorescence (IF) in a marmoset monkey ES cell line. Two of the three OCT4 antibodies also gave robust nuclear signals in testis-derived cells. However, the latter cells expressed no OCT4 mRNA as revealed by quantitative RT–PCR and turned out to be mesenchymal cells. When tested in western blot analyses, all antibodies detected heterologously expressed marmoset monkey OCT4 protein. But, importantly, those antibodies that resulted in non-specific signals in IF also showed additional non-specific bands in western blots. In summary, some commercially available OCT4 antibodies result in false-positive signals which may provoke erroneous conclusions when used in studies aiming at the generation of pluripotent cells in vitro. We conclude that (i) antibodies must be carefully characterized before use to prevent misleading observations and (ii) OCT4 expression must be monitored by a second antibody-independent method.
Keywords: OCT4, stem cell, pluripotency, reprogramming, testis, non-human primate
Introduction
Octamer-binding protein 4 (OCT4, also POU5F1) is a key player in pluripotent cells (Nichols et al., 1998; Niwa et al., 2000; Boyer et al., 2005). This transcription factor is essential for the reprogramming of differentiated cells into induced pluripotent stem cells (Takahashi and Yamanaka, 2006; Okita et al., 2007; Takahashi et al., 2007) and OCT4 alone is sufficient, but also essential to reprogram adult neural stem cells to pluripotency (Kim et al., 2009). Recent publications presented data indicating that OCT4 is quickly and robustly up-regulated in human cultured testis-derived cells even without any genetic modification of the cells (Conrad et al., 2008; Golestaneh et al., 2009; Gonzalez et al., 2009; Kossack et al., 2009; Mizrak et al., 2010). Altogether, these facts indicate the vital importance of specific and reliable detection of the key pluripotency marker OCT4 in cell culture experiments aiming at the generation of pluripotent cells from differentiated cells lacking OCT4. This issue is even more critical when studies with human or non-human primate cells are performed, where no transgenic OCT4-green fluorescent protein-reporter cells are available.
OCT4 exists basically as two isoforms that differ by the alternative use of the leader exons encoding the N-terminal part of the respective OCT4 proteins. Importantly, these different OCT4 proteins have different functional properties (Wang and Dai, 2010). While the OCT4A isoform is clearly related to stemness and pluripotency, OCT4B seems to be involved in the cellular stress response. The role of the recently discovered OCT4B1 isoform is still unclear. The basic cellular localization of OCT4A and -B is different. OCT4A is present in the nucleus, while OCT4B is mainly cytoplasmic (Cauffman et al., 2005). Important for functional analyses, the ‘pluripotency isoform’ OCT4A is the only isoform that includes exon 1. Thus, exon 1 is a unique and specific part of OCT4A and hence its presence is the only reliable indicator of pluripotency (Liedtke et al., 2008; Wang and Dai, 2010). All other OCT4 exons (and the corresponding parts of the proteins) are not meaningful with regard to pluripotency.
The common marmoset monkey (Callithrix jacchus) is a well-established non-human primate species in preclinical and especially in reproduction research, since its testicular development and histological organization are very similar to those of the human testis (Weinbauer et al., 2001; Millar et al., 2000; Mitchell et al., 2008). During our attempts to derive pluripotent cells from the common marmoset monkey testis, as it was recently published for the human testis (Conrad et al., 2008), we used three different antibodies to detect OCT4 in cultured cells by immunofluorescence (IF). However, our data were inconsistent, prompting us to further characterize the different frequently used polyclonal OCT4 antibodies. The first antibody (Abcam ab19857) was generated against the C-terminal part of the human OCT4, which is common to both OCT4A and OCT4B. The second antibody was from Santa Cruz (Sc-9081) and was generated against the OCT4A-specific part [amino acids (aa) 1-134 of OCT4A]. The third antibody was also from Santa Cruz (Sc-8628) and directed against the OCT4A-specific 19 N-terminal aa of OCT4A. Importantly, two of these antibodies (Abcam ab19857 and Sc-9081) generated signals in IF which can easily be misinterpreted and eventually lead to erroneous conclusions when used in studies aiming at the generation of pluripotent cells.
Materials and Methods
Cell culture
Embryonic stem cells
Common marmoset monkey (C. jacchus) embryonic stem cells (ESCs; line cjes001; Muller et al., 2009) were cultured on 3000-rad γ-irradiated mouse embryonic fibroblasts in medium consisting of 80% knockout Dulbecco's modified Eagle's medium (DMEM; GIBCO) and 20% Knockout Serum Replacement (GIBCO) supplemented with 2 mM GlutaMAX™ (GIBCO), 0.1 mM minimum essential medium (MEM) non-essential aa (GIBCO), 0.1 mM β-mercaptoethanol (GIBCO), 100 IU/ml penicillin and 100 mg/ml streptomycin as described previously (Muller et al., 2009).
Derivation and primary culture of testicular multipotent stromal cells
Common marmoset testis were kept on ice in DMEM/F-12 immediately after recovery and further processed in a sterile environment. After removing capsule and epididymis, the tubules were digested using DMEM/F-12 (GIBCO) containing 1 mg/ml hyaluronidase (Sigma), 1 mg/ml collagenase (Sigma) and 15 U/ml DNase (Roche) for 35–40 min at 37°C under constant mild rotation. Resulting single cells were seeded at a cell density of 2 × 105 cells/cm2 to plastic culture dishes. Testicular multipotent stromal cell (TMSC)-culture medium was based on a 1:1 mixture of DMEM (GIBCO) and F-12 nutrient mixture (GIBCO), which was supplemented with 10% fetal bovine serum (FBS; GIBCO), 100 U/l penicillin–streptomycin (GIBCO), 0.25 µg/ml Fungizone® Antimycotic (GIBCO) and 5 ng/ml recombinant human basic fibroblast growth factor (ProSpecTany). Following overnight incubation, any non-adherent cells were removed from the dish and remaining adherent cells were cultured until they reached confluency. Generally, TMSCs were cultured at 37°C under 5% CO2 and the medium was changed every 2–3 days. Passaging was performed with 0.05% trypsin in phosphate-buffered saline (PBS) (GIBCO) every 3–5 days (in general upon confluency) at a dilution of 1:3–1:5 (Eildermann et al., 2012a).
Cloning of marmoset monkey OCT4A and SOX2 and transfection into Human Embryonic Kidney-293 cells
Marmoset monkey OCT4A and SOX2 cDNA were amplified from single-strand cDNA of the marmoset ES cell line cjes001 (passage 51) with the following primers including restriction sites: OCT4 fw 5′-GATCGGATCCTTGGGGCGCCTTCCTTC-3′, OCT4 rev 5′-CTGATCTAGACTCCTCTCCCTGTCCCCC-3′; SOX2 fw 5′-GCTAGGATCCACAGCGCCCGCATG-3′, and SOX2 rev 5′-CCGCTCGAGAATGCCTCCCCCGTCCAGTTCG-3′, respectively. The OCT4-PCR product of 1159 bp and the SOX2-PCR product of 1023 bp were digested with BamHI/XbaI and BamHI/XhoI, respectively, cloned into the vector pBluecsriptII SK—(Stratagene) and sequenced. Compared with the published OCT4 sequence [UCSC Genome Bioinformatics, http://genome.ucsc.edu/, the March 2009 C. jacchus draft assembly (WUGSC 3.2 (GCA_000004665.1)] the amplified open reading frame (ORF) had two silent mutations: C363T and T1014A, whereas the sequence of the amplified SOX2 ORF is identical to the published one. The complete sequence of the amplified OCT4 ORF has been deposited in GenBank (http://www.ncbi.nlm.nih.gov/genbank/), accession number JQ627833. Subsequently, the OCT4 ORF and SOX2 ORF were amplified with primers OCT4 fw 5′-CGGGATCCCCACCATGGCGGGACACCTGGCTTCG, OCT4 rev 3′-GCTCTAGATCAGTTGGAATGCATGGGAGAGC; SOX2 fw 5′-CGGGATCCCCACCATGTACAACATGATGGAGACGGAG and SOX2 rev 3′-GCTCTAGATCACATGTGCGAGAGCGGCAG. These primers included additional restriction sites (BamHI/XbaI and BamHI/XhoI, respectively) for ligation into the expression vector pcDNA3.1+ (Invitrogen).
For a more robust expression of the OCT4 protein the cytomegalovirus (CMV) promoter was replaced by the CAG promoter, which was synthesized by GenScript (www.genscript.com) and cloned into the pUC57 vector by digestion with BamHI and EcoRI. Subsequent digestion of vectors pcDNA3.1+ -OCT4 and pcDNA3.1+-SOX2, respectively and pUC57-CAG with BglII and NheI allowed the replacement of the CMV promoter by the CAG promoter resulting in the final expression vectors pcDNA3.1+-CAG-OCT4 and pcDNA3.1+ -CAG-SOX2, respectively, which were sequenced and used in this study.
Twenty-four hours prior transfection 4.5 × 106 human embryonic kidney (HEK)-293 cells were seeded to a 9 cm culture dish and maintained in DMEM (GlutaMAX, Invitrogen) containing 10% fetal bovine serum (GIBCO/BRL), 1% penicillin/streptomycin (GIBCO/BRL) and 1% non-essential-aa (GIBCO/BRL) at 37°C under 5% CO2. The transfection was performed using 0.02 µg/µl pcDNA3.1+ -CAG-OCT4 or pcDNA3.1+ -CAG-SOX2 expression vector and the FuGENE HD Reagent (Promega) according to the manufacturer's manual. The cells were harvested for protein isolation 48 h post-transfection.
Immunofluorescence
For IF, cells were grown in 48-well-plastic dishes and fixed for 30 min in 4% paraformaldehyde. The cells were permeabilized with 0.04% Triton X-100 for 10 min. After rinsing with PBS, the primary antibodies (see Table I), diluted in PBS/5% bovine serum albumin (BSA), were applied for 1 h at 37°C. Following two PBS washing steps the appropriate Alexa fluor (AF) 488-linked secondary antibodies (Table II), diluted in PBS/5% BSA, were applied for 30 min at room temperature in the dark. Controls were performed omitting the primary antibody and with the corresponding immunoglobulin G (IgG) fraction at the same protein concentration as the primary antibodies. Cells were counter-stained with 4’,6-diamidino-2-phenylindole (DAPI), covered with citifluor (Citifluor Ltd) and images were taken on an Axio Observer Z1 fluorescence microscope from Zeiss (Germany).
Table I.
Primary antibodies used in this study.
| Immunogen/isoform specificity | Company | Cat. no. | Species/isotype | Times cited according to supplier's webpage |
|---|---|---|---|---|
| aa 300-360 (OCT4A and OCT4B | Abcam | #ab19857 | Rabbit IgG | 42 |
| aa 1-19 (OCT4A) | Santa Cruz | #sc-8628 | Goat IgG | 41 |
| aa 1-134 (OCT4A) | Santa Cruz | #sc-9081 | Rabbit IgG | 86 |
Table II.
Secondary antibodies for immunofluorescence.
| Antigen | Linked fluorophore | Host species | Company | Cat. no. |
|---|---|---|---|---|
| Anti-goat IgG | Alexa fluor 488 | Donkey | Invitrogen | #A11055 |
| Anti-rabbit IgG | Alexa fluor 488 | Donkey | Invitrogen | #A21206 |
| Anti-rabbit IgG | Alexa fluor 594 | Donkey | Invitrogen | #A21207 |
Western blot analysis
Western blots were basically performed as previously described (Eildermann et al. 2012b). Cytoplasmic and nuclear protein from wild-type, cjOCT4 or cjSOX2 expressing HEK-293 cells were isolated with the Qproteom nuclear protein kit from Qiagen (Hilden, Germany) according to the manufacturer's instructions. Protein concentrations were determined using the Bradford-assay and 20 µg of protein per sample was separated by electrophoresis on a 10% SDS–polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (Immobilon-P, Millipore). Membranes were incubated with one of the three different OCT4 primary antibodies (see Table I), followed by incubation with the according horse-radish peroxidise (HRP)-coupled secondary antibody (Table III). Signals were detected with enhanced chemiluminescence western blot detection reagents (GE Healthcare) and the ChemoCam-System (INTAS, Goettingen, Germany).
Table III.
Secondary antibodies for western blots.
| Antigen | Host species | Company | Cat. no. |
|---|---|---|---|
| Anti-rabbit IgG-HRP | Goat | R&D | #HAF008 |
| Anti-goat IgG-HRP | Rabbit | R&D | #HAF017 |
Quantitative (q) RT–PCR
Relative qRT-PCR was performed on common marmoset TMSCs, ESCs and testis material. Design and testing of the qRT-PCR primers (OCT4 fw 5′AAACCCACACTTCAGCAGATCA 3′, re 5′CACACGGACCACATCCTTCTC 3′; SOX2 fw 5′ GAGAACCCCAAGATGCACAAC 3′, re 5′TCTCGGACAGCAGCTTCCA 3′) as well as the qRT-PCR procedure was performed as previously described (Eildermann et al. 2012a). In brief, 2 µg testicular RNA were reverse-transcribed, using random hexamers, by Superscript II (Invitrogen, Karlsruhe, Germany) to obtain cDNA. Two microlitres of 1:2 diluted cDNA was used for each 20 µl PCR with Power SYBR Green Mastermix (Applied Biosystems), and different primer concentrations ranging between 50 and 900 nM. The extent of fluorescence of the Power SYBR green dye was detected and analyzed using the 2−(Delta Delta C(T)) method and the ABI Prism® 7000 SDS software (Applied Biosystems). Each sample was assayed in triplicate und normalized to glyceraldehyde-3-phosphate dehydrogenase expression.
Results
All three tested antibodies detect OCT4 in ES cells
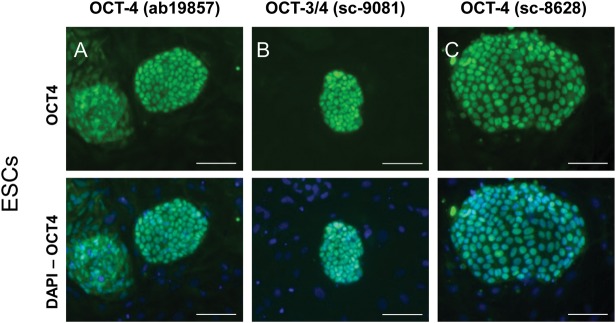
We used marmoset monkey ES cells as a positive control for OCT4-expressing cells (Muller et al., 2009). All three antibodies irrespective of the epitope used for immunization of the animals, detected OCT4 in the nuclei of the marmoset monkey ES cells (Fig. 1). Antibody ab19857 also produced a slight cytoplasmic signal (Fig. 1, left panel).
Figure 1.
OCT4 signals obtained in marmoset monkey ESCs with three different polyclonal antibodies. (A) Abcam ab19857 OCT4 antibody directed against the C-terminus recognizing OCT4A and OCT4B (upper panel). Nuclei were stained with DAPI, which is shown as an overlay in the respective lower panels. Scale bar: 100 µm in all panels. (B) Santa Cruz Sc-9081 OCT4 antibody directed against the N-terminus recognizing only OCT4A. (C) Santa Cruz Sc-8626 OCT4 antibody directed against the N-terminus recognizing only OCT4A.
Two out of three OCT4 antibodies stain primary TMSCs
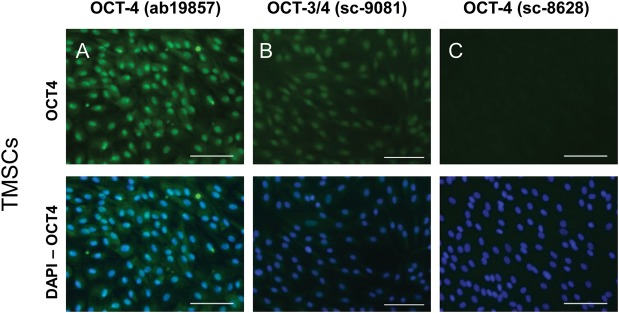
Importantly, two of the OCT4 antibodies also showed robust nuclear staining in marmoset monkey TMSCs, which are non-pluripotent cells from connective tissue (Fig. 2) (Eildermann et al., 2012b). The ab19857 OCT4 antibody displayed a strong nuclear signal in TMSCs, which was comparable with the one seen in pluripotent ES cells. Additionally, weak cytoplasmic staining was visible (Fig. 2, left panels). The sc-9081 OCT3/4 antibody displayed moderate nuclear and also faint cytoplasmic signals. Only the sc-8628 OCT4 antibody displayed no signal in TMSCs. A comparable result was obtained when HEK-293 cells of human origin were stained: very intense staining with the ab19857 OCT4 antibody, moderate staining with the sc-9081 OCT3/4 antibody and only faint signals with the sc-8628 OCT4 antibody (data not shown) indicating a staining pattern of the different OCT4 antibodies which is neither species- nor cell type-specific.
Figure 2.
OCT4 signals obtained in marmoset monkey TMSCs with three different polyclonal antibodies. (A) Abcam ab19857 OCT4 antibody (upper panel). This antibody showed strong signals in testis-derived stromal cells. Nuclei were stained with DAPI (respective lower panels). Scale bar: 100 µm in all panels. (B) Santa Cruz Sc-9081 OCT4 antibody showing signals of intermediate intensity. (C) Santa Cruz Sc-8626 OCT4 antibody showing no signals.
qRT-PCR reveals that TMSCs do not express the pluripotency markers OCT4A and SOX2
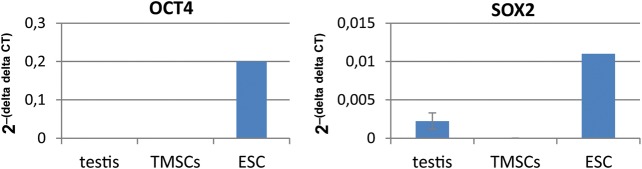
In order to either confirm or disprove the unexpected IF OCT4 stainings in marmoset testicular cells, we performed qRT-PCR for OCT4 (Fig. 3). The primers bind to exons 4 and 5 so that both OCT4A and OCT4B would be detected. Importantly, the TMSCs did not express any OCT4 mRNA. In contrast, pluripotent ES cells, which were included as positive controls, expressed OCT4 mRNA, while OCT4 was also not detectable in the adult marmoset testis. To further verify that the pluripotency-determining transcriptional network is not activated in the TMSCs, we also tested the expression of SOX2, another key player in pluripotency (Boyer et al., 2005). Confirming the OCT4 mRNA data, SOX2 was also absent from the TMSCs, while it was detectable at low levels in whole testis mRNA and at high levels in ES cell RNA.
Figure 3.
Quantitative real-time RT–PCR for OCT4 and SOX2 on marmoset monkey testis-, TMSC- and ESC RNA. (A) OCT4 was detected only in ES cell RNA, while it was undetectable in testis and testicular multipotent stromal cell RNA. (B) SOX2 was detected at high levels in ES cell RNA, at lower levels in testis RNA and was not detected in testicular multipotent stromal cell RNA.
Western blot analysis
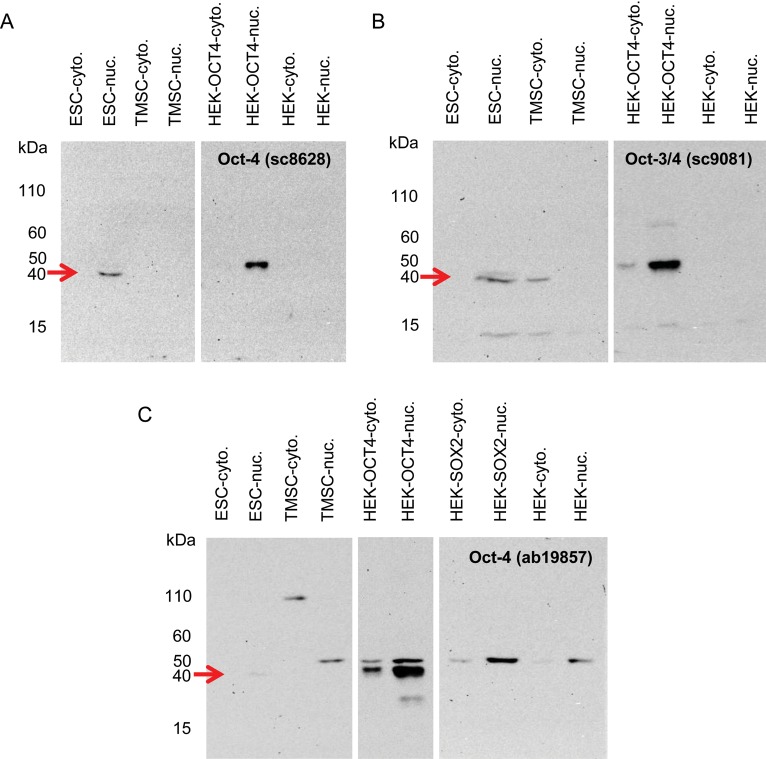
Following the contradictory results from IF and qRT-PCR, we tested the antibodies in western blot analysis on a variety of samples from which we isolated nuclear and cytoplasmic protein fractions. We included ES cells, TMSCs, OCT4A-transfected and non-transfected HEK-293 cells. As an additional control for the OCT4 ab19857 antibody, we included SOX2-transfected HEK-293 cells also (Fig. 4). The sc8626 OCT4 antibody, which was negative in IF on TMSCs, also revealed no band for TMSCs and non-transfected HEK-293 cells in western blot analysis (Fig. 4A). However, single bands of the expected size of ∼45 kDa were obtained with ES cells and OCT4-transfected HEK-293 cells. The same band was also obtained with the other antibodies. However, importantly, both antibodies also detected additional unspecific bands. The sc9081 OCT4 antibody showed an additional signal in the cytoplasmic fraction of TMSCs, misleadingly indicating the same apparent molecular weight as the band in ES cells (Fig. 4B). Moreover, there was an additional smaller band that was also present in the OCT4-transfected HEK cells. The OCT4 ab19857 antibody also showed an additional band at ∼50 kDa in the OCT4-transfected HEK-293 cells (Fig. 4C). It seems most likely that this band did not represent post-translational modification of OCT4 since it was also present in control cells transfected with SOX2. Moreover, the same band was also obtained with non-transfected HEK cells as well as TMSCs. In addition, in the cytoplasmic fraction of TMSCs there appeared a band of apparently ∼110 kDa.
Figure 4.
OCT4 antibody specificity analysis by western blot analysis. (A) Santa Cruz Sc-8626 OCT4 antibody detected exclusively and specifically one band in the positive controls (ES cells and transfected HEK-293 cells, red arrow). With non-transfected cells and TMSCs no signals were obtained. (B) Santa Cruz Sc-9081 OCT4 antibody detected the OCT4-positive controls (red arrow). Additional non-specific bands were present in OCT4-transfected HEK cells as well as in TMSCs and ES cells. (C) Abcam ab19857 OCT4 antibody detected the OCT4-positive control in transfected cells (red arrow). The ES cell positive control signal showed up only very weakly. Additional unspecific bands were detected in OCT4-transfected HEK cells. The upper unspecific band migrating at ∼50 kDa was also present in non-transfected and SOX2-transfected HEK cells as well as in the nuclear fraction of TMSCs. An additional unspecific band (∼110 kDa) was detected in the cytoplasmic fraction of TMSCs.
Discussion
The human and the marmoset OCT4 genes are very well conserved with regard to the gene structure and the encoded aa sequence. Both the human and the marmoset OCT4A isoform encodes a protein consisting of 360 aa. The marmoset OCT4 gene displays 97% nucleotide homology to the human gene. The protein products derived from the human OCT4B mRNA variant range between 164 and 265 aa depending on the translational start site and the use of an internal ribosomal entry site (Wang and Dai, 2010). Assuming that no post-translational modifications occur which significantly influence the migration during electrophoresis, the pluripotency-associated OCT4A isoform has a higher apparent molecular weight than those OCT4 isoforms that are not associated with pluripotency (Liedtke et al., 2008; Wang and Dai, 2010). In order to analyze whether the double bands on the western blot with the OCT4 antibody ab19857 resulted from post-translational modifications of OCT4, or whether they just represent unspecific binding of the antibody to an OCT4-unrelated epitope, we also probed HEK-293 cells overexpressing SOX2 instead of OCT4. The western blot analysis of the nuclear fraction of the SOX2-transfected cells exhibited also a predominant band of ∼50 kDa comparable with the bands present in the untransfected and OCT4 overexpressing HEK-293 cells. Importantly, this band was also present in the nuclear fraction of the TMSCs (Fig. 4), which have no OCT4 mRNA (Fig. 3). Altogether, this strongly suggests that the signals that were obtained in western blots above the OCT4A-positive controls are not related to OCT4 and are thus unspecific. However, to finally investigate the occurrence of post-translational modifications other than phospohorylation (Brumbaugh et al., 2012), substantial additional biochemical analyses have to be performed in future studies.
In general, the appearance of the presumably unspecific band at ∼50 kDa in the nuclear fraction in western blot analysis using the isoform-non-distinctive ab19857 OCT4 antibody correlates very well with the IF signals, which also show strong nuclear staining. Notably, this unspecific band was already observed earlier (Bhartiya et al., 2010), see also (http://www.abcam.com/index.html?pageconfig=reviews&intAbreviewID=22268&intAbID=19857). Similar false-positive results were obtained with the OCT4A-directed sc-9081 antibody, although the additional bands appeared at an apparent molecular weight below 15 kDa. Only the OCT4A-specific antibody sc-8628 detected specifically the positive control band in an ES cell sample and in OCT4-transfected HEK-293 cells. Furthermore, this antibody did not produce any signals with protein from TMSCs, further confirming its specificity. These western blot results correlate very well with the IF signals in ESCs and TMSCs. In summary, all three tested antibodies detect OCT4 in ESCs and in OCT4-transfected HEK293 cells. However, if they are used to demonstrate the pluripotency-indicating OCT4A isoform, two of the tested antibodies are inappropriate since they also may detect OCT4B or an even completely OCT4-unrelated epitope. This may result in false-positive signals, which probably provoke erroneous interpretations when they are used in studies on pluripotent cells.
For the OCT4 analysis on protein levels, antibodies with possibly insufficient specificity were used in studies on testis-derived cells. Conrad and colleagues (Conrad et al., 2008) used a rabbit polyclonal OCT4 antibody from abcam, which was not further specified in this report. However, it is likely that this antibody was the same as in the present study. Importantly, this antibody does not discriminate between OCT4A and OCT4B and detects an additional epitope unrelated to OCT4 (this study Figs 2 and 4 and Bhartiya et al., 2010). Mizrak et al. (2010) used an antibody (Santa Cruz Sc-8629) that was directed against the C-terminal epitope of human OCT4. Hence, this antibody was also unable to differentiate between OCT4A and OCT4B. Meanwhile, this group questioned its own data and showed that those cells reported previously (Mizrak et al., 2010) are indeed not pluripotent but multipotent cells (Chikhovskaya et al., 2012). Gonzalez et al. (2009) used an ‘OCT3/4 H-134’ antibody from Santa Cruz, which is probably the same (sc-9081) we used in the present study. In our study, this antibody produced false-positive signals delusively suggesting the presence of OCT4A. Golestaneh et al. (2009) also used an OCT3/4 antibody from Santa Cruz, which was also not further specified. This lack of information prevents an evaluation of the validity of the signals. The same is true for Kossack et al. (2009). Altogether, the expression analysis of the pluripotency factor OCT4A as well as the corresponding conclusions with regard to the derivation of pluripotent stem cells in recent publications on testis-derived multi- or pluripotent cells should be received with caution.
Over the past years, other researchers investigated the expression of OCT4 in different human tissues and cell lines (e.g. Cantz et al., 2008; Kristensen et al., 2010). All studies showed, like our present one, that the antibodies against OCT4 must be carefully characterized. However, our present study provides additional significant information. Cantz et al. (2008) focused on the expression of OCT4 in somatic tumor cell lines, which exhibit some characteristics of (pluripotent) stem cells. Therefore, the focus of the study by Cantz et al. (2008) was whether tumor cell lines and pluripotent stem cells also share the expression of OCT4 or whether this is a differential characteristic of both cell types. Moreover, in that study, OCT4 antibodies different from the ones used in our study were characterized. Also, those highly relevant studies on testis-derived stem cells that were the starting point for this investigation were not yet published when Cantz et al. (2008) investigated OCT4 in tumor cells. Kristensen et al. (2010) focused on OCT4 expression in human somatic urogential epihelia from prostate and epididymis. Hence, in contrast to the previous studies, we show here for the first time that OCT4 staining of primary cells from a primate testis with insufficiently characterized, but frequently used antibodies can result in severely misleading data. This is of great relevance for reproductive and stem cell research, especially with regard to the ‘launch’ of pluripotent cells from the human testis. We currently believe that these reports are based in part on misinterpretations which in turn are based on insufficiently controlled experiments (see also Eildermann et al., 2012a).
To prevent misleading antibody-based studies on OCT4 in pluripotent cells researchers should consider (i) appropriate positive and negative controls including western blot analyses, (ii) the use of the appropriate OCT4A-specific antibody and (iii) confirming their results with an independent and meaningful method on the DNA or RNA level circumventing different pitfalls like the detection of pseudogenes (Liedtke et al., 2008; Tapia et al., 2011).
Authors' roles
R.W.: study design, generation of data, data analysis and interpretation, manuscript writing and final approval of manuscript. K.E.: study design, generation of data, data analysis and interpretation and final approval of manuscript. K.D.: study design, contribution of study material and final approval of manuscript. R.B.: study design, data analysis and interpretation, manuscript writing and final approval of the manuscript.
Funding
This work was supported by grants from the German Ministry of Education and Science to R.B. (FKZ:01GN0817) and to R.B. and Stefan Schlatt (FKZ:01GN0810) and by the German Research Foundation by an unrestricted grant within the Research Unit ‘Germ Cell Potential’ (FOR-BE 2296/6-2). Funding to pay the Open Access publication charges for this article was provided by the German Primate Center, which is a Leibniz Institute financed by the Bundesrepublik Deutschland and the Bundesländer.
Conflict of interest
None declared.
Acknowledgements
We thank Jörg Gromoll for generating real-time RT–PCR data and Angelina Berenson, Magdalena Wienken and Nicole Terwort for excellent technical assistance.
References
- Bhartiya D, Kasiviswanathan S, Unni SK, Pethe P, Dhabalia JV, Patwardhan S, Tongaonkar HB. Newer insights into premeiotic development of germ cells in adult human testis using Oct-4 as a stem cell marker. J Histochem Cytochem. 2010;58:1093–1106. doi: 10.1369/jhc.2010.956870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumbaugh J, Hou Z, Russell JD, Howden SE, Yu P, Ledvina AR, Coon JJ, Thomson JA. Phosphorylation regulates human OCT4. Proc Natl Acad Sci USA. 2012;109:7162–7168. doi: 10.1073/pnas.1203874109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantz T, Key G, Bleidissel M, Gentile L, Han DW, Brenne A, Scholer HR. Absence of OCT4 expression in somatic tumor cell lines. Stem Cells. 2008;26:692–697. doi: 10.1634/stemcells.2007-0657. [DOI] [PubMed] [Google Scholar]
- Cauffman G, Van de Velde H, Liebaers I, Van Steirteghem A. Oct-4 mRNA and protein expression during human preimplantation development. Mol Hum Rreprod. 2005;11:173–181. doi: 10.1093/molehr/gah155. [DOI] [PubMed] [Google Scholar]
- Chikhovskaya JV, Jonker MJ, Meissner A, Breit TM, Repping S, van Pelt AM. Human testis-derived embryonic stem cell-like cells are not pluripotent, but possess potential of mesenchymal progenitors. Hum Reprod. 2012;27:210–221. doi: 10.1093/humrep/der383. [DOI] [PubMed] [Google Scholar]
- Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M, Aicher W, Buhring HJ, Mattheus U, Mack A, et al. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456:344–349. doi: 10.1038/nature07404. [DOI] [PubMed] [Google Scholar]
- Eildermann K, Gromoll J, Behr R. Misleading and reliable markers to differentiate between primate testis-derived multipotent stromal cells and spermatogonia in culture. Hum Reprod. 2012a;27:1754–1767. doi: 10.1093/humrep/des091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eildermann K, Aeckerle N, Debowski K, Godmann M, Christiansen H, Heistermann M, Schweyer S, Bergmann M, Kliesch S, Gromoll J, et al. Developmental expression of the pluripotency factor SALL4 in the monkey, human, and mouse testis: restriction to premeiotic germ cells cells tissues organs. Cells Tissues Organs. 2012b doi: 10.1159/000335031. doi:10.1159/000335031. [DOI] [PubMed] [Google Scholar]
- Golestaneh N, Kokkinaki M, Pant D, Jiang J, DeStefano D, Fernandez-Bueno C, Rone JD, Haddad BR, Gallicano GI, Dym M. Pluripotent stem cells derived from adult human testes. Stem Cells Dev. 2009;18:1115–1126. doi: 10.1089/scd.2008.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Griparic L, Vargas V, Burgee K, Santacruz P, Anderson R, Schiewe M, Silva F, Patel A. A putative mesenchymal stem cells population isolated from adult human testes. Biochem Biophys Res Commun. 2009;385:570–575. doi: 10.1016/j.bbrc.2009.05.103. [DOI] [PubMed] [Google Scholar]
- Kim JB, Greber B, Arauzo-Bravo MJ, Meyer J, Park KI, Zaehres H, Scholer HR. Direct reprogramming of human neural stem cells by OCT4. Nature. 2009;461:649–653. doi: 10.1038/nature08436. [DOI] [PubMed] [Google Scholar]
- Kossack N, Meneses J, Shefi S, Nguyen HN, Chavez S, Nicholas C, Gromoll J, Turek PJ, Reijo-Pera RA. Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells. 2009;27:138–149. doi: 10.1634/stemcells.2008-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen DM, Nielsen JE, Kalisz M, Dalgaard MD, Audouze K, Larsen ME, Jacobsen GK, Horn T, Brunak S, Skakkebaek NE, et al. OCT4 and downstream factors are expressed in human somatic urogenital epithelia and in culture of epididymal spheres. Mol Hum Reprod. 2010;16:835–845. doi: 10.1093/molehr/gaq008. [DOI] [PubMed] [Google Scholar]
- Liedtke S, Stephan M, Kogler G. Oct4 expression revisited: potential pitfalls for data misinterpretation in stem cell research. Biol Chem. 2008;389:845–850. doi: 10.1515/BC.2008.098. [DOI] [PubMed] [Google Scholar]
- Millar MR, Sharpe RM, Weinbauer GF, Fraser HM, Saunders PT. Marmoset spermatogenesis: organizational similarities to the human. Int J Androl. 2000;23:266–277. doi: 10.1046/j.1365-2605.2000.00236.x. [DOI] [PubMed] [Google Scholar]
- Mitchell RT, Cowan G, Morris KD, Anderson RA, Fraser HM, McKenzie KJ, Wallace WH, Kelnar CJ, Saunders PT, Sharpe RM. Germ cell differentiation in the marmoset (Callithrix jacchus) during fetal and neonatal life closely parallels that in the human. Hum Reprod. 2008;23:2755–2765. doi: 10.1093/humrep/den295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrak SC, Chikhovskaya JV, Sadri-Ardekani H, van Daalen S, Korver CM, Hovingh SE, Roepers-Gajadien HL, Raya A, Fluiter K, de Reijke TM, et al. Embryonic stem cell-like cells derived from adult human testis. Hum Reprod. 2010;25:158–167. doi: 10.1093/humrep/dep354. [DOI] [PubMed] [Google Scholar]
- Muller T, Fleischmann G, Eildermann K, Matz-Rensing K, Horn PA, Sasaki E, Behr R. A novel embryonic stem cell line derived from the common marmoset monkey (Callithrix jacchus) exhibiting germ cell-like characteristics. Hum Reprod. 2009;24:1359–1372. doi: 10.1093/humrep/dep012. [DOI] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tapia N, Arauzo-Bravo MJ, Ko K, Scholer HR. Concise review: challenging the pluripotency of human testis-derived ESC-like cells. Stem Cells. 2011;29:1165–1169. doi: 10.1002/stem.669. [DOI] [PubMed] [Google Scholar]
- Wang X, Dai J. Concise review: isoforms of OCT4 contribute to the confusing diversity in stem cell biology. Stem Cells. 2010;28:885–893. doi: 10.1002/stem.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbauer GF, Aslam H, Krishnamurthy H, Brinkworth MH, Einspanier A, Hodges JK. Quantitative analysis of spermatogenesis and apoptosis in the common marmoset (Callithrix jacchus) reveals high rates of spermatogonial turnover and high spermatogenic efficiency. Biol Reprod. 2001;64:120–126. doi: 10.1095/biolreprod64.1.120. [DOI] [PubMed] [Google Scholar]