Abstract
Aim
To develop a suitable buccal bioadhesive gel formulation containing cyclosporine A solid lipid nanoparticles (CsA SLNs) for the treatment of recurrent aphthous stomatitis.
Methods
The suitability of the prepared formulations for buccal application was assessed by means of rheological studies, textural profile analysis, and ex vivo drug-release studies. Plastic flows, typical gel-like spectra, and suitable mechanical properties were obtained from prepared formulations. The retention time was explored in in vivo distribution studies and the effect of the gel containing CsA SLNs on the healing of oral mucosal ulceration was investigated in an animal model. In vivo distribution studies are a very important indicator of the retention time of formulations at the application site.
Results
Distribution studies showed that 64.76% ± 8.35% of the formulation coded “F8+SLN” remained on the buccal mucosa 6 hours after application. For the second part of the in vivo experiments, 36 rabbits were separated into three groups: the first group was treated with the gel formulation without the active agent; the second group with the gel formulation containing CsA SLNs; and the third group, used as the control group, received no treatment. Wound healing was established by scoring of the rate of wound healing on Days 3, 6, 9, and 12. Histological observations were made on the same days as the scoring studies. The bioadhesive gel formulation that included CsA SLNs increased the rate of mucosal repair significantly.
Conclusion
This study has shown that the bioadhesive gel formulation containing CsA SLNs reported here is a promising candidate for the topical treatment of recurrent aphthous stomatitis.
Keywords: SLNs, buccal application, wound healing, oral mucosal disease
Introduction
Recurrent aphthous stomatitis (RAS) is a common oral mucosal disease state characterized by the development of oral aphthosis.1 Clinically, it presents as recurring, very painful, solitary or multiple ulcers, covered by a grayish white pseudomembrane encircled by an erythematous halo. These lesions are mostly observed in nonkeratinizing mucosa.2,3 RAS has two forms of manifestation – simple and complex aphthosis – and three morphological types – minor, major, and herpetiform aphthous ulcers, which can appear in patients with simple or complex aphthosis. The severity and duration of symptoms and the frequency and number of lesions are higher in the complex form of the disease.1 RAS or aphthous-like ulcers observed in this more serious form of aphthosis usually appear after childhood and do not disappear with age; are generally associated with underlying auto-inflammatory systemic conditions such as inflammatory bowel disease, celiac disease, Behçet’s syndrome, and human immunodeficiency virus infection; and systemic complaints, especially fever and malaise, are also observed.1,4
Even though its exact etiology is not clearly established, a T-cell-mediated immune response dysfunction, in which tumor necrosis factor α plays a major role, is considered the main disorder that destroys the oral epithelium.2,3,5,6
Because of its vague etiology and versatile clinical presentation, treatment of RAS is mostly palliative and aims to reduce the severity and duration of the symptoms and decrease the frequency of RAS attacks. The lack of a specific and definitive prophylactic remedy has led to the development of several treatments that use different drugs, antibacterial agents (eg, minocycline, tetracycline, cephalexin), anti-acidic agents (eg, sucralfate), and antineoplastics (eg, thalidomide).7–9 In particular, anti-inflammatory and immunomodulatory agents such as clofazimine, thalidomide, pentoxifylline, colchicine, levamisole, azathioprine, amlexanox, and steroids have been administered with varying clinical benefits.10–20
Cyclosporine A (CsA) is a potent immunosuppressive agent that interferes with both B- and T-cell activation. It is widely used to prevent organ transplant rejection and in the treatment of various systemic and local immune diseases. Side effects of CsA such as nephrotoxicity, cholestasis, hypertension, hirsutism, and neuropathy are common, even with careful monitoring of the patient. Hence, it was suggested that the potential toxicity of CsA could be reduced by topical application, minimizing the side effects. Unfortunately, the low water solubility of CsA has limited the development of a topical dosage form formulated for buccal mucosa. The currently available dosage forms for topical administration of CsA employ oils that possess poor bioavailability.20–22
One approach to overcome the bioavailability problems of poorly soluble drugs is to formulate them as solid lipid nanoparticles (SLNs). SLNs are alternative nanoparticulate systems to polymeric nanoparticles and liposomes, and are developed for lipophilic or hydrophilic drugs. They are biocompatible, stable during storage and manufacturing, and suitable for up scaling. With these features, SLN formulations have the potential to improve the bioavailability of drugs for topical therapy.23,24
Recent trials have focused primarily on local and topical treatments since these generally carry lower risks of systemic adverse effects; thus, these should be considered the first-line treatment.1 The use of systemic drugs for topical use is especially attractive to dermatologists because of the potential for efficacy with relatively mild systemic adverse effects during topical application.9
In this context, the objective of this investigation was to develop a bioadhesive gel formulation containing CsA-loaded SLNs and optimize its efficacy for the treatment of RAS by evaluation of the in vivo conditions in rabbits.
Materials and methods
Materials
Compritol® 888 ATO (glyceryl behenate) was generously supplied by Gattefossée (Nanterre, France). Pluronic® F-68 (poloxamer 188) was kindly gifted by BASF Chemical Company (Ludwigshafen, Germany). Tween® 80 (polysorbate 80) and CsA were obtained from Sigma-Aldrich (Munich, Germany). Carbopol® 974 P NF polymer was obtained from Noveon (Cleveland, OH). Hydroxypropylmethylcellulose (HPMC) K 100M was kindly gifted by Colorcon (Dartford, UK). All high-performance liquid chromatography (HPLC) reagents were purchased from Sigma-Aldrich. IRDye® 800RS carboxylate was obtained from LI-COR Biosciences (Lincoln, NE). All other chemicals were purchased from Sigma-Aldrich.
Methods
Preparation of SLNs
CsA-loaded SLNs were prepared by high shear homogenization. Production parameters such as mixing time, ultrasonication time (3 minutes), total formulation volume (25 mL), and lipid/aqueous phase ratio were established in accordance with Gokce et al’s study.25 Compritol 888 ATO (C888) and CsA as lipid phase and poloxamer 188 (P188) and Tween 80 (Tw 80) as the aqueous phase in bidistilled water were heated to 85°C, separately. Then, after being stirred at 24,000 rpm using a T 25 Ultra-Turrax® homogenizer (IKA, Germany) for 3 minutes, the aqueous phase was poured into the lipid phase. The particles were dispersed in bidistilled water at 4°C ± 0.5. The SLNs were kept at 4°C ± 0.5. SLNs including IRDye 800RS carboxylate were prepared by replacing the same amount of CsA (1 μg) with dye. Blank SLNs were prepared in a similar way without incorporation of the drug.
Measurement of particle size (PS) and polydispersity index (PI)
The PS and PI were measured at 25°C using a Nano-ZS Zetasizer (Malvern Instruments, Malvern, UK) at an angle of 173° after the dilution of formulations with bidistilled and filtered (0.45 μm) water. Each experiment was carried out six times.
Measurement of zeta potential (ZP)
The ZP of the SLN dispersions was measured at 25°C, under an electrical field of 40 V/cm using the Nano-ZS Zetasizer. The measurements were carried out six times.
Drug entrapment efficiency (EE%)
The SLN dispersion was ultracentrifuged for 1.5 hours at 55,000 rpm using an Optima L100 XP (Beckman Coulter, Brea, CA). The supernatant was used for CsA analysis by HPLC and the quantity of free drug was determined. The encapsulated amount of CsA was calculated by subtracting the free amount of CsA from the total amount in the dispersion. Each batch was evaluated six times. EE% was calculated using the following equation:
| (1) |
where Wi is the amount of initial drug and Wf is the amount of free drug.
The drug amount was determined using a validated HPLC method with a Hewlett Packard series 1200 HPLC apparatus (Palo Alto, CA) equipped with an ultraviolet detector set at 214 nm and a column oven set at 55°C using a C18 column (5 μm; 250 mm × 4.6 mm). The injection volume was 20 μl. The mobile phase, fluxed at 1.0 mL/min, was a mixture of methanol:water (90:10).26
Differential scanning calorimetry (DSC) analysis
DSC analysis was performed with a Mettler Toledo STAR system (model DSC821, Columbus, Ohio). The samples were sealed in aluminum pans under a nitrogen-air atmosphere at a flow rate of 50 mL/min and evaluated in 30°C–230°C temperature ranges. CsA, C888, and CsA-loaded SLN formulations were evaluated.
Preparation of gels containing CsA-loaded SLNs
Carbopol 974 P NF and HPMC K 100M were used at 1.0%–1.5% to 2.0%–2.5%. Carbopol 974 P NF and HPMC K 100M were added into the SLN solution with continued stirring for 3–5 hours at room temperature. After 24 hours, the gels were neutralized with triethanolamine to give a carbopol gel matrix with a pH value of 7.0. The final concentration of CsA in the gels was 1 μg/mL. The composition of the gels is given in Table 1.
Table 1.
Composition of gel formulations
| Formulation | HPMC K100M (%) | Carbopol 974 P NF (%) |
|---|---|---|
| F1 | 1 | – |
| F2 | 1.5 | – |
| F3 | 2 | – |
| F4 | 2.5 | – |
| F5 | – | 1 |
| F6 | – | 1.5 |
| F7 | – | 2 |
| F8 | – | 2.5 |
Abbreviation: HPMC, hydroxypropylmethylcellulose.
Mechanical properties of gels containing CsA-loaded SLNs
Textural profile analysis (TPA) was performed using a software-controlled penetrometer (TA-TX Plus, Stable Micro Systems, Godalming, UK) equipped with a 500 g load cell in texture profile analysis mode. Formulations were transferred into jacketed glass vials (20 mL) at 20°C. An analytical probe was compressed twice into these formulations to a defined depth (15 mm) and at a defined rate (2 mm/s), allowing a delay period (15 seconds) between the end of the first and beginning of the second compression. Mechanical parameters (hardness, compressibility, adhesiveness, cohesiveness, and elasticity) were derived from the resultant force–time curve. Experiments were carried out at least three times.27,28
Evaluation of the mucoadhesive properties
The mucoadhesion measurements were performed using the penetrometer TA-TX Plus equipped with a load cell of 500 g, a cylinder probe of 1 cm, and an A/MUC measuring system.29 The A/MUC measuring system consisted of a ring in which the biological support could be fixed. In this case, the support was a filter paper disc wetted with 50 μL of mucin dispersion 8% w/w in simulated saliva fluid (mucin type: type II crude; Sigma-Aldrich). A total of 50 μg of each sample was applied to the cylinder probe. The sample and biologic substrate were put in contact with a preload of 6000 mN for 3 minutes. The cylinder probe was moved upwards at a predetermined speed of 2.5 mm/min to the complete separation of the mucoadhesive interface (mucin–sample). Maximum detachment force was obtained from the force–distance graph. The area under the curve as the mucoadhesion took place was calculated from the force–distance plot. The tests were conducted at 37°C and each experiment was carried out six times.
Rheological studies
All formulations were characterized rheologically using a Haake Mars rheometer (Thermo Fisher, Karlsruhe, Germany). Continuous shear analysis of each formulation was performed at 20°C ± 0.1, in flow mode, and in conjunction with parallel steel plate geometry (diameter 40 mm) with a gap of 0.3 mm. Samples were carefully applied to the lower plate, ensuring that shearing of the formulation was minimized and that it was allowed to equilibrate for at least 1 minute prior to analysis. Upward and downward flow curves were measured over a range of shear rates (10–1000s−1). The flow properties of at least six replicate samples were determined.30,31
Oscillatory analysis of each formulation was performed at 20.0°C ± 0.1 after determination of its linear viscoelastic region, where the stress was directly proportional to the strain and the storage modulus remained constant. Frequency sweep analysis was performed over the frequency range of 0.1–10.0 Hz following application of a constant stress. The standard gap size was 0.3 mm for each sample. Storage modulus (G′) and loss modulus (G″), the dynamic viscosity (η′), and the loss tangent (tan δ) were determined. In each case, the dynamic rheological properties of at least six replicates were examined.32,33
Ex vivo drug-release studies
CsA release rates from 0.5 mL of gel were determined through Franz diffusion cells (model VTC 200, Logan Instruments Corp. Somerset, NJ) using cow buccal mucosa (0.5 cm2). A mixture of 5 mL of simulated saliva fluid (2.38 g Na2HPO4, 0.19 g KH2PO4, and 8.00 g NaCl per liter of distilled water adjusted with phosphoric acid to pH 6.75) and methanol (60:40) was used as the receptor phase, allowing sink conditions at 37°C ± 0.5. The samples withdrawn directly at appropriate time intervals were analyzed by a validated HPLC method as previously described. At the end of the experiment, buccal mucosa was taken and extracted with receptor medium to find the amount of CsA in the mucosa.34
In vivo studies
The housing care and experimental protocol of the study were approved by the Ege University Animal Ethics Committee (2009-51 and 2012-066). Adequate measures were taken to minimize the pain or discomfort of the animals. Experiments were carried out in accordance with the guidelines laid down by the Helsinki Declaration regarding the care and use of animals for experimental procedures and in accordance with local laws and regulations.
In vivo distribution of gels containing CsA-loaded SLNs
The in vivo distribution of gels containing CsA-loaded SLNs was investigated with the near-infrared dye IRDye 800RS carboxylate. Animals were anaesthetized with administration of pentobarbital (50 mg/kg). A MousePOD™ adapter (LI-COR Biosciences-GmbH, Nebraska, USA) was equipped with temperature regulators to maintain animal temperature during scanning. Gels containing dye-loaded SLNs (0.1 mL of each) were applied on the rat buccal mucosa. The buccal mucosas of the animals were scanned for 6 hours, in two infrared channels simultaneously (700 nm and 800 nm), with the 700 nm channel used for normalization of the measured infrared intensities. The intensities were displayed in pseudo colors by a LI-COR infrared imaging system (ODYSSEY) to isolate regions of interest.35
In vivo efficacy study of gels containing CsA-loaded SLNs on oral ulcer model
Young adult male New Zealand rabbits (36), weighing 2.5–3.0 kg were used, similarly to how they were used in our previous study, to create an oral ulcer model on the gingiva.36 The observation intervals and the days of histological sample collection were predetermined according to previous data.36 Since in that study an oral ulcerative wound was found to have completed healing in 12 days, the same model was utilized in the present investigation.
A round filter paper, 6 mm in diameter, was soaked in 15 μL of 50% acetic acid and was used to cause aseptic tissue necrosis. To create round ulcers, the acid-soaked paper was pressed onto the labial gingival tissue of the rabbits for 60 seconds. The rabbits were separated into three groups consisting of 12 animals and twice per day treatment was started 24 hours after ulcer initiation. The first group was treated with only the gel base, which did not contain any active agent; the second group was treated with gel containing CsA-loaded SLNs; and the third group received no treatment and their measurements served as the control. Gel containing 0.1 μg CsA (0.1 mL), was applied to the ulcerative area using a syringe without a needle. The remnants of the previous gel application were removed before each new application.
The clinical healing of each oral ulcer was evaluated by subtracting the most recent reading from the initial ulcer measurement at each observation interval. Thus, wound healing was defined using the decrease in the ulcer area. The greater the difference between the initial measurement and the observation day, the better the wound healing.
Photographs of the ulcers were taken with a digital camera. In each photograph, a filter paper disc of 6 mm diameter was included to provide calibration of the ulcer area measurement between the images. ImageJ software37 was used to measure the area of ulceration in cm2. All measurements were performed by the same researcher (PG) in a blinded fashion three times on the same day and the mean ulcer area was calculated.
For the histological examination, three animals selected randomly from each group were sacrificed with an excess dose of pentobarbital at Days 3, 6, 9, and 12 and gingival specimens were taken. Specimens were fixed in 10% formalin in phosphate buffer for 24–48 hours, processed by routine histological methods, and embedded in paraffin blocks. All the following histological analyses described were performed by two investigators, blind to the animals’ treatment. Sections (5 μm) were taken using a rotary microtome (RM 2255, Leica, Wetzlar, Germany). Tissue sections were deparaffinized; hydrated through xylenes and graded alcohol series; hematoxylin-eosin and Masson’s trichrome stained; and cleared and mounted with Entellan®. After the staining process was completed, sections were examined using a pre-established histological score. According to this previously developed scoring system, the scoring protocol was developed to establish the histological level of healing. In this system, a score of 1 means presence of epithelial necrosis, but no signs of inflammation; 2 means the inflammatory reaction has started, with no new capillary proliferation; 3 means the inflammatory reaction is prominent with few capillary proliferations on the basis of the ulcer but no epithelialization at the surface; 4 means the inflammatory reaction is decreased, new capillary proliferation has reached the surface, and epithelialization has started at the surface; and 5 means the epithelialization is complete.36 The images were analyzed using a computer-assisted image analyzer system consisting of a microscope (Olympus BX-51, Tokyo, Japan) equipped with a high-resolution video camera (Olympus DP-71) and all sections were digitally photographed.
Statistical analysis
The differences between the groups were analyzed using one-way analysis of variance. Pair-wise comparisons were completed using the Duncan test and the paired test was used to compare observation periods. The Friedman test was used to compare histological scores for each test group. In all statistical analyses, P was set as 0.05 and SPSS for Windows software (v 10.0, SPSS, Chicago, IL) was used for data analysis.
Results
Measurement of PS, PI, and ZP
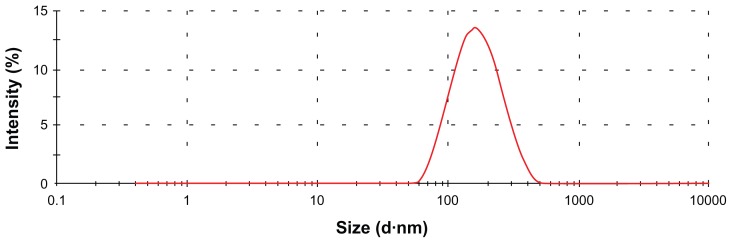
In our study, SLNs were successfully prepared using a high shear homogenization method. Table 2 shows the PS, PI, and ZP of the SLN formulations. Figure 1 shows the PS distributions of the formulations.
Table 2.
The particle size (PS), polydispersity index (PI), and zeta potential (ZP) of blank and cyclosporine A (CsA)-loaded solid lipid nanoparticle (SLN) formulations
| PS ± SD (nm) | PI ± SD | ZP ± SD (mV) | |
|---|---|---|---|
| SLNs | 165.3 ± 3.759 | 0.295 ± 0.006 | -29.7 ± 0.603 |
| CsA-loaded SLNs | 203.8 ± 2.350 | 0.374 ± 0.027 | -26.5 ± 0.624 |
Abbreviation: SD, standard deviation.
Figure 1.
The particle size distributions of the cyclosporine A-loaded solid lipid nanoparticle formulations.
Drug EE%
A validated HPLC method was used to measure the concentration of CsA in the aqueous phase. The EE% of CsA (the percentage of CsA encapsulated with respect to the total amount of CsA added to the system) was as high as 94.81%. This result was expected because of the high solubility of the CsA in lipid matrices.
DSC analysis
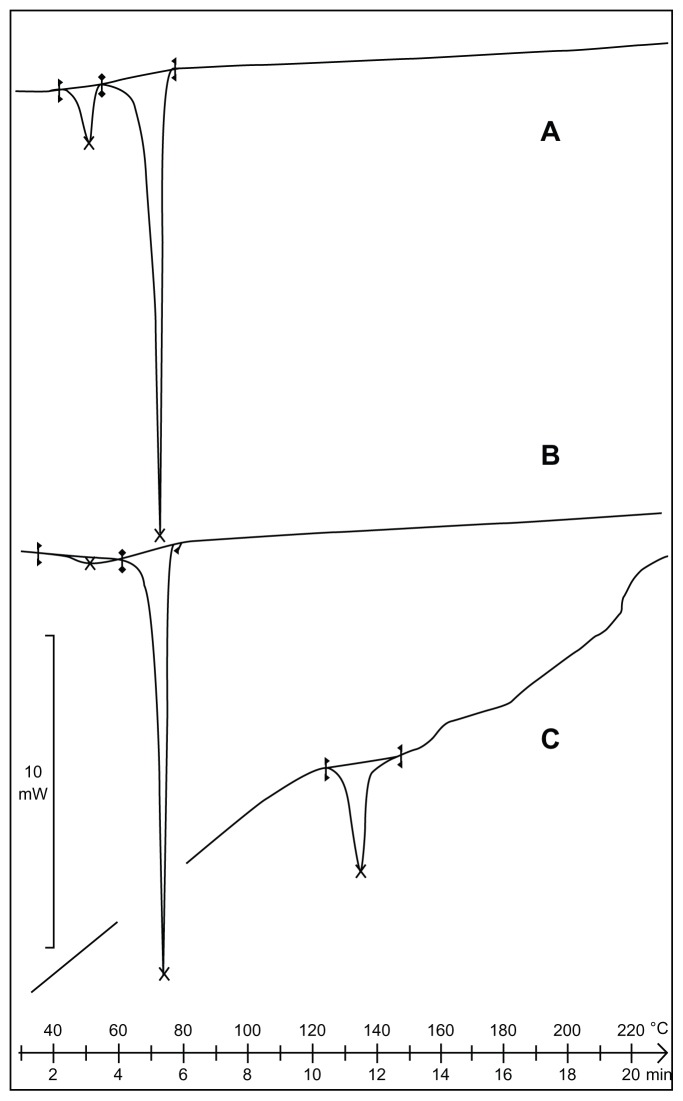
DSC was used to investigate the melting and crystallization behavior of materials and SLNs. The thermograms of the bulk lipid C888, CsA, and SLN formulations are shown in Figure 2. The melting process for C888 and CsA took place with maximum peaks at 72.31°C and 129.83°C, respectively.
Figure 2.
Differential scanning calorimetry thermograms of (A) cyclosporine A-loaded solid lipid nanoparticles, (B) C888, and (C) cyclosporine A.
Mechanical and mucoadhesive properties of gels containing CsA-loaded SLNs
The mechanical and mucoadhesive properties of the formulations are presented in Table 3. For all of the formulations, increase in polymeric concentration altered their mechanical properties, resulting in an increase in all mechanical properties.
Table 3.
Mechanical and mucoadhesive properties of the formulations
| Formulation | H (g) ± SD | C (g.sec) ± SD | A (g.sec) ± SD | Ch ± SD | E ± SD | M (mN.mm) ± SD |
|---|---|---|---|---|---|---|
| F1 | 0.417 ± 0.003 | 0.345 ± 0.007 | 3.147 ± 0.027 | 0.829 ± 0.019 | 0.640 ± 0.012 | 94.716 ± 15.432 |
| F2 | 0.576 ± 0.005 | 0.362 ± 0.006 | 7.868 ± 0.071 | 0.940 ± 0.036 | 1.273 ± 0.043 | 184.914 ± 22.647 |
| F3 | 0.986 ± 0.004 | 0.815 ± 0.018 | 10.451 ± 0.089 | 1.139 ± 0.017 | 1.593 ± 0.027 | 263.758 ± 30.204 |
| F4 | 1.422 ± 0.001 | 1.251 ± 0.031 | 15.272 ± 0.025 | 1.313 ± 0.097 | 2.083 ± 0.028 | 302.522 ± 23.242 |
| F5 | 7.727 ± 0.075 | 6.546 ± 0.073 | 7.188 ± 0.023 | 1.048 ± 0.028 | 1.488 ± 0.025 | 206.492 ± 12.348 |
| F6 | 11.524 ± 0.091 | 12.930 ± 0.081 | 12.435 ± 0.041 | 1.120 ± 0.018 | 1.539 ± 0.074 | 230.553 ± 40.919 |
| F7 | 14.046 ± 0.048 | 16.936 ± 0.091 | 15.227 ± 0.075 | 1.315 ± 0.062 | 1.746 ± 0.061 | 252.792 ± 57.439 |
| F8 | 19.110 ± 0.092 | 30.791 ± 0.028 | 22.316 ± 0.097 | 1.442 ± 0.046 | 1.936 ± 0.057 | 329.768 ± 39.013 |
| F1+SLN | 0.686 ± 0.014 | 1.085 ± 0.049 | 4.446 ± 0.051 | 0.932 ± 0.025 | 1.104 ± 0.026 | 229.790 ± 18.269 |
| F2+SLN | 0.976 ± 0.018 | 1.319 ± 0.039 | 5.761 ± 0.029 | 1.019 ± 0.083 | 1.382 ± 0.061 | 317.681 ± 24.622 |
| F3+SLN | 1.878 ± 0.022 | 2.809 ± 0.035 | 8.874 ± 0.015 | 1.442 ± 0.036 | 1.761 ± 0.048 | 467.836 ± 27.809 |
| F4+SLN | 3.515 ± 0.063 | 4.875 ± 0.061 | 14.961 ± 0.052 | 1.686 ± 0.042 | 2.652 ± 0.063 | 512.471 ± 34.079 |
| F5+SLN | 12.537 ± 0.043 | 24.615 ± 0.083 | 10.437 ± 0.041 | 1.143 ± 0.018 | 1.816 ± 0.037 | 936.872 ± 30.443 |
| F6+SLN | 24.593 ± 0.109 | 54.936 ± 0.039 | 35.531 ± 0.013 | 1.237 ± 0.074 | 2.177 ± 0.083 | 2092.069 ± 38.332 |
| F7+SLN | 29.340 ± 0.029 | 68.814 ± 0.090 | 42.226 ± 0.096 | 1.927 ± 0.064 | 2.659 ± 0.076 | 3071.017 ± 43.695 |
| F8+SLN | 32.935 ± 0.094 | 88.977 ± 0.080 | 55.154 ± 0.081 | 2.095 ± 0.010 | 3.084 ± 0.059 | 3766.281 ± 67.091 |
Abbreviations: H, hardness; C, compressibility; A, adhesiveness; E, elasticity; Ch, cohesiveness; g, gram; M, mucoadhesion; SD, standard deviation; sec, second.
Rheological studies
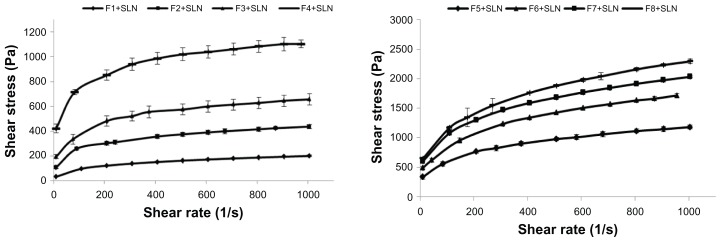
The rheological properties of formulations affect both the ease of application and retention on the mucosal surface. Rheological properties also determine the time-dependent recovery of the product following administration. Representative flow curves of the formulations are graphically presented in Figure 3.
Figure 3.
Flow curves of the formulations.
Abbreviation: SLN, solid lipid nanoparticle.
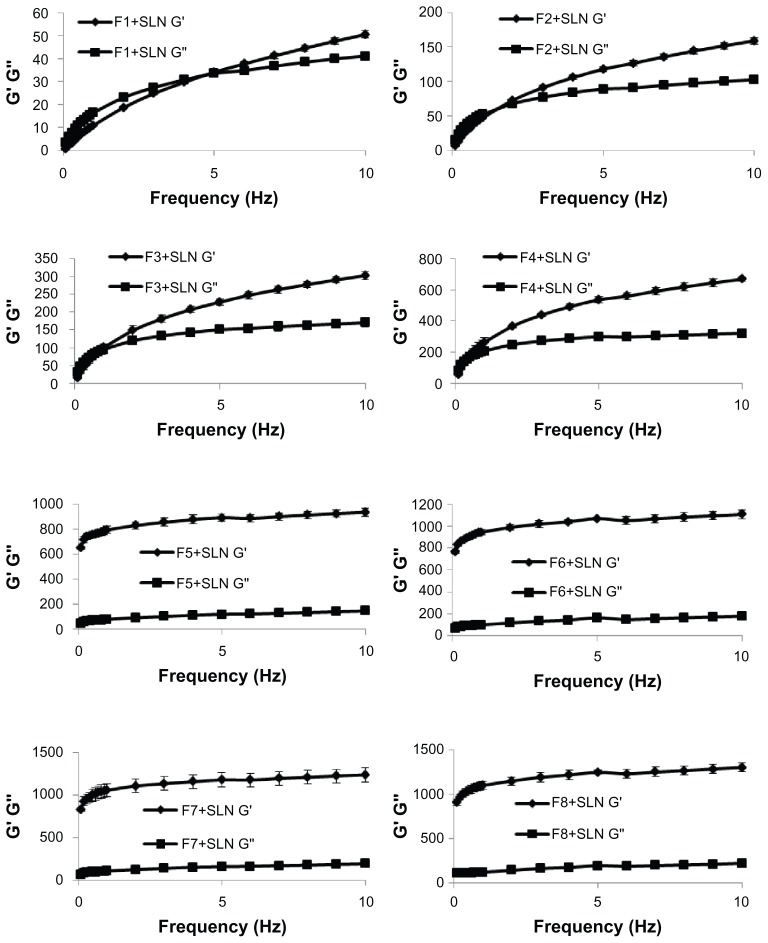
Oscillatory analysis is a dynamic mechanical technique involving the application of stress to a sample within a small strain range. It is useful to understand the relationship between viscous and elastic flow properties of samples. Figure 4 shows the rheological properties of the formulations.
Figure 4.
Frequency-dependent changes in viscoelastic properties of the formulations.
Abbreviations: G′, storage modulus; G″, loss modulus; SLN, solid lipid nanoparticle.
Ex vivo drug-release studies
The amount of CsA detected in the receptor medium was under the detection limits during the ex vivo drug-release studies (the limit of detection value was 0.011). At the end of the study after 24 hours, the buccal mucosa was extracted and the amount of CsA in the mucosa was found to be 71.69% ± 1.05% of the initial dose.
In vivo studies
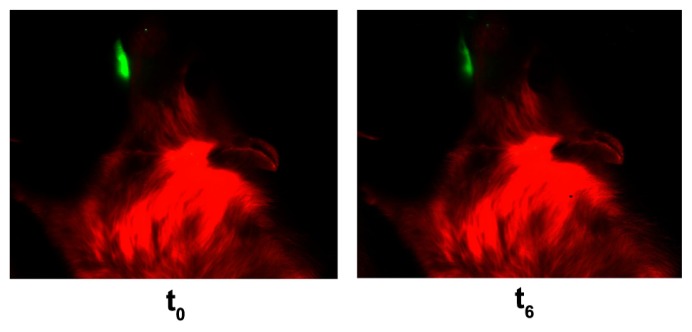
The in vivo distribution studies showed that the F8+SLN gel formulation containing CsA-loaded SLNs remained on the rabbit buccal mucosa 6 hours after application (Figure 5). Mean density % of three animals was found to be 64.76% ± 8.35% at the end of the study.
Figure 5.
In vivo distribution studies of bioadhesive gel formulations containing cyclosporine A-loaded solid lipid nanoparticles.
Notes: t0: At the beginning of the experiment. t6: After 6 hours from application of the formulation.
The ulcer model produced uniform wounds in almost identical positions on the gingival mucosa of the rabbits and allowed the healing phases to be examined by measuring ulcer size. The mean ulcer area values of the animals on Days 3, 6, 9, and 12 are presented in Table 4. Throughout the examination period, the mean ulcer area measurement in each group ranged from 31.92 mm2 to 0.00 mm2. The largest mean ulcer area values were observed on Day 3, and a significant decrease was found on Day 9, with the smallest ulcer areas on Day 12 (P < 0.05).
Table 4.
The mean ulcer area values for three animals in each test group on Days 3, 6, 9, and 12
| Group | Day 3 (mm2) | Day 6 (mm2) | Day 9 (mm2) | Day 12 (mm2) |
|---|---|---|---|---|
| Control | ||||
| Min | 18.766 | 12.552 | 3.988 | 1.150 |
| Max | 31.923 | 20.683 | 8.542 | 1.205 |
| X ± SH | 24.945 ± 0.861a,A | 17.210 ± 0.699b,A | 5.798 ± 0.542c,A | 1.181 ± 0.009d,A |
| Gel formulation (without active agent) | ||||
| Min | 17.145 | 7.144 | 2.232 | 0.000 |
| Max | 28.648 | 20.363 | 7.788 | 2.249 |
| X ± SH | 21.396 ± 0.621a,B | 13.292 ± 0.895b,B | 4.876 ± 0.484c,A | 1.119 ± 0.500d,A |
| Gel formulation | ||||
| Min | 8.397 | 7.354 | 1.591 | 0.000 |
| Max | 29.947 | 19.724 | 4.897 | 0.384 |
| X ± SH | 21.867 ± 0.991a,B | 12.117 ± 0.692b,B | 3.531 ± 0.351c,B | 0.116 ± 0.058d,B |
Notes:
Different letters on the same rows show statistically meaningful differences within the sample (P < 0.05).
Different letters on the same columns show statistically meaningful differences within the sample (P < 0.05).
According to the previously developed scoring system already discussed, if the score is high, the healing is better. The scores of the specimens on Days 3, 6, 9, and 12 are presented in Table 5. The control group had lower scores until the end of the observation period. An increment was observed for the score of this group, which changed from 1.67 to 2.33 over Days 3–6 and from 3.33 to 4.33 over Days 9–12. The first group (treated solely with the gel base) had a score of 2 on Day 3. This score increased from 2 to 2.67, 4, and 5 on Days 6, 9 and 12, respectively. The score given to the group treated with the gel containing CsA-loaded SLNs was significantly higher than the other groups on Day 3, indicating improved healing. The score of this group increased from 3 to 3.67, 4.67, and 5.00 on Days 6, 9 and 12, respectively. The average value of all measurements on Days 3, 6, 9, and 12 of the group treated with the gel containing CsA-loaded SLNs was higher than that of the control group (P < 0.05).
Table 5.
Histological healing scores for three animals in each test group on Days 3, 6, 9, and 12
| Groups | Day 3 | Day 6 | Day 9 | Day 12 |
|---|---|---|---|---|
| Control | ||||
| Min | 1 | 2 | 3 | 4 |
| Max | 1 | 3 | 4 | 5 |
| X ± SH | 1.67 ± 0.33c,B | 2.33 ± 0.33b,c,B | 3.33 ± 0.33a,b,B | 4.33 ± 0.33a,A |
| Gel formulation (without active agent) | ||||
| Min | 2 | 2 | 4 | 5 |
| Max | 2 | 3 | 4 | 5 |
| X ± SH | 2 ± 0.00d,B | 2.67 ± 0.33c,A,B | 4 ± 0.00b,A,B | 5 ± 0.00a,A |
| Gel formulation | ||||
| Min | 3 | 3 | 4 | 5 |
| Max | 3 | 4 | 5 | 5 |
| X ± SH | 3 ± 0.00b,A | 3.67 ± 0.33b,A | 4.67 ± 0.33a,A | 5 ± 0.00a,A |
Notes:
Different letters on the same rows show statistically meaningful differences within the sample (P < 0.05).
Different letters on the same columns show statistically meaningful differences within the sample (P < 0.05).
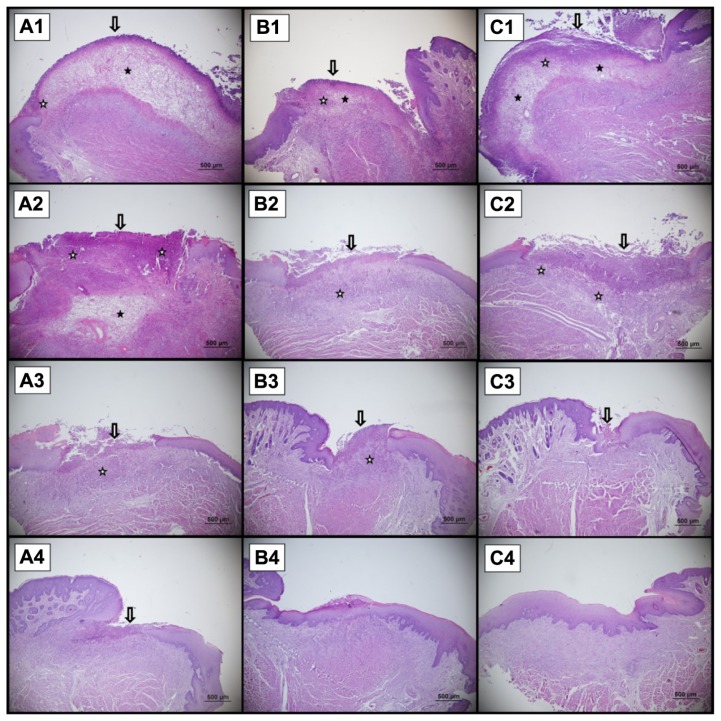
Statistically significant differences were observed between the control group, gel base group (first group), and gel containing CsA-loaded SLNs group (second group) and expressed as scores for evaluation of the histological healing (Figure 6).
Figure 6.
A sample of the histological presentation of the ulcer area. There is epithelial necrosis at the surface and subepithelial inflammatory activity (hematoxylin and eosin staining). (A) Control, (B) gel formulation (without active agent), (C) gel formulation. A1, B1, and C1 show Day 3; A2, B2, and C2 show Day 6; A3, B3, and C3 show Day 9; A4, B4, and C4 show Day 12.
Notes: Arrow denotes epidermal injury. ★ shows neutrophil and mononucleer cell infiltration. ✰ shows odema in lamina propria.
Discussion
Recognition of the commonness and relapse frequency of RAS has led to a search for alternative treatment options. For this reason, a bioadhesive gel formulation containing CsA-loaded SLNs may be a good alternative candidate for RAS treatment.
The studies have shown that as the PS decreases, the total area of the active substance and the level of interaction with body fluids increase. This phenomenon results in the enhancement of bioavailability.38 The PS of blank prepared SLNs was around 160 nm. The PS was increased by up to 200 nm by the addition of CsA and this increment was statistically significant. However, the size was still suitable for endocytosis. In a study by Silva et al, risperidone SLNs were produced for similar purposes and it was found that SLNs with a PS of around 200 nm were stable in hydrogels for 30 days.39 In our study, the addition of the drug did not perturb the distribution of nanoparticles and the produced system was still homogeneous, as indicated by the PI being smaller than 0.5.
In this study, the prepared CsA-loaded SLNs had high drug EE%, as expected. Previous studies have shown that CsA can be successfully loaded into SLNs. In the studies of Müller et al40 and Gokce et al,25 the entrapment efficiencies were similar – 96.1% and 95.6%, respectively.
DSC studies showed that the lipid C888 was completely in crystalline form. The CsA peak was lost in the formulation, indicating the solubilization of CsA in the lipid.
ZP is an important parameter that gives an indication of the potential stability of colloidal systems. It is the overall charge required in a specific medium for the particles to be suspended without being aggregated. If all the particles have a large negative or positive ZP, they will repel each other and dispersion is considered stable.41 The addition of CsA into the SLNs decreased the magnitude of ZP. A ZP of below −30 mV or above +30 mV is required for stability and the SLNs prepared in this study were on the border of this value range. Therefore, it was thought that incorporation of the SLNs into hydrogels would be beneficial for the stability of the nanosystem. In addition, the hydrogels would help adhesiveness and prolong the residence time of the applied system, permitting drug absorption and, in most cases, improving bioavailability.
The mechanical properties of buccal formulations have been proven relevant to the performance of formulations. For this reason, TPA – which explains the resistance of the formulations to compressive stresses and subsequent relaxation – is frequently used to identify formulations that may be suitable for clinical application.42 TPA analysis revealed that our formulations had suitable mechanical properties, with suitable hardness, elasticity, high cohesiveness, and high adhesiveness values. However, the polymer concentration of the formulations and the addition of SLNs affected their mechanical properties. This can be explained by concentration-dependent effects on the formulations’ viscosity. In the literature, Lucero et al43 and Jones et al44 have described a correlation between a formulation’s viscosity and its mechanical properties. In our study, the formulation coded “F8+SLN” exhibited the greatest cohesiveness and adhesiveness values, thus offered optimal performance in this regard.
A gel formulation for buccal application should exhibit high mucoadhesiveness to be able to fix the formulation on the buccal mucosa, allowing enough time for prolonged drug release, due to the appropriate mechanical properties.45 Of the various formulations under examination in this study, F8+SLN showed the highest mucoadhesion. This is a very important advantage for a buccal formulation to have, as it means that it can withstand salivation, tongue movement, and swallowing for some time.
The rheological properties of the gel-type dosage forms are important for predicting their behavior in vivo. In particular, the flow property of a gel formulation is fundamental, since this property influences pharmaceutical development and thus the formulation’s success as a drug delivery system. In this study, the shear stress changes versus shear rates were used to determine whether the flow properties of the formulations were Newtonian or non-Newtonian. All of the prepared formulations were determined to have a non-Newtonian plastic behavior, as observed in their flow curves. These systems started flowing after achieving a certain yield value, which is a typical behavior of non-Newtonian plastic fluids. After these yield values, the formulations showed shear thickening behavior and their viscosity values decreased (data not shown). Moreover, in accordance with the polymer concentration, the yield values increased for all of the formulations. Among all formulations, the highest shear stress values were obtained for the F8+SLN formulation.
The rheological properties of gel formulations affect both the ease of application and retention on the mucosal surface. Following local application to the buccal mucosa, it is accepted that the equilibrium rheological properties of the formulations will dominate the subsequent physicochemical properties.46,47 In oscillatory rheometry, the effects of oscillatory stress on the viscoelastic properties are measured as the storage modulus (G′), a measure of the elasticity, and the loss modulus (G′), representing viscous components at a given frequency of oscillation.42,48 An ideal gel formulation should exhibit a solid-like mechanical spectrum, where G′ > G″ with little frequency dependence.49 According to the rheological studies conducted, the formulations prepared with Carbapol 974 P NF were found to be frequency independent and they exhibited typical solid-like mechanical spectra (G′ > G″). For the formulations prepared with HPMC K 100M, the same results were observed after certain frequency values. It was also determined that the highest elasticity value, when compared to other formulations, was obtained for the F8+SLN formulation. The greater elasticity of this formulation would result in enhanced retention in the buccal mucosa.
The release of the active drug from the SLN-incorporated gel was studied ex vivo in this work. The in vitro release studies showed that, under the study conditions, there was not any permeation through the buccal mucosa, which suggests that the prepared formulation might potentially be used as a vehicle for local delivery. Since CsA is highly lipophilic, it was dissolved in the matrix of SLNs and no release could be seen in vitro. The release of CsA from the SLNs has been tested previously by Gokce et al.25 In their study, CsA was not released from the SLNs due to the high affinity of drug to lipid. After the addition of lipase to the medium, CsA release could be observed and it was found that the release correlated with degradation of the lipid matrix. In terms of cellular uptake, it was determined that the release of CsA from the SLNs would be dependent on the lipase and the uptake ability was shown on corneal epithelial cells. Therefore, it is very likely that in this study, SLNs would diffuse to the mucosa and that the release of CsA would, similarly, occur after endocytosis.
In this study, it was thought that an SLN-incorporated gel would be beneficial for increasing the retention time and penetration (ie, the enhanced permeability and retention effect) of the SLNs at the application area. The ex vivo studies showed that although CsA was not detected in receptor phase, the fact that 71.69% ± 1.05% of the CsA was extracted from the mucosa confirmed that the SLNs were localized in this tissue. The remaining CsA remained in the gel.
As a result of the in vitro characterization studies, the F8+SLN formulation was chosen for in vivo studies due to its suitable mechanical properties, high mucoadhesion values, and ideal rheological properties. The contact time of a formulation on the buccal mucosa is of high importance for buccal drug delivery and in vivo distribution studies are a very important indicator of the retention time of the formulation at the application site. In this work, in vivo distribution studies were conducted with IRDye. The benefit of this method is that live animals are used and that they are not exposed to radiation as they are in radio-imaging studies. Our studies showed that 64.759% ± 8.35% of the F8+SLN formulation remained on the buccal mucosa 6 hours after application.
In the present investigation, a 12-day healing period was used, since preliminary investigations revealed that ulcer healing is completed in 12 days. To determine the speed of wound healing, the ulcer area was measured on each observation day, then the same animals were sacrificed and their wound areas evaluated histologically to determine the healing in each group. According to results, on Days 3, 6, and 9, the ulcer area sizes of the first group (treated with the gel base only) and the second group (treated with the gel containing CsA-loaded SLNs) were smaller than those of the control group. On Day 12, the smallest ulcer area measurements were derived from the treatment groups (P < 0.05). According to the histological studies, on Day 3, many destructive changes occurred in the gingival tissue of animals in all groups. It was observed that there was damage in the epithelium and lamina propria, such as ulceration, mononuclear cell infiltration, and generalized edema. The ulceration and inflammatory cell infiltration could still be determined on Day 6; however, a decrement in tissue edema could be observed in the group treated with the gel containing CsA-loaded SLNs. On Day 12, it was seen that epithelialization had been completed in both treatment groups while incomplete epithelialization was observed in the control group.
Conclusion
A bioadhesive gel formulation containing CsA-loaded SLNs for the treatment of RAS was prepared successfully in the work described here. The rheological behavior of the formulations revealed a plastic flow and typical gel spectra. In addition, by texture analysis, the developed formulations were shown to have an appropriate consistency, with high adhesiveness and cohesiveness values. This study also demonstrated a rapid decrease in ulcer size with the application of the ideal gel containing CsA-loaded SLNs. In the experimental standard mucosal wound animal study, the gel containing CsA-loaded SLNs showed a statistically significant increased rate of mucosal repair. When lesions were covered with the gel formulation containing no active agent, moderate improvement was observed, compared with untreated animals, suggesting that the gel base provided a protective layer over the lesion. Thus, even though the advantage of this preparation over the most commonly used topical agents for RAS treatment should be investigated in vivo in further studies, the novel bioadhesive gel formulation containing CsA-loaded SLNs discussed here appears to be a candidate for the topical treatment of RAS.
Acknowledgments
The authors wish to thank the Research Foundation of Ege University (09/ECZ/026) and the Novartis Drug Company for financial support given to this study.
Footnotes
Disclosure
Other than the funding outlined in the Acknowledgments, the authors declare no conflicts of interest in this work.
References
- 1.Baccaglini L, Lalla RV, Bruce AJ, et al. Urban legends: recurrent aphthous stomatitis. Oral Dis. 2011;17(8):755–770. doi: 10.1111/j.1601-0825.2011.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scully C, Shotts R. ABC of oral health Mouth ulcers and other causes of orofacial soreness and pain. BMJ. 2000;321(7254):162–165. doi: 10.1136/bmj.321.7254.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonsalves WC, Chi AC, Neville BW. Common oral lesions: Part I. Superficial mucosal lesions. Am Fam Physician. 2007;75(4):501–507. [PubMed] [Google Scholar]
- 4.Scully C, Hodgson T. Recurrent oral ulceration: aphthous-like ulcers in periodic syndromes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(6):845–852. doi: 10.1016/j.tripleo.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Brice SL. Clinical evaluation of the use of low-intensity ultrasound in the treatment of recurrent aphthous stomatitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83(1):14–20. doi: 10.1016/s1079-2104(97)90084-6. [DOI] [PubMed] [Google Scholar]
- 6.Eversole LR. Immunopathogenesis of oral lichen planus and recurrent aphthous stomatitis. Semin Cutan Med Surg. 1997;16(4):284–294. doi: 10.1016/s1085-5629(97)80018-1. [DOI] [PubMed] [Google Scholar]
- 7.Gorsky M, Epstein J, Rabenstein S, Elishoov H, Yarom N. Topical minocycline and tetracycline rinses in treatment of recurrent aphthous stomatitis: a randomized cross-over study. Dermatol Online J. 2007;13(2):1. [PubMed] [Google Scholar]
- 8.Gorsky M, Epstein J, Raviv A, Yaniv R, Truelove E. Topical minocycline for managing symptoms of recurrent aphthous stomatitis. Spec Care Dentist. 2008;28(1):27–31. doi: 10.1111/j.1754-4505.2008.00006.x. [DOI] [PubMed] [Google Scholar]
- 9.Chiu HY, Tsai TF. Topical use of systemic drugs in dermatology: a comprehensive review. J Am Acad Dermatol. 2011;65(5):1048. e1–e22. doi: 10.1016/j.jaad.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 10.de Abreu MA, Hirata CH, Pimentel DR, Weckx LL. Treatment of recurrent aphthous stomatitis with clofazimine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(5):714–721. doi: 10.1016/j.tripleo.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Escudier M, Nunes C, Sanderson JD. Disorders of the mouth. Medicine. 2011;39(3):127–131. [Google Scholar]
- 12.Thornhill MH, Baccaglini L, Theaker E, Pemberton MN. A randomized, double-blind, placebo-controlled trial of pentoxifylline for the treatment of recurrent aphthous stomatitis. Arch Dermatol. 2007;143(4):463–470. doi: 10.1001/archderm.143.4.463. [DOI] [PubMed] [Google Scholar]
- 13.Davatchi F, Shahram F, Chams-Davatchi C, et al. How to deal with Behcet’s disease in daily practice. Int J Rheum Dis. 2010;13(2):105–116. doi: 10.1111/j.1756-185X.2010.01462.x. [DOI] [PubMed] [Google Scholar]
- 14.Pakfetrat A, Mansourian A, Momen-Heravi F, et al. Comparison of colchicine versus prednisolone in recurrent aphthous stomatitis: A double-blind randomized clinical trial. Clin Invest Med. 2010;33(3):E189–E195. doi: 10.25011/cim.v33i3.13725. [DOI] [PubMed] [Google Scholar]
- 15.Picciani BL, Silva-Junior GO, Barbirato DS, Ramos RT, Cantisano MH. Regression of major recurrent aphthous ulcerations using a combination of intralesional corticosteroids and levamisole: a case report. Clinics (Sao Paulo) 2010;65(6):650–652. doi: 10.1590/S1807-59322010000600015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Zeng X, Chen Q, et al. An evaluation on the efficacy and safety of amlexanox oral adhesive tablets in the treatment of recurrent minor aphthous ulceration in a Chinese cohort: a randomized, double-blind, vehicle-controlled, unparallel multicenter clinical trial. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(4):475–481. doi: 10.1016/j.tripleo.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Murray B, Biagioni PA, Lamey PJ. The efficacy of amlexanox OraDisc on the prevention of recurrent minor aphthous ulceration. J Oral Pathol Med. 2006;35(2):117–122. doi: 10.1111/j.1600-0714.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- 18.Lin P, Liang G. Behçet disease: recommendation for clinical management of mucocutaneous lesions. J Clin Rheumatol. 2006;12(6):282–286. doi: 10.1097/01.rhu.0000249894.03016.de. [DOI] [PubMed] [Google Scholar]
- 19.Femiano F, Buonaiuto C, Gombos F, Lanza A, Cirillo N. Pilot study on recurrent aphthous stomatitis (RAS): a randomized placebo-controlled trial for the comparative therapeutic effects of systemic prednisone and systemic montelukast in subjects unresponsive to topical therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(3):402–407. doi: 10.1016/j.tripleo.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 20.Elad S, Epstein JB, von Bültzingslöwen I, Drucker S, Tzach R, Yarom N. Topical immunomodulators for management of oral mucosal conditions, a systematic review; Part II: miscellaneous agents. Expert Opin Emerg Drugs. 2011;16(1):183–202. doi: 10.1517/14728214.2011.528390. [DOI] [PubMed] [Google Scholar]
- 21.Sahebjamee M, Arbabi-Kalati F. Management of oral lichen planus. Arch Iran Med. 2005;8(4):252–256. [Google Scholar]
- 22.Spolidório LC, Merzel J, Villalba H, Vargas PA, Coletta RD, Almeida OP. Morphometric evaluation of gingival overgrowth and regression caused by cyclosporin in rats. J Periodont Res. 2001;36(6):384–389. doi: 10.1034/j.1600-0765.2001.360606.x. [DOI] [PubMed] [Google Scholar]
- 23.Italia JL, Bhardwaj V, Kumar MN. Disease, destination, dose and delivery aspects of ciclosporin: the state of the art. Drug Discov Today. 2006;11(17–18):846–854. doi: 10.1016/j.drudis.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Sinha VR, Srivastava S, Goel H, Jindal V. Solid lipid nanoparticles (SLN’S): trends and implications in drug targeting. Int J Adv Pharma Sci. 2011;1(3):212–238. [Google Scholar]
- 25.Gokce EH, Sandri G, Bonferoni MC, et al. Cyclosporine A loaded SLNs: evaluation of cellular uptake and corneal cytotoxicity. Int J Pharm. 2008;364(1):76–86. doi: 10.1016/j.ijpharm.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 26.Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47(2–3):165–196. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q, Yie G, Li Y, Yang Q, Nagai T. Studies on the cyclosporin A loaded stearic acid nanoparticles. Int J Pharm. 2000;200(2):153–159. doi: 10.1016/s0378-5173(00)00361-6. [DOI] [PubMed] [Google Scholar]
- 28.Chang JY, Oh YK, Choi HG, Kim YB, Kim CK. Rheological evaluation of thermosensitive and mucoadhesive vaginal gels in physiological conditions. Int J Pharm. 2002;241(1):155–163. doi: 10.1016/s0378-5173(02)00232-6. [DOI] [PubMed] [Google Scholar]
- 29.Jones DS, Woolfson AD, Djokic J, Coulter WA. Development and mechanical characterization of bioadhesive semi-solid, polymeric systems containing tetracycline for the treatment of periodontal diseases. Pharm Res. 1996;13(11):1734–1738. doi: 10.1023/a:1016413428473. [DOI] [PubMed] [Google Scholar]
- 30.Sandri G, Bonferoni MC, Rossi S, et al. Platelet lysate formulations based on mucoadhesive polymers for the treatment of corneal lesions. J Pharm Pharmacol. 2011;63(2):189–198. doi: 10.1111/j.2042-7158.2010.01208.x. [DOI] [PubMed] [Google Scholar]
- 31.Jones DS, Woolfson AD, Brown AF. Textural analysis and flow rheometry of novel, bioadhesive antimicrobial oral gels. Pharm Res. 1997;14(4):450–457. doi: 10.1023/a:1012091231023. [DOI] [PubMed] [Google Scholar]
- 32.Andrews GP, Gorman SP, Jones DS. Rheological characterisation of primary and binary interactive bioadhesive gels composed of cellulose derivatives designed as ophthalmic viscosurgical devices. Biomaterials. 2005;26(5):571–580. doi: 10.1016/j.biomaterials.2004.02.062. [DOI] [PubMed] [Google Scholar]
- 33.Andrews GP, Jones DS. Rheological characterization of bioadhesive binary polymeric systems designed as platforms for drug delivery implants. Biomacromolecules. 2006;7(3):899–906. doi: 10.1021/bm050620y. [DOI] [PubMed] [Google Scholar]
- 34.Ankarao A, Jitendra Kumar P, Babu Rao Ch, Devanna N, Venkata Phani Deepthi B. Formulation and evaluation of buccoadhesive bilayered tablets of carvedilol. International Journal of Research in Pharmaceutical and Biomedical Sciences. 2011;1:6–11. [Google Scholar]
- 35.He X, Ma J, Mercado AE, Xu W, Jabbari E. Cytotoxicity of Paclitaxel in biodegradable self-assembled core-shell poly(lactide-co-glycolide ethylene oxide fumarate) nanoparticles. Pharm Res. 2008;25(7):1552–1562. doi: 10.1007/s11095-007-9513-z. [DOI] [PubMed] [Google Scholar]
- 36.Karavana Hizarcioğlu SY, Sezer B, Güneri P, et al. Efficacy of topical benzydamine hydrochloride gel on oral mucosal ulcers: an in vivo animal study. Int J Oral Maxillofac Surg. 2011;40(9):973–978. doi: 10.1016/j.ijom.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 37.Rasband WS. ImageJ [software] Bethesda, MD: US National Institutes of Health; [Accessed 23 October 2012]. pp. 1997–2012. Available from: http://imagej.nih.gov/ij/ [Google Scholar]
- 38.Sjöström B, Bergenståhl B, Kronberg B. A method for the preparation of submicron particles of sparingly water-soluble drugs by precipitation in oil-in-water emulsions. II: Influence of the emulsifier, the solvent, and the drug substance. J Pharm Sci. 1993;82(6):584–589. doi: 10.1002/jps.2600820608. [DOI] [PubMed] [Google Scholar]
- 39.Silva AC, Amaral MH, González-Mira E, Santos D, Ferreira D. Solid lipid nanoparticles (SLN) – based hydrogels as potential carriers for oral transmucosal delivery of risperidone: preparation and characterization studies. Colloids Surf B Biointerfaces. 2012;93:241–248. doi: 10.1016/j.colsurfb.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Müller RH, Runge S, Ravelli V, Mehnert W, Thünemann AF, Souto EB. Oral bioavailability of cyclosporine: solid lipid nanoparticles (SLN) versus drug nanocrystals. Int J Pharm. 2006;317(1):82–89. doi: 10.1016/j.ijpharm.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 41.Riddick TM. Zeta Potential. Staunton, VA: Zeta-Meter Inc; 2007. Control of colloid stability; pp. 198–200. [Google Scholar]
- 42.Baloglu E, Karavana SY, Senyigit ZA, et al. In-situ gel formulations of econazole nitrate: preparation and in-vitro and in-vivo evaluation. J Pharm Pharmacol. 2011;63(10):1274–1282. doi: 10.1111/j.2042-7158.2011.01315.x. [DOI] [PubMed] [Google Scholar]
- 43.Lucero MJ, Vigo J, León MJ. A study of shear and compression deformations on hydrophilic gels of α-tocopherol. Int J Pharm. 1994;111(3):261–269. [Google Scholar]
- 44.Jones DS, Woolfson AD, Brown AF. Textural, viscoelastic and mucoadhesive properties of pharmaceutical gels composed of cellulose polymers. Int J Pharm. 1997;151(2):223–333. [Google Scholar]
- 45.Serra L, Doménech J, Peppas NA. Engineering design and molecular dynamics of mucoadhesive drug delivery systems as targeting agents. Eur J Pharm Biopharm. 2009;71(3):519–528. doi: 10.1016/j.ejpb.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruschi ML, Jones DS, Panzeri H, Gremião MP, de Freitas O, Lara EH. Semisolid systems containing propolis for the treatment of periodontal disease: in vitro release kinetics, syringeability, rheological, textural, and mucoadhesive properties. J Pharm Sci. 2007;96(8):2074–2089. doi: 10.1002/jps.20843. [DOI] [PubMed] [Google Scholar]
- 47.Ross-Murphy SB. Physical gelation of synthetic and biological macromolecules. In: DeRossi D, Kajiwara K, Osada Y, Yamauchi A, editors. Polymer Gels: Fundamentals and Biomedical Application. New York, NY: Plenum Press; 1991. pp. 21–39. [Google Scholar]
- 48.Jones DS, Woolfson AD, Brown AF, Coulter WA, McClelland C, Irwin CR. Design, characterisation and preliminary clinical evaluation of a novel mucoadhesive topical formulation containing tetracycline for the treatment of periodontal disease. J Control Release. 2000;67(2–3):357–368. doi: 10.1016/s0168-3659(00)00231-5. [DOI] [PubMed] [Google Scholar]
- 49.Ikeda S, Nishinari K. “Weak gel”-type rheological properties of aqueous dispersions of nonaggregated kappa-carrageenan helices. J Agric Food Chem. 2001;49(9):4436–4441. doi: 10.1021/jf0103065. [DOI] [PubMed] [Google Scholar]