Abstract
CCN proteins play crucial roles in development, angiogenesis, cell motility, matrix turnover, proliferation, and other fundamental cell processes. Early embryonic lethality in CCN5 knockout and over-expressing mice led us to characterize CCN5 distribution in early development. Previous papers in this series showed that CCN5 is expressed widely in mice from E9.5 to adult; however, its distribution before E9.5 has not been studied. To fill this gap in our knowledge of CCN5 expression in mammals, RT-PCR was performed on preimplantation murine embryos: 1 cell, 2 cell, 4 cell, early morula, late morula, and blastocyst. CCN5 mRNA was not detected in 1, 2, or 4 cell embryos. It was first detected at the early morula stage and persisted to the preimplantation blastocyst stage. Immunohistochemical staining showed widespread CCN5 expression in post-implantation blastocysts (E4.5), E5.5, E6.5, and E7.5 stage embryos. Consistent with our previous study on E9.5 embryos, this expression was not limited to a particular germ layer or cell type. The widespread distribution of CCN5 in early embryos suggests a crucial role in development.
Keywords: CCN2, CCN5, CTGF, Embryo expression pattern, WISP-2
Introduction
CCN5 is a member of the cysteine-rich 61/connective tissue growth factor/nephroblastoma-overexpressed (CCN) family of genes (Perbal 2005). The six members of this family are matricellular proteins that have important functions in numerous cell and physiologic processes, including embryonic development, cell motility and proliferation, angiogenesis, and extracellular matrix biology (Leask and Abraham 2006; Perbal 2004; Perbal 2005; Rachfal and Brigstock 2005). CCN5, also known as WISP-2 (Pennica et al. 1998), rCop-1(Zhang et al. 1998), COP-1(Delmolino et al. 2001), and CTGF-L (Kumar et al. 1999), is highly conserved among vertebrates and is the only CCN protein lacking the C-terminal domain (Gray 2005).
Originally discovered as a heparin-induced gene in vascular smooth muscle cells (SMC), CCN5 behaves as a growth-arrest-specific gene in this cell type. CCN5 is highly expressed in quiescent, non-proliferating rat aortic SMC and expression levels drop rapidly as cells are stimulated to re-enter the cell cycle. SMC CCN5 expression decreases in uterine fibroids and after vascular injury, two in vivo models of SMC proliferation (Delmolino et al. 2001; Lake et al. 2003; Mason et al. 2004b). CCN5 over-expression inhibits SMC proliferation and motility in vitro, and CCN5 knock-down dysregulates proliferation and increases basal motility (Lake et al. 2003; Lake and Castellot 2003; Mason et al. 2004b). CCN5 is strongly up-regulated by estrogen in both SMC and epithelial cells (Fritah et al. 2006; Inadera et al. 2000; Mason et al. 2004a).
Efforts to develop transgenic mice overexpressing CCN5 resulted in early embryonic lethality. Further analysis indicated that embryos developed until the blastocyst stage and were implanted, but did not progress beyond this point and appeared to be undergoing resorption. The crucial role of other CCN proteins is also suggested by gene manipulation studies in mice. Targeted deletion of the CCN1 gene in mice resulted in embryonic lethality due to vascular defects (Mo et al. 2002), whereas CCN2-null mice die perinatally due to respiratory failure (Ivkovic et al. 2003). Targeted deletion of CCN3 gene is not embryonic lethal in mice. However, it results in abnormal skeletal and cardiac development, cardiomyopathy, muscle atrophy and cataracts (Heath et al. 2008).
To better understand the role of CCN proteins in pre-implantation development, and to complement earlier studies of embryonic expression by our group and others (Jones et al. 2007; Katsube et al. 2001; Pin 2006; Surveyor et al. 1998), we assessed the onset of CCN5 transcription using real time PCR in preimplantation embryos. These results are reported in this communication and indicate that CCN5 expression begins in early morulae, while CCN2 expression was detected one cell division earlier, at the 4-cell stage.
Materials and methods
Immunohistochemistry
5 μm frozen sagittal sections of C57BL/6 post-implantation embryos were obtained from Folio Bio (Columbus, OH). Sections were cleared with xylene and endogenous peroxidase activity was quenched by treatment with two changes of 0.6 % hydrogen peroxide (H2O2) in ethanol for 5 min. The slides were then rehydrated and treated with the Avidin/Biotin Blocking Kit (Vector Laboratories) in blocking serum (4 % bovine serum albumin and 2 % goat serum in phosphate-buffered saline) and then incubated in primary antibody in blocking serum overnight at 4 °C. CCN5 protein was detected using a well characterized, highly specific, peptide affinity-purified rabbit polyclonal antibody to a polypeptide fragment from amino acids 103–117 of the von Willebrand Factor-C (VWC) domain of CCN5 (Gray 2005; Lake et al. 2003; Lake and Castellot 2003; Mason et al. 2004a). This recognizes full-length 27 kDa CCN5 protein on western blot (Gray 2005; Lake et al. 2003; Lake and Castellot 2003; Mason et al. 2004a). Embryos did not immunostain when mixed IgG was used in place of the CCN5 primary antibody. Slides were developed using the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) and the 3,3’-diaminobenzidine (DAB) substrate kit (Vector Laboratories) and counterstained with Harris modified hematoxylin with acetic acid (Fisher). All slides were dehydrated and embedded in permanent mounting medium (#13510; DPX Mountant; Electron Microscopy Sciences; Hatfield, PA) and photographed using a microscope (Zeiss Axioscope) and a digital camera system (SPOT; Diagnostic Instruments). Antibody concentrations and substrate exposure times were carefully titrated to minimize artifacts. Directly compared images are from slides processed in a single experiment with a matched negative control.
Pre-implantation embryo collection
C57BL/6J female mice (approximately 5 weeks old) were superovulated using a 5 IU IP injection of pregnant mare’s serum gonadotropin (PMSG) (NIH National Hormone & Peptide Program, Torrance, CA) followed by 5 IU of human chorionic gonadotropin (hCG) (Sigma Aldrich, St. Louis, MO) 48 h later. These females were mated to C57BL/6J males immediately after hCG injection. Pregnant mice were then sacrificed with carbon dioxide (CO2) overdose at various time points depending on the embryo stage desired [1 cell (18 h post hCG injection), 2 cell (42 h), 4 cell (54 h), morula (66 h), late morula (70 h), blastocyst (90 h)]. Oviducts and uterine horns were dissected from the pregnant mice and embryos were collected as described (Nagy 2003).
mRNA isolation
Messenger RNAs from mouse embryos were extracted by Dynabeads mRNA Direct Micro Kit (Invitrogen, Carlsbad, California) according to the manufacturer’s protocol. Briefly, mouse embryo masses were dissociated in 0.3 mg/mL hyaluronidase (Chemicon, Billerica, MA) in M2 media (Chemicon, Billerica, MA) for 2 min. Dissociated embryos were washed 3X in PBS. Fifteen to 30 embryos were transferred with a minimal volume of medium into 100 μL of lysis buffer (100 mM Tris–HCl, pH 7.5; 500 mM LiCl, 10 mM EDTA, 1 % lithium dodecylsulfate [LiDS], and 5 mM dithiothreitol) and mixed with 20 μL of Dynabeads oligo(dT)25 for 3–5 min. The bound mRNAs were washed twice in washing buffer containing LiDS (10 mM Tris–HCl; pH 7.5, 0.15 M LiCl, 1 mM EDTA, and 0.1 % LiDS), then three times in washing buffer (10 mM Tris–HCl, pH 7.5; 0.15 M LiCl, 1 mM EDTA). The bound mRNAs were then resuspended and eluted in 10 μL of 10 μM Tris–HCl at 80 °C.
Reverse transcriptase PCR (RT-PCR)
The mRNA isolated from pre-implantation embryos using Dynabeads were reverse transcribed into cDNA using the Retroscript kit (Ambion, Naugatuck, CT) according to their protocol. PCR reactions were made using the iTaq kit (BioRad, Hercules, CA) according to manufacturer’s protocol. Primers were as follows: CCN5: GTTGGATACTCGGGTGGCTA and ATACAGGTGCCAGGAAGGTG, CCN2: ATCCGAATTCCAGAACTGCAGCGGGCCGTGCCGGTGCCCG and ATACGGATCCCTCATGCCATGTCTCCGTACATCTTCCTGTHPRT: GCTTGCTGGTGAAAAGGACCTCTCGAAG and CCCTGAAGTACTCATTATAGTCAAGGGCAT PCR was performed using the following conditions: 94 ° C for 4 min, (94 °C for 30 s, 65 °C for 30 s, and 72 °C for 1 min) for 35 cycles, then 72 °C for 5 min. PCR reactions were then run on a 1 % agarose gel and stained with ethidium bromide.
Results
CCN5 Transcription in Pre-implantation embryos
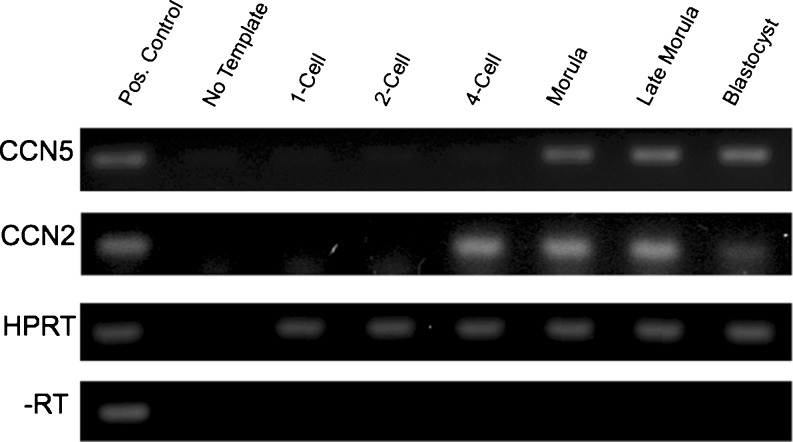
Our efforts to generate transgenic mice overexpressing CCN5 produced extremely small litter sizes, due to an apparent early embryonic lethality. Following pronuclear injection of overexpression constructs and subsequent dissection of uterine horns, we observed that the transgenic mice developed until the blastocyst stage and were implanted. However, no development past the blastocyst stage was ever observed, and the small number of embryos that were implanted appeared to be undergoing resorption into the decidua. To assess the embryonic stage at which CCN5 transcription begins, we superovulated C57BL/6J female mice and collected 1-cell, 2-cell, 4-cell, early morula (12–16 cell), late morula (32–48 cell), and blastocyst embryos. Total mRNA was extracted from these embryos and reverse transcribed. The resulting cDNA was PCR amplified using primers for CCN5 and then loaded on an agarose gel (Fig. 1). CCN5 mRNA was not detected in 1-cell, 2-cell, and 4-cell staged embryos. It was first detected in the early morula and persisted through the later morula and blastocyst stages. To compare this expression pattern with CCN2, we used the same embryonic extracts and RT-PCR approach. CCN2 transcription began slightly earlier than that of CCN5, at the 4-cell stage.
Fig. 1.
CCN5 mRNA transcription begins at the morula stage of development. C57BL/6 embryos were harvested and pooled as described in Materials and Methods. Total mRNA was extracted and then reverse transcribed. PCR was performed using primers specific to CCN5, CCN2, or HPRT (loading control)
Observations by others indicate that the CCN1 transcript is absent in 1-cell embryos, appears first in 2-cell embryos and remains to the blastocyst stage (Pin 2006). These investigators also found that CCN2 transcript was not consistently present until the 4-cell stage, and then persisted to the blastocyst stage and beyond. Interestingly, these investigators observed transient transcription of CCN3 at the 4-cell and early morula stage, which was not detected by the late morula stage.
CCN5 Expression pattern in post-implantation embryos
We further studied the temporal and spatial pattern of CCN5 expression in post-implantation embryos using a well-characterized anti-CCN5 antibody (Lake et al. 2003; Mason et al. 2004b). C57BL/6 embryos (E4.5, E5.5, E6.5, and E7.5) were harvested and prepared as described in Materials and Methods. No color reaction was detected when pooled rabbit IgG replaced primary antibody, corroborating the specificity of the antibody (Jones et al. 2007).
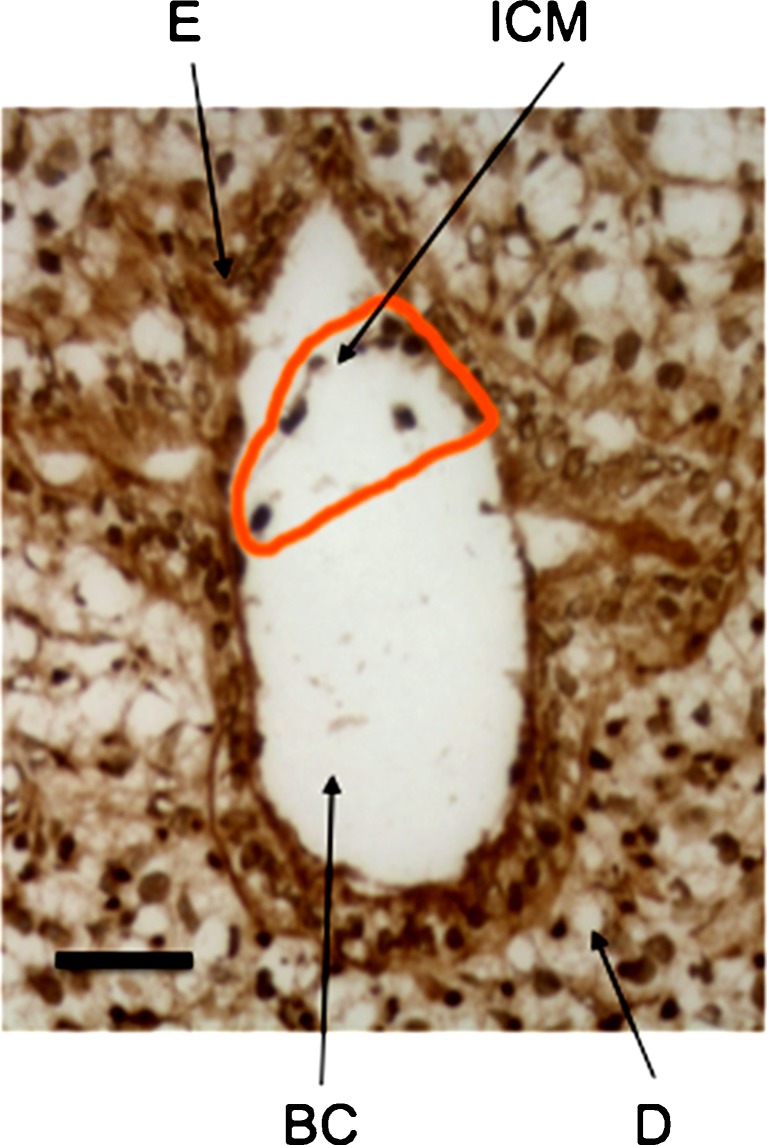
Implantation of the blastocyst in the uterine horn occurs at E4.5 (Fig. 2). CCN5 expression was widespread at this stage. It was present within the extraembryonic maternal cells of both the decidua and endometrium surrounding the implanted blastocyst. We saw continued expression of CCN5 in the decidua in every stage studied (up to E7.5). At least some of the inner cell mass (ICM) cells, which eventually become the embryo proper, expressed CCN5 protein.
Fig. 2.
CCN5 is expressed in E4.5 embryos (implanted blastocysts). 5 μm frozen sagittal sections of embryo were processed as described in Materials and Methods. BC = Blastocoel; D = Decidua; E = Endometrium; ICM = Inner Cell Mass. Scale Bar = 50 μm. Brown staining indicates CCN5 immunoreactivity. Nuclei are visualized by hematoxylin (blue) staining
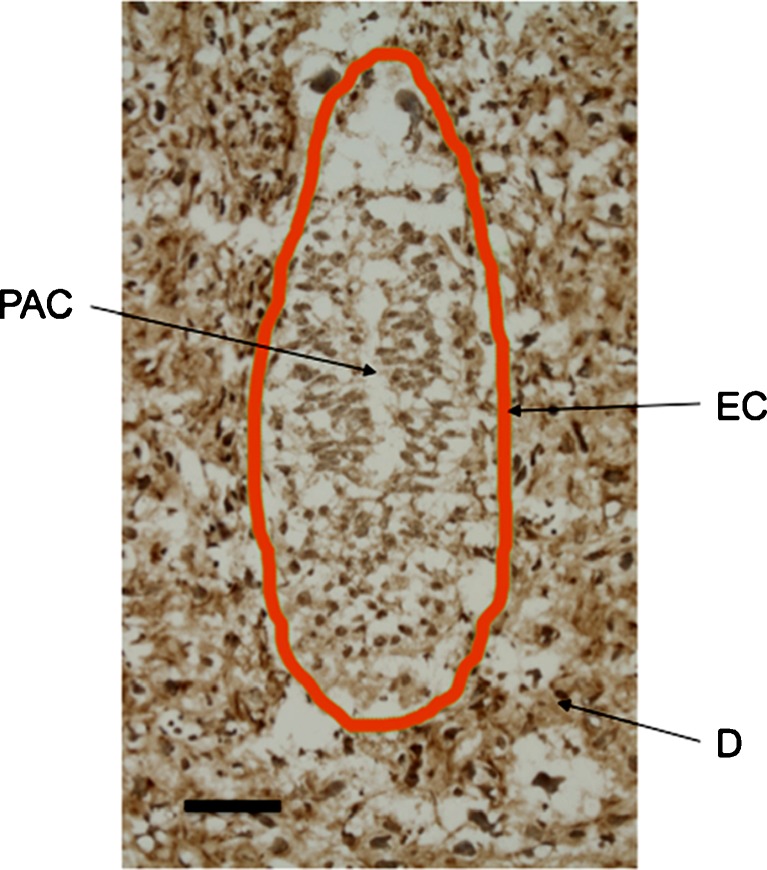
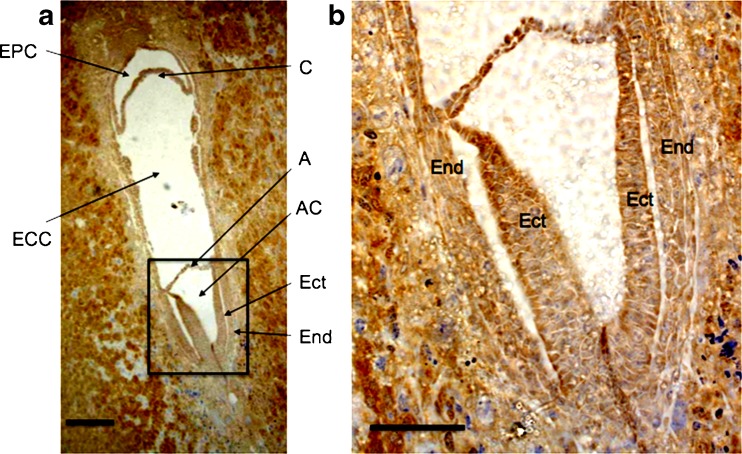
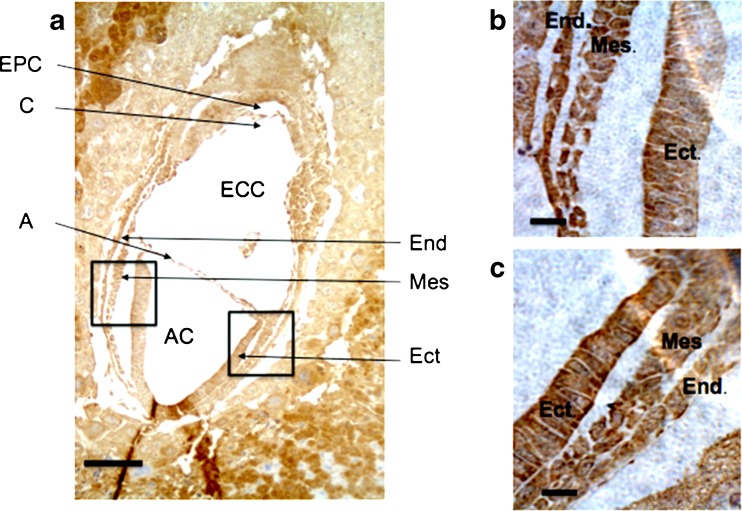
Once the embryo reached embryonic stage E5.5 the ICM cells proliferated rapidly, giving rise to the epiblast that subsequently formed the egg cylinder. The cells of the egg cylinder expressed CCN5 (Fig. 3). The egg cylinder differentiates at E6.5. After formation of the amniotic cavity, gastrulation begins and the first mesoderm cells are evident. At this stage CCN5 continued to display widespread expression, and was not germ layer specific as it was present in all three germ layers: endoderm, mesoderm and ectoderm (Fig. 4). The widespread expression of CCN5 persisted into stage E7.5 as gastrulation concluded (Fig. 5).
Fig. 3.
CCN5 is expressed in E5.5 embryos (formation of egg cylinder). 5 μm frozen sagittal sections of embryo were processed as described in Materials and Methods. EC = Egg Cylinder Stage Embryo Outlined in Orange; D = Decidua; PAC = Proamniotic Cavity. Scale Bar = 50 μm. CCN5 immunoreactivity is indicated by brown staining. Nuclei are stained with hematoxylin (blue)
Fig. 4.
CCN5 is expressed in E6.5 embryos (early stages of egg cylinder differentiation). a 5 μm frozen sagittal sections of embryo were processed as described in Materials and Methods. A = Amnion; AC = Amniotic Cavity; C = Chorion; ECC = Exocoelomic Cavity; Ect = Ectoderm; EPC = Ectoplacental Cavity; End = Endoderm. b Zoom of black box from (a) showing germ layers. Scale Bars = 50 μm (a), 25 μm (b). CCN5 immunoreactivity is indicated by brown staining. Nuclei are shown by hematoxylin (blue) stain
Fig. 5.
CCN5 is expressed in E7.5 embryos (gastrulation complete). a 5 μm frozen sagittal sections of embryo were processed as described in Materials and Methods. A = Amnion; AC = Amniotic Cavity; C = Chorion; ECC = Exocoelomic Cavity; Ect = Ectoderm; End = Endoderm; EPC = Ectoplacental Cavity; Mes = Mesoderm. b Zoom of left black box from (a) showing germ layers. c Zoom of right black box from (a) showing germ layers. Scale Bars = 50 μm (a), 10 μm (b and c). CCN5 immunoreactivity is indicated by brown staining. Nuclei are shown by hematoxylin (blue) staining
Discussion
In this study we show that CCN5 transcription initiates at the early morula stage and persists through the pre- and post-implantation embryo stages. The onset of CCN5 transcription at the early morula (12-16 cell) stage is consistent with the onset of a major transcriptional wave that begins at approximately the 4-cell stage in mice (Clegg and Piko 1983). The CCN5 expression patterns we observed are very similar to those of CCN2 (Surveyor et al. 1998). In the newly implanted blastocyst (E4.5), these investigators observed robust CCN2 expression in the inner cell mass, and at E5.5, CCN2 displayed widespread expression throughout the embryo. At E6.5, CCN2 showed widespread expression in all three germ layers.
Furthermore, we confirm that CCN5 transcription begins later than CCN1, CCN2, and CCN3 in mouse embryos (Table 1). These data complement the CCN5 expression data in later stage embryos (E9.5 through 18) recently published by our group (Jones et al. 2007), as well as data on CCN5 expression in adult rodents (Gray et al. 2007). With the data provided in this communication, we now have a reasonably complete map of the cell and tissue-specific expression pattern of CCN5 in rodents from the zygote to the adult.
Table 1.
Summary and Comparison of CCN5 Transcription in Preimplantation Embyos
| 1-cell | 2-cell | 4-cell | Morula | Late Morula | Blastocyst | |
|---|---|---|---|---|---|---|
| This paper | ||||||
| CCN2 | − | − | + | + | + | + |
| CCN5 | − | − | − | + | + | + |
| Pin et al. 2006 | ||||||
| CCN1 | − | + | + | + | + | + |
| CCN2 | − | variable | + | + | + | + |
| CCN3 | − | − | + | + | − | − |
There is a potentially important difference between the transcription initiation points of CCN5 compared to other CCN family members, such as CCN1, CCN2, and CCN3. Prior to the early morula stage, the embryo is a relatively loosely associated group of equal-volume cells that undergo synchronous proliferation. Beginning at the 8-cell stage in mice, compaction begins and with it asymmetric embryo development, characterized by unequal cell volumes, proliferation and migration rates, and positions within the embryonic morula. It is possible that CCN5, a molecule with antiproliferative and antimotility properties, begins transcription at the early morula stage to counteract or balance the effects of the mitogenic and motogenic activities of the other CCN family members.
To date, only the expression patterns of CCN2 and CCN5 have been examined in very early mammalian development (Surveyor et al. 1998). CCN3 has been studied in early avian development. Robust CCN3 expression is seen in the neuroepithelium (E3) and neural tube (E3-E7) in chick embryos (Katsube et al. 2001).
There are several pathologic conditions in which CCN2 and CCN5 have inversely coordinated expression patterns, including asthma (unpublished observations; (van den Brule et al. 2007)) uterine leiomyoma (fibroids) (De Falco et al. 2006; Mason et al. 2004b), and hepatocellular carcinoma (Cervello et al. 2004; Hirasaki et al. 2001). However, in other diseases, including arthritis (Manns et al. 2006; Tanaka et al. 2005) and viral hepatitis (Fukutomi et al. 2005; Shin et al. 2005), CCN2 and CCN5 are similarly expressed. CCN2 and CCN5 demonstrate different biological activity profiles in vascular SMC. CCN2 over-expression induces vascular SMC proliferation and increases MMP-2 expression, whereas CCN5 over-expression reduces proliferation and MMP-2 expression. CCN5 expression decreases and CCN2 expression increases in vascular SMC during the proliferative phase of balloon angioplasty injury (Ando et al. 2004; Fan and Karnovsky 2002; Fan et al. 2000; Lake et al. 2003; Lake and Castellot 2003). We are actively investigating the potential interplay between CCN5, CCN2, and other CCN family members in early development.
Our examination of post-implantation CCN5 expression yielded interesting results. In early post-implantation E4.5 mouse embryos, we observe CCN5 expression in the inner cell mass, which ultimately forms the embryo proper. The observed ICM expression is consistent with our earlier findings that CCN5 is widely expressed within fetal tissues at E8.5 and later. CCN5 was also present within the extraembryonic maternal cells of both the decidua and endometrium surrounding the implanted blastocyst.
We see continued expression of CCN5 in the decidua in every stage we studied (up to E7.5). At the E5.5 stage, when the egg cylinder formed, there is robust and ubiquitous expression of CCN5. Between E5.5 and 6.5, the egg cylinder differentiated. After formation of the amniotic cavity, gastrulation began and the first mesoderm cells were evident. At this stage, CCN5 continued to display widespread expression, and was not germ layer specific as it is present in mesoderm, ectoderm, and endoderm. The widespread expression of CCN5 continued through stage E7.5; as gastrulation concluded, CCN5 was found in all three germ layers.
Given the early onset of CCN5 transcription, ubiquitous expression in all three germ layers, and embryonic lethality in both CCN5-null and CCN5 over-expressor mice, CCN5 appears to play a critical role in early embryonic development. Further studies are required to define the mechanisms and specific roles played by CCN5 in this context.
Acknowledgments
The authors dedicate this paper to the memory of Dr. Mark Gray, whose help with the antibody staining and frequent discussion were instrumental in completing this work. We thank Jennifer Jones for characterizing the anti-CCN5 antibody. We are grateful to Lan Wei, Cassandra Baughman, Joshua Russo, and Kristina Weisman for many helpful discussions. This work was supported in part by NIH grants HD046251and HL49973 to JJC.
Glossary
- CCN
Cysteine-rich 61/Connective Tissue Growth Factor/Nephroblastoma-overexpressed family of Proteins
- E[#]
Embryonic day [number]
- RT-PCR
Reverse transcriptase polymerase chain reaction
- SMC
Smooth muscle cells
Footnotes
Concise Summary
CCN5 mRNA was not detected in 1, 2, or 4 cell embryos. It was first detected at the early morula stage and persisted to the preimplantation blastocyst stage. Immunohistochemical staining showed widespread CCN5 expression in post-implantation blastocysts (E4.5), E5.5, E6.5, and E7.5 stage embryos.
References
- Ando H, Fukuda N, Kotani M, Yokoyama S, Kunimoto S, Matsumoto K, Saito S, Kanmatsuse K, Mugishima H. Chimeric DNA-RNA hammerhead ribozyme targeting transforming growth factor-beta 1 mRNA inhibits neointima formation in rat carotid artery after balloon injury. Eur J Pharmacol. 2004;483:207–14. doi: 10.1016/j.ejphar.2003.10.035. [DOI] [PubMed] [Google Scholar]
- Cervello M, Giannitrapani L, Labbozzetta M, Notarbartolo M, D’Alessandro N, Lampiasi N, Azzolina A, Montalto G. Expression of WISPs and of their novel alternative variants in human hepatocellular carcinoma cells. Ann N Y Acad Sci. 2004;1028:432–9. doi: 10.1196/annals.1322.051. [DOI] [PubMed] [Google Scholar]
- Clegg KB, Piko L. Quantitative aspects of RNA synthesis and polyadenylation in 1-cell and 2-cell mouse embryos. J Embryol Exp Morphol. 1983;74:169–82. [PubMed] [Google Scholar]
- Falco M, Staibano S, D’Armiento FP, Mascolo M, Salvatore G, Busiello A, Carbone IF, Pollio F, Lieto A. Preoperative treatment of uterine leiomyomas: clinical findings and expression of transforming growth factor-beta3 and connective tissue growth factor. J Soc Gynecol Investig. 2006;13:297–303. doi: 10.1016/j.jsgi.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Delmolino LM, Stearns NA, Castellot JJ., Jr COP-1, a member of the CCN family, is a heparin-induced growth arrest specific gene in vascular smooth muscle cells. J Cell Physiol. 2001;188:45–55. doi: 10.1002/jcp.1100. [DOI] [PubMed] [Google Scholar]
- Fan WH, Karnovsky MJ. Increased MMP-2 expression in connective tissue growth factor over-expression vascular smooth muscle cells. J Biol Chem. 2002;277:9800–5. doi: 10.1074/jbc.M111213200. [DOI] [PubMed] [Google Scholar]
- Fan WH, Pech M, Karnovsky MJ. Connective tissue growth factor (CTGF) stimulates vascular smooth muscle cell growth and migration in vitro. Eur J Cell Biol. 2000;79:915–23. doi: 10.1078/0171-9335-00122. [DOI] [PubMed] [Google Scholar]
- Fritah A, Redeuilh G, Sabbah M. Molecular cloning and characterization of the human WISP-2/CCN5 gene promoter reveal its upregulation by oestrogens. J Endocrinol. 2006;191:613–24. doi: 10.1677/joe.1.07009. [DOI] [PubMed] [Google Scholar]
- Fukutomi T, Zhou Y, Kawai S, Eguchi H, Wands JR, Li J. Hepatitis C virus core protein stimulates hepatocyte growth: correlation with upregulation of wnt-1 expression. Hepatology. 2005;41:1096–105. doi: 10.1002/hep.20668. [DOI] [PubMed] [Google Scholar]
- Gray MR, Castellot JJ., Jr . Function and regulation of CCN5. In: Perbal BV, Takigawa ME, editors. CCN proteins: a new family of cell growth and differentiation regulators. London: Imperial College Press; 2005. [Google Scholar]
- Gray MR, Malmquist JA, Sullivan M, Blea M, Castellot JJ., Jr CCN5 Expression in mammals. II. Adult rodent tissues. J Cell Commun Signal. 2007;1:145–58. doi: 10.1007/s12079-007-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath E, Tahri D, Andermarcher E, Schofield P, Fleming S, Boulter CA. Abnormal skeletal and cardiac development, cardiomyopathy, muscle atrophy and cataracts in mice with a targeted disruption of the Nov (Ccn3) gene. BMC Dev Biol. 2008;8:18. doi: 10.1186/1471-213X-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasaki S, Koide N, Ujike K, Shinji T, Tsuji T. Expression of Nov, CYR61 and CTGF genes in human hepatocellular carcinoma. Hepatol Res. 2001;19:294–305. doi: 10.1016/S1386-6346(00)00101-7. [DOI] [PubMed] [Google Scholar]
- Inadera H, Hashimoto S, Dong HY, Suzuki T, Nagai S, Yamashita T, Toyoda N, Matsushima K. WISP-2 as a novel estrogen-responsive gene in human breast cancer cells. Biochem Biophys Res Commun. 2000;275:108–14. doi: 10.1006/bbrc.2000.3276. [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130:2779–91. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JA, Gray MR, Oliveira BE, Koch M, Castellot JJ., Jr CCN5 expression in mammals : I. Embryonic and fetal tissues of mouse and human. J Cell Commun Signal. 2007;1:127–43. doi: 10.1007/s12079-007-0012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsube K, Chuai ML, Liu YC, Kabasawa Y, Takagi M, Perbal B, Sakamoto K. The expression of chicken NOV, a member of the CCN gene family, in early stage development. Brain Res Gene Expr Patterns. 2001;1:61–5. doi: 10.1016/S1567-133X(01)00009-6. [DOI] [PubMed] [Google Scholar]
- Kumar S, Hand AT, Connor JR, Dodds RA, Ryan PJ, Trill JJ, Fisher SM, Nuttall ME, Lipshutz DB, Zou C, Hwang SM, Votta BJ, James IE, Rieman DJ, Gowen M, Lee JC (1999) Identification and cloning of a connective tissue growth factor-like cDNA from human osteoblasts encoding a novel regulator of osteoblast functions. J Biol Chem 274:17123–17131 [DOI] [PubMed]
- Lake AC, Castellot JJ., Jr CCN5 modulates the antiproliferative effect of heparin and regulates cell motility in vascular smooth muscle cells. Cell Commun Signal. 2003;1:5. doi: 10.1186/1478-811X-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake AC, Bialik A, Walsh K, Castellot JJ., Jr CCN5 is a growth arrest-specific gene that regulates smooth muscle cell proliferation and motility. Am J Pathol. 2003;162:219–31. doi: 10.1016/S0002-9440(10)63813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–10. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- Manns JM, Uknis AB, Rico MC, Agelan A, Castaneda J, Arango I, Barbe MF, Safadi FF, Popoff SN, DeLa Cadena RA. A peptide from thrombospondin 1 modulates experimental erosive arthritis by regulating connective tissue growth factor. Arthritis Rheum. 2006;54:2415–22. doi: 10.1002/art.22021. [DOI] [PubMed] [Google Scholar]
- Mason HR, Grove-Strawser D, Rubin BS, Nowak RA, Castellot JJ., Jr Estrogen induces CCN5 expression in the rat uterus in vivo. Endocrinology. 2004;145:976–82. doi: 10.1210/en.2003-0823. [DOI] [PubMed] [Google Scholar]
- Mason HR, Lake AC, Wubben JE, Nowak RA, Castellot JJ., Jr The growth arrest-specific gene CCN5 is deficient in human leiomyomas and inhibits the proliferation and motility of cultured human uterine smooth muscle cells. Mol Hum Reprod. 2004;10:181–7. doi: 10.1093/molehr/gah028. [DOI] [PubMed] [Google Scholar]
- Mo FE, Muntean AG, Chen CC, Stolz DB, Watkins SC, Lau LF. CYR61 (CCN1) is essential for placental development and vascular integrity. Mol Cell Biol. 2002;22:8709–20. doi: 10.1128/MCB.22.24.8709-8720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A. Manipulating the Mouse Embryo: A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press, Cold Spring Harbor; 2003. [Google Scholar]
- Pennica D, Swanson TA, Welsh JW, Roy MA, Lawrence DA, Lee J, Brush J, Taneyhill LA, Deuel B, Lew M, Watanabe C, Cohen RL, Melhem MF, Finley GG, Quirke P, Goddard AD, Hillan KJ, Gurney AL, Botstein D, Levine AJ. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc Natl Acad Sci U S A. 1998;95:14717–22. doi: 10.1073/pnas.95.25.14717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62–4. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- Perbal BV, Takigawa ME. CCN Proteins: a new family of cell growth and differentiation regulators. London: Imperial College Press; 2005. [Google Scholar]
- AA Pin, A Watson (2006) Expression of Gene Products Encoding CCN Proteins During the First Week of Development. In Proceedings of the Annual Conference of the International Embryo Transfer Society. Vol. 18. Reproduction, Fertility and Development Orlando, FL. 179–179
- Rachfal AW, Brigstock DR. Structural and functional properties of CCN proteins. Vitam Horm. 2005;70:69–103. doi: 10.1016/S0083-6729(05)70003-0. [DOI] [PubMed] [Google Scholar]
- Shin JY, Hur W, Wang JS, Jang JW, Kim CW, Bae SH, Jang SK, Yang SH, Sung YC, Kwon OJ, Yoon SK. HCV core protein promotes liver fibrogenesis via up-regulation of CTGF with TGF-beta1. Exp Mol Med. 2005;37:138–45. doi: 10.1038/emm.2005.19. [DOI] [PubMed] [Google Scholar]
- Surveyor GA, Wilson AK, Brigstock DR. Localization of connective tissue growth factor during the period of embryo implantation in the mouse. Biol Reprod. 1998;59:1207–13. doi: 10.1095/biolreprod59.5.1207. [DOI] [PubMed] [Google Scholar]
- Tanaka I, Morikawa M, Okuse T, Shirakawa M, Imai K. Expression and regulation of WISP2 in rheumatoid arthritic synovium. Biochem Biophys Res Commun. 2005;334:973–8. doi: 10.1016/j.bbrc.2005.06.196. [DOI] [PubMed] [Google Scholar]
- Brule S, Heymans J, Havaux X, Renauld JC, Lison D, Huaux F, Denis O. Profibrotic effect of IL-9 overexpression in a model of airway remodeling. Am J Respir Cell Mol Biol. 2007;37:202–9. doi: 10.1165/rcmb.2006-0397OC. [DOI] [PubMed] [Google Scholar]
- Zhang R, Averboukh L, Zhu W, Zhang H, Jo H, Dempsey PJ, Coffey RJ, Pardee AB, Liang P. Identification of rCop-1, a new member of the CCN protein family, as a negative regulator for cell transformation. Mol Cell Biol. 1998;18:6131–41. doi: 10.1128/mcb.18.10.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]