Abstract
Fluid flow stress (FSS) is a major mechanical stress that induces bone remodeling upon orthodontic tooth movement, whereas CCN family protein 2 (CCN2) is a potent regenerator of bone defects. In this study, we initially evaluated the effect of laminar FSS on Ccn2 expression and investigated its mechanism in osteoblastic MC3T3-E1 cells. The Ccn2 expression was drastically induced by uniform FSS in an intensity dependent manner. Of note, the observed effect was inhibited by a Rho kinase inhibitor Y27632. Moreover, the inhibition of actin polymerization blocked the FSS-induced activation of Ccn2, whereas inducing F-actin formation using cytochalasin D and jasplakinolide enhanced Ccn2 expression in the same cells. Finally, F-actin formation was found to induce osteoblastic differentiation. In addition, activation of cyclic AMP-dependent kinase, which inhibits Rho signaling, abolished the effect of FSS. Collectively, these findings indicate the critical role of actin polymerization and Rho signaling in CCN2 induction and bone remodeling provoked by FSS.
Keywords: Osteoblasts, Connective tissue growth factor, CTGF, CCN2, Fluid shear stress, Actin polymerization
Introduction
CCN family protein 2 (CCN2) belongs to the CCN family (Perbal and Takigawa 2005), which is comprised of secretory proteins that contain 38 conserved cysteine residues in four distinct modules. Depending upon the cell type, this protein performs various cellular functions including the promotion of cell proliferation, chemotaxis, differentiation, adhesion, migration, extracellular matrix formation, and survival (Moussad and Brigstock 2000; Kubota and Takigawa 2007). Recent studies have shown that Ccn2 is expressed in normal bones during development, growth, and remodeling, and treating osteoblast cultures with recombinant CCN2 enhances their proliferation and differentiation (Nishida et al. 2000; Safadi et al. 2003; Kubota and Takigawa 2011). According to another report, the overexpression of Ccn2 in ST-2 cells increased alkaline phosphatase activity, the mRNA levels of osteocalcin and alkaline phosphatase, and mineralized nodule formation (Smerdel-Ramoya et al. 2008). These studies have collectively demonstrated that Ccn2 is expressed in bone tissue and that its gene product exerts diverse modulatory functions on osteoblast differentiation and proliferation.
The mass and structure of bone changes in response to mechanical stimuli. It is now commonly recognized that fluid shear stress due to interstitial fluid flow through the lacuno-canalicular network after bone loading is a candidate for the major mechanical trigger affecting the behavior of osteoblasts (Burger and Klein-Nulend 1999). We previously reported that osteoblasts and osteocytes in alveolar bone showed increased Ccn2 mRNA expression during experimental tooth movement (Yamashiro et al. 2001). These findings suggested that Ccn2 plays a role in the regulation of osteoblastic function during the mechanical stimulation of bone. Considering these findings together, we hypothesized that Ccn2 mRNA expression in osteocytes and osteoblasts is induced by continuous fluid shear stress (FSS) caused by orthodontic force, which might play a significant role in triggering bone remodeling (Sakai et al. 2009).
Recently, the actin cytoskeleton has been shown to be involved in many important cellular functions induced by mechanical stimuli in osteoblasts (Jackson et al. 2008). Therefore, we suspected that actin cytoskeleton reorganization might also mediate mechanical stress-induced Ccn2 expression in osteoblasts.
In this study, we continuously loaded different levels of laminar fluid shear stress on an osteoblastic cell line, MC3T3-E1, to elucidate its effect on the expression of Ccn2 mRNA over time and its signal transduction pathway. Here, we show that uniform fluid shear stress induced Ccn2 expression in osteoblastic cells and that this induction was entirely dependent on the formation of actin stress fibers that eventually promoted osteoblastic differentiation.
Materials and methods
Cell culture
MC3T3-E1 cells (Sudo et al. 1983) were purchased from RIKEN CELL BANK (Tsukuba, Japan). To analyze the expression of Ccn2 mRNA in response to fluid shear stress, 8 × 105 cells were seeded onto a slide glass coated with poly-D-lysine and fibronectin and cultured in α-MEM containing 10 % fetal bovine serum (FBS) for 3 days before the fluid shear stress loading. The culture medium was substituted for that containing 0.5 % FBS 24 h before the fluid shear stress experiment. All reagents used for the signal transduction analysis were applied to the cells 30 min before the fluid shear stress loading and were maintained during the fluid flow experiment.
Reagents for signal transduction analysis
Forskolin (10 μM; Sigma, St. Louis, MO) was used to induce cAMP production. Y-27632 (10 μM; Sigma) was used as a specific ROCK inhibitor. Cytochalasin D (10 μM; Calbiochem, San Diego, CA) and latrunculin A (10 μM; Calbiochem) were used to inhibit F-actin formation. Jasplakinolide (10, 50, or 200 nM; Molecular Probes, Eugene, OR) was used to accelerate actin polymerization.
Induction of fluid shear stress
MC3T3-E1 cells were subjected to fluid flow in a flow chamber connected to a flow apparatus (Frangos et al. 1985). The cells were exposed to a defined laminar shear stress of 1.25 Pa for the specified time, as monitored by a flow meter (L-500CCM-D, Alicat Scientific Inc., Tucson, AZ). This level of shear stress is within the predicted physiological range (Weinbaum et al. 1994). The control cells were maintained in the same flow chamber without fluid flow.
RT-PCR analysis
The expression of Ccn2 mRNA was detected and analyzed by quantitative real-time RT-PCR. Total RNA was isolated from the cells with ISOGEN (Nippon Gene, Tokyo, Japan) according to the manufacturer’s instruction. Total RNA was reverse transcribed with M-MLV reverse transcriptase (Invitrogen, Rockville, MD) for 50 min at 42 °C. For quantitative real-time PCR, each synthesized cDNA was analyzed using the LightCycler system (Roche, Mannheim, Germany). The FastStart DNA Master SYBR Green I Kit (Roche) was employed. The oligonucleotide primers used for Ccn2, alkaline phosphatase (alp), osteocalcin (oc) and glyceraldehyde 3 phosphate dehydrogenase (g3pdh) were as follows: 5'-AGAAGGGCAAAAAGTGCATCCG-3' (forward) and 5'-GCCATGTCTCCGTACATCTTCCTG-3' (reverse) for Ccn2; 5'-GCTGATCAT TCCCACGTTTT-3' (forward) and 5'-CTGGGCCTGGTAGTTGTT GT-3' (reverse) for alp; 5'-AAGCAGGAGGGCAATAAGGT-3' (forward) and 5'-TTTGTAGGCGGTCTTCAAGC-3' (reverse) for oc; and 5'-ACCACAGTCCATGCC ATCA-3' (forward) and 5'-TCCACCACCCTGTTGCTGTA-3' (reverse) for g3pdh, respectively. After the denaturation at 95 °C for 10 min, the reaction was carried out under the following conditions: one cycle at 95 °C for 30 s and 35 cycles of 95 °C for 15 s/60 °C for 5 s/72 °C for 10 s with a single fluorescence detection point at the end of the relevant annealing or extension segment. Thereafter, one cycle of melting curve analysis was performed to confirm the integrity of the analysis.
Cytochemical
After FSS, the cells were washed in phosphate buffered-saline (PBS), fixed in 4 % paraformaldehyde in PBS for 15 min, and permeabilized for 5 min in PBS containing 0.15 % Triton X-100 at 37 °C, before being washed twice with PBS. To prevent nonspecific interactions, we immersed the specimens in a blocking solution of 1 % bovine serum albumin (BSA) in PBS for 2 h. Thereafter, the cells were incubated with Alexa 594-phalloidin (Molecular Probes) for 30 min to visualize F-actin and then were washed three times with PBS.
Fluorescent images
Images of the MC3T3-E1 cells were obtained with an inverted Olympus IX 70 microscope, using a 100×(NA1.4) objective lens (Olympus, Tokyo, Japan). Images of the cells were recorded with a 1024 × 1024 Hamamatsu CCD camera controlled by Aquacosmos software (Hamamatsu photonics, Hamamatsu, Japan) for the quantification of the cytochemical data.
Statistical analysis
The data represent the mean ± SD calculated from three to four independent experiments. Unless otherwise specified, differences were analyzed by the Student’s t-test using StatView software (SAS Institute Inc., Cary, NC). Differences between the data from “No Flow” and “Flow” groups were analyzed by two way ANOVA. The significance of differences was accepted at p < 0.05.
Results
CCN2 response to continuous fluid shear stress in osteoblastic cells
A recent study described that basal Ccn2 expression was strongly repressed by long term exposure to laminar fluid flow stress in human umbilical vein endothelial cells (HUVEC)(Cicha et al. 2008). In osteoblastic MC3T3-E1 cells, the basal Ccn2 expression level was quite low, and Ccn2 mRNA was undetectable in steady cultures. However, the application of continuous 1.25 Pa laminar fluid shear stress to MC3T3-E1 cells produced a rapid increase in the expression of Ccn2 mRNA. The expression of Ccn2 mRNA was significantly increased within an hour of flow application, peaked at 2 h at >10-fold higher than the control (Fig.1a). Furthermore, Ccn2 mRNA expression was increased in an intensity-dependent manner by a fluid shear stress of 0.75–1.75 Pa, when it was applied for 2 h (Fig. 1b).
Fig. 1.
Induction of ccn2 expression in osteoblastic MC3T3-E1 cells by FSS loading. a MC3T3-E1 cells were exposed to 1.25 Pa fluid shear stress, and Ccn2 mRNA expression was quantified over time by real time RT-PCR. Relative expression levels were computed by standardizing the data against those observed at 2 h with FSS (100 %). The mean and SD from 3 slides are given for each time point. The solid circles and squares denote the results obtained with and without FSS, respectively. **significant effect confirmed by comparing the flow treated and no flow control at each time point; p < 0.01. b MC3T3-E1 cells were exposed to indicated fluid shear stress for 2 h and then were analyzed as described for panel a. Relative expression levels were computed by standardizing the data against those observed with FSS at 1.25 Pa (100 %). The mean and SD from 3 slides are given for each stress intensity. * significant effect confirmed by comparing the flow treated and no flow control at each time point; p < 0.05. c Effect of Y27632, a Rho GTPase inhibitor, on the Ccn2-induction by FSS. The results are represented as percentages of the Ccn2 mRNA expression observed after FSS exposure for 2 h, against those induced by FSS without the drug (=100). The mean and SD values from 3 slides are displayed. *significant effect of Y27632 treatment; p < 0.05
The role of ROCK in Ccn2 mRNA induction in response to fluid shear stress
Previous studies have shown that Rho proteins are involved in the regulation of Ccn2 induction by LPA (Hahn et al. 2000). Suspecting a similar regulatory mechanism, we evaluated the effect of Y-27632, an inhibitor of the Rho-associated kinase ROCK, on the effect of laminar FSS on Ccn2 expression in MC3T3-E1 cells. As a result, we found that Y-27632 reduced both steady state and FSS-induced Ccn2 mRNA expression, indicating that the RhoA-ROCK pathway is involved in the regulation of Ccn2 gene expression by FSS in osteoblastic cells (Fig. 1c).
Differential effect of latrunculin A and cytochalasin D on actin stress fiber formation and Ccn2 expression in MC3T3-E1 cells
It is known that the activation of Rho signaling induces actin polymerization, which eventually increases Ccn2 expression (Hahn et al. 2000; Woods et al. 2009). In order to elucidate whether actin polymerization directly mediated the FSS-induced increase in Ccn2 mRNA expression, we pretreated MC3T3-E1 cells with latrunculin A that inhibits F-actin formation (Xu et al. 2010). In fact, in latrunculin A treated cells, the formation of actin stress fibers by FSS was significantly inhibited (Fig. 2a). As expected, latrunculin A repressed basal Ccn2 mRNA expression in the control cell culture with no flow, as well as the flow-induced Ccn2 mRNA expression (Fig. 2b).
Fig. 2.
Effect of Latrunculin A or Cytochalasin D on actin fiber formation and ccn2 expression in MC3T3-E1 cells in the absence or presence of FSS. a Fluorescent F-actin staining of the cells subjected to FSS in presence of Latrunculin A or Cytochalasin D. Fluid shear stress induced the development of prominent actin stress fibers, which were oriented roughly parallel to the long axis of the cell (a and d), whereas stress fibers failed to develop in the cells treated with 1 μM Latrunculin A (b and e). In contrast, even in the absence (a and c) of fluid flow over the cells, treatment with 10 μM Cytochalasin D induced the formation of abnormally short and thick stress fibers (c). b MC3T3-E1 cells were pretreated with Latrunculin A (1 μM), and Ccn2 mRNA expression was analyzed. The results are shown as percentages of the Ccn2 mRNA expression level observed after 2 h of FSS loading in the absence of the drug. Each column indicates the mean, and the SD is shown as an error bar. All experiments were performed three times with independent samples. *significant differences between the data indicated by brackets; p < 0.05; **p < 0.01. c MC3T3-E1 cells were pretreated with Cytochalasin D (10 μM). After 2 h of FSS loading, Ccn2 mRNA expression was analyzed. The results are shown as percentages of the Ccn2 mRNA expression noted in the flow experiments without the drug (=100). Each column and error bar indicates the mean and SD values computed from the results of three independent experiments, respectively. *significant difference from the control without Cytochalasin D. **p < 0.01. No significant difference was estimated between the Cytochalasin D-treated cells by ANOVA
In contrast to latrunculin A, cytochalasin D causes the formation of atypical actin stress fibers (Ott et al. 2003). Indeed, in cytochalasin D treated cells, irregular, thick, and short actin stress fibers were observed (Fig. 2a). Under the same conditions without FSS, strong induction of Ccn2 occurred (Fig. 2c). Considering these findings together, FSS-induced Ccn2 expression is mediated by actin polymerization events.
Induction of Ccn2 expression and osteoblastic differentiation by actin polymerization induced by jasplakinolide
Next, we evaluated the effect of forced F-actin formation on Ccn2 expression in the same cells. As shown in Fig. 3a, a large number of stress fibers were formed when MC3T3-E1 cells were treated with jasplakinolide (Fig. 3a, b)(Woods et al. 2009). At the same time, Jasplakinolide remarkably increased Ccn2 expression (Fig. 3c). Since CCN2 is known to promote osteoblastic differentiation, we subsequently evaluated the effect of stress fiber formation on the osteoblastic phenotype. As shown in Fig. 4, jasplakinolide strongly provoked alp expression representing early osteoblastic differentiation, whereas induction of a marker for late osteoblastic differentiation, oc, was not statistically significant. These results indicate that actin polymerization is sufficient to enhance Ccn2 expression and osteoblastic phenotype in MC3T3-E1 cells.
Fig. 3.
Induction of Ccn2 expression by actin polymerization forced by jasplakinolide. a Dose-dependent effects of jasplakinolide on MC3T3-E1 cells. Fluorescence images of MC3T3-E1 cells stained with Alexa 488-phalloidin to visualize actin filaments (a-d). a: control cells, b–d: cells treated for 30 min with 10 nM (b), 50 nM (c), and 200 nM (d) of jasplakinolide. b Quantitative analysis of the effect of jasplakinolide on the fluorescence intensity representing actin stress fiber formation in MC3T3-E1 cells. Each column and error bar represents the mean and SD, respectively, from 3 slides. **significant difference from the control; p < 0.01. c Dose-dependent effect of jasplakinolide on the level of Ccn2 expression. MC3T3 cells were incubated in the presence of jasplakinolide at the indicated concentrations
Fig. 4.
Induction of osteoblastic marker gene expression by actin polymerization. MC3T3-E1 cells were treated with Jasplakinolide (200 nM), and expression of osteoblastic marker genes were evaluated by real-time RT-PCR. The results are shown as percentages of each mRNA level in the control cells (=100). Each column and error bar represents the results from three independent experiments. ALP and OC denote alkaline phosphatase and osteocalcin, respectively. *significant difference from the data indicated by the bracket; p < 0.05
Role of cAMP in the Ccn2 mRNA response to fluid shear stress
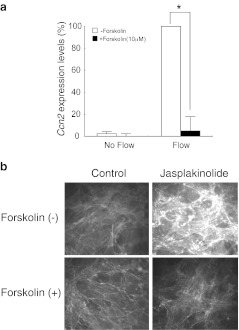
It is reported that the cAMP pathway, one of the major intracellular signal transduction cascades, regulates osteoblastic function and metabolism (Wong 1979). Moreover, cAMP-mediated regulation of Ccn2 was indicated in human renal fibroblasts (Heusinger-Ribeiro et al. 2001). Therefore, we investigated the effects of this second messenger of intracellular signal transduction on the Ccn2 mRNA response to FSS. The adenylate cyclase activator forskolin (10 μM) did not show significant effects on Ccn2 mRNA expression in the no-flow control. However, it completely inhibited the flow-induced Ccn2 response (Fig. 5a). These findings suggest that the Ccn2 mRNA response to FSS is negatively regulated by the intracellular cAMP level. Next, in order to determine whether cAMP is located upstream to regulate actin polymerization, or not, effects of forskolin on the jasplakinolide-induced F-actin formation was evaluated. The results showing no F-actin accumulation by jasplakinolide in the presence of forskolin (Fig. 5b) clearly indicate that F-actin formation is under the negative regulation by cAMP.
Fig. 5.
Regulatory role of cAMP in FSS-mediated Ccn2 induction. a MC3T3-E1 cells were pretreated with Forskolin (10 μM). Ccn2 mRNA expression was quantitatively analyzed, as described elsewhere. The results are shown as percentages of the Ccn2 mRNA level observed after FSS loading in the absence of Forskolin (=100). Each column denotes the mean and SD (error bars) value computed from the data of three independent experiments. *significant difference from the data indicated by the brackets; p < 0.05. No statistically significant difference was estimated between the untreated cells and the Forskolin-treated cells with FSS by two way ANOVA. b The same MC3T3-E1 cells were treated with Jasplakinolide (200 nM) in the absence or presence of Forskolin (10 μM). F-actin staining revealed the inhibition of actin polymerization by Forskolin
Discussion
Recently, much attention has focused on the regenerative function of CCN2. CCN2 promotes the proliferation and differentiation of endothelial cells, fibroblasts, chondrocytes, and osteoblasts. Moreover, recent reports have demonstrated that the local application of CCN2 promoted the regeneration of bone as well as articular cartilage in vivo (Kubota and Takigawa 2011). Of note, we also reported that experimental tooth movement increased the expression of Ccn2 mRNA in osteocytes and osteoblasts around the periodontal ligament, and intense expression of Ccn2 extended to osteocytes situated deep in the alveolar bone matrix far from the periodontal ligament (Yamashiro et al. 2001). These findings strongly suggest that CCN2 expression induced by orthodontic force plays a significant role in alveolar bone remodeling.
To mimic the events occurring on the bone-forming side of the moving tooth, we employed the osteoblastic cell line, MC3T3-E1 cells; exposed these cells to fluid shear stress; and examined the mechanism of Ccn2 expression in response to mechanical stress in vitro. We showed for the first time that continuous fluid shear stress immediately increased Ccn2 mRNA expression in the osteoblastic cell line in a dose dependent manner. A recent report demonstrated a positive effect of applying pulsatile stress at 1 Hz on Ccn2 expression in osteoblastic cells (Thi et al. 2007). This type of stress loading is a good model of the physiological stress loaded upon teeth during mastication, whereas our continuous FSS model represents orthodontic stress in the bone generating area. Therefore, our present study clearly indicates a critical role of orthodontic force in supplying the CCN2 that promotes bone regeneration.
Recently, the actin cytoskeleton was found to be associated with mechanotransduction in a variety of cells (Myers et al. 2007; Fu et al. 2008; Li et al. 2008). Previous reports have indicated that the small GTPase RhoA plays a pivotal role in mechanotransduction in osteoblasts. We have also confirmed that FSS-induced Ccn2 mRNA expression was inhibited by the Rho-kinase inhibitor Y-27632, suggesting the contribution of Rho signaling to the Ccn2 induction. The involvement of Rho signaling in Ccn2 regulation has been described in several types of cells. Interestingly, it was reported that Ccn2 expression was strongly repressed by the long-term exposure of HUVEC to laminar FSS, which was also mediated by Rho signaling. The apparent discrepancy between the results obtained with HUVEC and MC3T3-E1cells can be explained from two viewpoints. First, these two types of cells possess fundamentally different backgrounds with different basal Ccn2 expression levels. Second, our evaluation was performed over a time course of 4 h, while the HUVEC were exposed to laminar flow stress for 18 h. Since we observed a decline in Ccn2 expression after 2 h, a further reduction would be anticipated at 18 h. Probably, not only the laminar flow per se, but also the switch from no flow to flow may be a critical factor to induce maximal and durable induction of Ccn2 in osteoblasts. In this context, repeated FSS loaded at a very low frequency may be the optimal conditions for maximizing CCN2 production by osteoblasts.
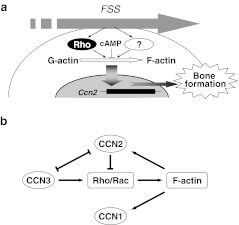
Rho signaling provokes a number of intracellular events. Thus, to determine whether the observed Ccn2 induction was principally caused by the RhoA-dependent actin reorganization events, we initially used 2 reagents with different effects. Both latrunculin A and cytochalasin D disrupt normal actin stress fibers; however, their actions on actin polymerization are different. G-actin subunits assemble into long filamentous polymers entitled F-actin. Latrunculin A affects the polymerization of pure actin in vitro via the formation of an equimolar complex between latrunculin A and G-actin (Coue et al. 1987). On the other hand, cytochalasin D “caps” actin filaments by binding to the plus (faster-growing) end and cleaving them (Cassimeris et al. 1990). Therefore, although this compound does not allow the formation of intact F-actin, continuous cleavage and polymerization of actin occurs in the presence of cytochalasin D, which result in the accumulation of aberrant fibers. As a result, the latrunculin A treated cells displayed strikingly decreased Ccn2 expression concomitant with the disruption of their actin stress fibers. In contrast, the cytochalasin D treated cells showed irregular, short but aggregated thick stress fibers with maximally upregulated Ccn2 expression, even in the absence of flow stress, and no further increase was conferred by FSS. These findings together suggest that rather than the proper F-actin formation, actin polymerization process itself or a decrease in the G-actin level is essential for Ccn2 mechanoinduction in osteoblasts. Moreover, the fact that accelerated F-actin formation by jasplakinolide promotes not only Ccn2 expression, but also osteoblastic differentiation further indicates the significant role of actin polymerization in stress-induced osteogenesis. Additionally, considering that the inhibition of FSS-induced Ccn2 expression by Y27632 was partial, involvement of signaling pathways other than the ROCK-mediated one is suggested therein (Fig. 6a). In this point of view, regulation by cAMP level is particularly of note.
Fig. 6.
a Schematic representation of the mechanism of Ccn2 induction during orthodontic bone remodeling. FSS activates Rho signaling and unknown pathways to promote actin polymerization, which induces CCN2 production toward bone formation. Cyclic AMP activates inhibitory signaling against this process. b Possible molecular interplay among the CCN family members in the actin fiber-mediated regulation of CCN2 production. Arrows or T-shaped lines indicate induction or reduction of a signal and molecules, respectively. Note that a negative feed back loop for CCN2 induction is formed via CCN2 molecule itself
Considering the critical role of CCN2 in bone remodeling, this actin-mediated regulation of Ccn2 expression should be under the strict control, since bone is always loaded by mechanical stresses. In fact, it was reported that CCN2 adversely affected actin polymerization through the Rho signaling pathways (Crean et al. 2006), which may form a negative feed back loop for CCN2 production (Fig. 6b). Moreover, the gene expression, as well as the molecular action of CCN2 is counteracted by another CCN family member CCN3, which also promotes the Rac 1 signaling to accelerate actin reorganization (Perbal and Takigawa 2005; Sin et al. 2009). Of note, CCN1, another classical member of the CCN family, is also induced by actin polymerization (Hanna et al. 2009). This possible regulatory network for the tight regulation of the CCN2 production by multiple CCN family proteins is summarized in Fig. 6b.
Fluid shear stress after bone loading, which is the principal orthodontic methodology, is known to determine the behavior of osteoblasts (Burger and Klein-Nulend 1999). Together with our previous studies, our present results further indicate the critical involvement of CCN2 in orthodontic tooth movement and possible orthodontic utility of this factor.
Acknowledgments
We thank Dr. M. Miyamoto for providing technical assistance in designing and completing the real time PCR protocols. This study was supported in part by grants-in-aid (20249081T.T-Y) for scientific research from the Japan Society for the Promotion of Science.
References
- Burger EH, Klein-Nulend J. Mechanotransduction in bone–role of the lacuno-canalicular network. FASEB J. 1999;13:S101–S112. [PubMed] [Google Scholar]
- Cassimeris L, McNeill H, Zigmond SH. Chemoattractant-stimulated polymorphonuclear leukocytes contain two populations of actin filaments that differ in their spatial distributions and relative stabilities. J Cell Biol. 1990;110:1067–1075. doi: 10.1083/jcb.110.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicha I, Goppelt-Struebe M, Muehlich S, Yilmaz A, Raaz D, Daniel WG, Garlichs CD. Pharmacological inhibition of RhoA signaling prevents connective tissue growth factor induction in endothelial cells exposed to non-uniform shear stress. Atherosclerosis. 2008;196:136–145. doi: 10.1016/j.atherosclerosis.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Coue M, Brenner SL, Spector I, Korn ED. Inhibition of actin polymerization by latrunculin A. FEBS Lett. 1987;213:316–318. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- Crean JK, Furlong F, Mitchell D, McArdle E, Godson C, Martin F. Connective tissue growth factor/CCN2 stimulates actin disassembly through Akt/protein kinase B-mediated phosphorylation and cytoplasmic translocation of p27(Kip-1) FASEB J. 2006;20:1712–1714. doi: 10.1096/fj.05-5010fje. [DOI] [PubMed] [Google Scholar]
- Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227:1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- Fu Q, Wu C, Shen Y, Zheng S, Chen R. Effect of LIMK2 RNAi on reorganization of the actin cytoskeleton in osteoblasts induced by fluid shear stress. J Biomech. 2008;41:3225–3228. doi: 10.1016/j.jbiomech.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Hahn A, Heusinger-Ribeiro J, Lanz T, Zenkel S, Goppelt-Struebe M. Induction of connective tissue growth factor by activation of heptahelical receptors. Modulation by Rho proteins and the actin cytoskeleton. J Biol Chem. 2000;275:37429–37435. doi: 10.1074/jbc.M000976200. [DOI] [PubMed] [Google Scholar]
- Hanna M, Liu H, Amir J, Sun Y, Morris SW, Siddiqui MA, Lau LF, Chaqour B. Mechanical regulation of the proangiogenic factor CCN1/CYR61 gene requires the combined activities of MRTF-A and CREB-binding protein histone acetyltransferase. J Biol Chem. 2009;284:23125–23136. doi: 10.1074/jbc.M109.019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusinger-Ribeiro J, Eberlein M, Wahab NA, Goppelt-Struebe M. Expression of connective tissue growth factor in human renal fibroblasts: regulatory roles of RhoA and cAMP. J Am Soc Nephrol. 2001;12:1853–1861. doi: 10.1681/ASN.V1291853. [DOI] [PubMed] [Google Scholar]
- Jackson WM, Jaasma MJ, Tang RY, Keaveny TM. Mechanical loading by fluid shear is sufficient to alter the cytoskeletal composition of osteoblastic cells. Am J Physiol Cell Physiol. 2008;295:C1007–C1015. doi: 10.1152/ajpcell.00509.2007. [DOI] [PubMed] [Google Scholar]
- Kubota S, Takigawa M. Role of CCN2/CTGF/Hcs24 in bone growth. Int Rev Cytol. 2007;257:1–41. doi: 10.1016/S0074-7696(07)57001-4. [DOI] [PubMed] [Google Scholar]
- Kubota S, Takigawa M. The role of CCN2 in cartilage and bone development. J Cell Commun Signal. 2011;5:209–217. doi: 10.1007/s12079-011-0123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhao Z, Wang J, Chen G, Yang J, Luo S. The role of extracellular matrix, integrins, and cytoskeleton in mechanotransduction of centrifugal loading. Mol Cell Biochem. 2008;309:41–48. doi: 10.1007/s11010-007-9641-0. [DOI] [PubMed] [Google Scholar]
- Moussad EE, Brigstock DR. Connective tissue growth factor: what’s in a name? Mol Genet Metab. 2000;71:276–292. doi: 10.1006/mgme.2000.3059. [DOI] [PubMed] [Google Scholar]
- Myers KA, Rattner JB, Shrive NG, Hart DA. Osteoblast-like cells and fluid flow: cytoskeleton-dependent shear sensitivity. Biochem Biophys Res Commun. 2007;364:214–219. doi: 10.1016/j.bbrc.2007.09.109. [DOI] [PubMed] [Google Scholar]
- Nishida T, Nakanishi T, Asano M, Shimo T, Takigawa M. Effects of CTGF/Hcs24, a hypertrophic chondrocyte-specific gene product, on the proliferation and differentiation of osteoblastic cells in vitro. J Cell Physiol. 2000;184:197–206. doi: 10.1002/1097-4652(200008)184:2<197::AID-JCP7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Ott C, Iwanciw D, Graness A, Giehl K, Goppelt-Struebe M. Modulation of the expression of connective tissue growth factor by alterations of the cytoskeleton. J Biol Chem. 2003;278:44305–44311. doi: 10.1074/jbc.M309140200. [DOI] [PubMed] [Google Scholar]
- Perbal B, Takigawa M. CCN protein -A new family of cell growth and differentiation regulators. London: Imperial College Press; 2005. pp. 1–311. [Google Scholar]
- Safadi FF, Xu J, Smock SL, Kanaan RA, Selim AH, Odgren PR, Marks SC, Jr, Owen TA, Popoff SN. Expression of connective tissue growth factor in bone: its role in osteoblast proliferation and differentiation in vitro and bone formation in vivo. J Cell Physiol. 2003;196:51–62. doi: 10.1002/jcp.10319. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Balam TA, Kuroda S, Tamamura N, Fukunaga T, Takigawa M, Takano-Yamamoto T. CTGF and apoptosis in mouse osteocytes induced by tooth movement. J Dent Res. 2009;88:345–350. doi: 10.1177/0022034509334649. [DOI] [PubMed] [Google Scholar]
- Sin WC, Tse M, Planque N, Perbal B, Lampe PD, Naus CC. Matricellular protein CCN3 (NOV) regulates actin cytoskeleton reorganization. J Biol Chem. 2009;284:29935–29944. doi: 10.1074/jbc.M109.042630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smerdel-Ramoya A, Zanotti S, Deregowski V, Canalis E. Connective tissue growth factor enhances osteoblastogenesis in vitro. J Biol Chem. 2008;283:22690–22699. doi: 10.1074/jbc.M710140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo H, Kodama HA, Amagai Y, Yamamoto S, Kasai S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 1983;96:191–198. doi: 10.1083/jcb.96.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thi MM, Iacobas DA, Iacobas S, Spray DC. Fluid shear stress upregulates vascular endothelial growth factor gene expression in osteoblasts. Ann N Y Acad Sci. 2007;1117:73–81. doi: 10.1196/annals.1402.020. [DOI] [PubMed] [Google Scholar]
- Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27:339–360. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Wong GL. Induction of metabolic changes and down regulation of bovine parathyroid hormone-responsive adenylate cyclase are dissociable in isolated osteoclastic and osteoblastic bone cells. J Biol Chem. 1979;254:34–37. [PubMed] [Google Scholar]
- Woods A, Pala D, Kennedy L, McLean S, Rockel JS, Wang G, Leask A, Beier F. Rac1 signaling regulates CTGF/CCN2 gene expression via TGFbeta/Smad signaling in chondrocytes. Osteoarthr Cartil. 2009;17:406–413. doi: 10.1016/j.joca.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Xu J, Millard M, Ren X, Cox OT, Erdreich-Epstein A. c-Abl mediates endothelial apoptosis induced by inhibition of integrins alphavbeta3 and alphavbeta5 and by disruption of actin. Blood. 2010;115:2709–2718. doi: 10.1182/blood-2009-05-223776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro T, Fukunaga T, Kobashi N, Kamioka H, Nakanishi T, Takigawa M, Takano-Yamamoto T. Mechanical stimulation induces CTGF expression in rat osteocytes. J Dent Res. 2001;80:461–465. doi: 10.1177/00220345010800021201. [DOI] [PubMed] [Google Scholar]