Abstract
Platelet-activating factor (PAF) is a potent phospholipid mediator involved in specific disease states such as allergic asthma, atherosclerosis and psoriasis. The human PAF receptor (PAFR) is a member of the G protein-coupled receptor (GPCR) family. Following PAF stimulation, cells become rapidly desensitized; this refractory state can be maintained for hours and is dependent on PAFR phosphorylation, internalization and trafficking. EBP50/NHERF1 has been found to interact with a variety of proteins and these interactions are involved in a growing range of functions including the assembly of signalling complexes, receptor recycling and transport of proteins to the cell surface. Crucial roles of EBP50 in GPCR physiology include its involvement in internalization, recycling, and downregulation. We were interested in identifying the role of EBP50 in PAFR trafficking. Our results showed that EBP50 binds the PAFR in its basal state, while stimulation decreased the ratio of interaction between the two proteins. We also demonstrated that EBP50 could bind PAFR via its PDZ 2 domain. In addition, we studied the role of EBP50 in various functions of the PAFR such as PAF-induced inositol phosphate accumulation and receptor internalization: EBP50 decreased the WT PAFR response and rescued the function of internalization-deficient mutant receptors, as previously described for the arrestins and the GRKs. These results suggest new roles for EBP50, some of which could help understanding the complex formation after receptor activation.
Keywords: Arrestin, EBP50/NHERF1, GPCR, GRK, Internalization, PAFR
Introduction
Platelet-activating factor (PAF) is a potent phospholipid mediator released from activated basophils, platelets, macrophages, polymorphonuclear neutrophils and many other cell types (Braquet and Rola-Pleszczynski 1987). In humans, various diseases have been associated with PAF, such as allergic asthma, endotoxic shock, acute pancreatitis and dermal inflammation such as psoriasis and pruritis (Ishii and Shimizu 2000). PAF is known to be involved in a variety of biological activities related to inflammatory and immune responses, respiratory and nervous system physiology as well as circulatory system disorders such as atherosclerosis (Brocheriou et al. 2000). PAF biological actions are mediated through the binding and activation of a specific G-protein coupled receptor (GPCR), high-affinity receptor (PAFR) on the target cell surface. Cell responsiveness to agonists of GPCRs is usually characterized by a rapid desensitization to subsequent exposures, followed by a resensitization in the absence of stimulation (Goldstein et al. 1985; Pearse and Robinson 1990; Trowbridge 1991; Smythe and Warren 1991). The internalization of GPCRs is believed to be responsible, at least in part, for desensitization and/or for resensitization (Pippig et al. 1995; Krueger et al. 1997; Zhang et al. 1997; Vogler et al. 1998; Ishii et al. 1998; Oakley et al. 1999) of many GPCRs (Pippig et al. 1995; Krueger et al. 1997). Members of the arrestin family play an important role in the process of GPCR internalization (Laporte et al. 1999, 2000; Luttrell et al. 1999). After agonist-activation, the receptors are phosphorylated by GPCR-specific kinases (GRKs) and second messenger-dependent kinases (Hausdorff et al. 1990). Phosphorylation of the receptor and arrestin binding prevent coupling of the receptor to the heterotrimeric G-protein and are believed to be the key elements of a rapid desensitization (Lin et al. 1997; Barak et al. 1997; Freedman and Lefkowitz 1996). We have shown, in several studies from our group, the mechanisms employed by the PAFR for its internalization and down-regulation processes (Perron et al. 2003; Dupre et al. 2003; Chen et al. 2002; Le Gouill et al. 1997).
EBP50, also known as NHERF1, is a phosphoprotein of 50 kDa first identified as a cofactor essential for protein kinase A-mediated inhibition of Na+/H+ exchanger isoform 3 (Weinman et al. 1995). EBP50 contains two PDZ domains (PDZ1 and PDZ2) involved in multiple protein-protein interactions and an ERM domain that binds to the actin-associated ERM proteins (ezrin-radixin-moesin-merlin) (Murthy et al. 1998; Reczek et al. 1997). EBP50 has been found to interact with a variety of proteins such as G proteins (Rochdi and Parent 2003; Rochdi et al. 2002), receptors (Hall et al. 1998a; Maudsley et al. 2000; Li et al. 2002; Hammad et al. 2010), phospholipase Cβ1/2 (Tang et al. 2000), TRP4/5 calcium channels (Tang et al. 2000), cystic fibrosis transmembrane conductance regulator (CFTR) (Moyer et al. 2000), GRK6A (Hall et al. 1999),and NHERF2 (Yun et al. 1997; Poulat et al. 1997; Hwang et al. 2000) among others. These interactions are involved in a range of functions including the assembly of signalling complexes, receptor recycling and transport of proteins to the cell surface (Shenolikar and Weinman 2001). Cao et al. have shown that EBP50 can participate in GPCR trafficking by its involvement in the recycling steps towards the cell surface following agonist-induced internalization (Cao et al. 1999). A role of EBP50 in down-regulation of the kappa opioid receptor (Li et al. 2002) and in the activation-independent internalization of the parathyroid hormone receptor (Sneddon et al. 2003) and its desensitization (Wang et al. 2009) has also been shown. Class I PDZ proteins, such as NHERF1, recognize target proteins with the sequence motif (D/E)(S/T)XΦ, where X represents any amino acid and Φ is a hydrophobic residue, generally Leu/Ile/Val (Songyang et al. 1997). The platelet-activating factor receptor C-terminal tail contains a PDZ recognition motif constituted by an asparagine, a serine, a leucine and a lysine. We demonstrate here that EBP50 can interact with the PAFR to regulate its function. We characterized the interaction domains implicated for this complex formation. We also verified the implication of each PDZ domain of EBP50 in this interaction and the role of EBP50 in various functions of the PAFR such as PAF-induced inositol phosphate response and receptor internalization.
Experimental procedures
Dowex AG1-X8 was from Bio-RAD Laboratories Ltd (Hercules CA, USA). 3H-PAF and 3H-myo-inositol were obtained from Amersham-Pharmacia Biotech (Piscataway NJ, USA) while 3H-WEB2086 was from NEN (Boston MA, USA). Dulbecco’s modified Eagle’s medium and DMEM high glucose with/without sodium phosphate or inositol were from Invitrogen Canada Inc (Burlington ON, Canada) and FuGENE-6 was from Roche Diagnostics (Laval QC, Canada). FBS was from Sigma-Aldrich Canada Ltd (Oakville ON, Canada).
cDNA constructions, cell culture and transfections
The WT, D63N, D289A, Y293A cDNAs were cloned in PJ3M expression vector in frame with a cmyc epitope and then transferred to pcDNA3 as previously described (Parent et al. 1996a, c) (Le Gouill et al. 1997). The STOP4 (Q334STOP), N338A, S339A, L340A, K341R, D289A/S339A were constructed using T7 primer and mutation containing primers on a pcDNA3 myc-PAFR template. PcDNA3 GRK2, GRK3, GRK5, GRK6, arrestin2, arrestin3 and dominant negative constructs (C-terminus 318–419) were kind gifts from Dr. Benovic (Thomas Jefferson University, Philadelphia, PA, USA) while dominant negative arrestin2 V53D was made by site-directed mutagenesis (Chen et al. 2003). pcDNA3 HA-NHERF/EBP50 WT and dominant negative constructs were kind gifts from Dr. von Zastrow (University of California, San Francisco, CA, USA) and Dr. Bretscher (Cornell University, Ithaca, NY, USA) while the PDZ1-HA, PDZ2-HA and ERM-HA constructs were obtained from Dr. Jean-Luc Parent (Université de Sherbrooke, Canada). HEK293 cells were grown in DMEM high glucose supplemented with 10 % FBS. HEK293 cells were transfected with constructions encoding myc-tagged WT or mutant PAF receptor in 30-mm dishes (3 × 105 cells/dish) using 2 μl of FuGENE-6 and 1 μg of DNA. Assays were performed 48 h post-transfection.
Inositol phosphate determination
HEK293 cells were transfected as described above with the cDNAs of WT or mutant receptors and EBP50 and labeled the following day for 18–24 h with 3H-myo-inositol at 5 μCi/ml in DMEM (high glucose, without inositol). After labeling, cells were washed once in PBS and preincubated with 20 mM of LiCl for 10 min at 37 °C followed by a stimulation with PAF at 1 × 10−6M for 15 min. The reaction was terminated by the addition of perchloric acid followed by an incubation of 30 min on ice. Inositol phosphates were extracted and separated on Dowex AG1-X8 columns. Total 3H-labeled inositol phosphates were then counted by liquid scintillation.
Radioligand binding assay
3H-WEB2086 binding reactions were performed, as previously described by Parent et al. (Parent et al. 1996b), on HEK293 cells transfected with cDNA encoding a cmyc epitope tagged (N-terminus) WT PAFR in pcDNA3. Briefly, cells were harvested, washed twice with PBS and resuspended in HEPES-Tyrode’s buffer (140 nM NaCl, 2.7 mM KCl, 1 mM CaCl2, 12 mM NaHCO3, 5.6 mM D-glucose, 0.49 mM MgCl2, 0.37 mM NaH2PO4, 25 mM Hepes, pH 7.4) containing 0.1 % BSA. The binding assays were done on 5 × 104 cells in a total volume of 0.25 ml of the same buffer, containing 10 nM 3H-WEB2086, at RT for 90 min. Binding reactions were stopped by centrifugation. The cell-associated radioactivity was measured by liquid scintillation.
Ligand internalization
The evaluation of ligand internalization kinetics was done on HEK293 cells transiently transfected in 12-well dishes with constructions encoding mutant or WT receptors and EBP50, arrestin or GRK constructs. 48 h post-transfection, cells were incubated at 37 °C with 2nM 3H-hexadecyl-PAF in a buffer containing 150 mM choline chloride, 10 mM Tris–HCl, pH 7.5 10 mM MgCl2, and 0.25 % lipid-free BSA (Ye et al. 1991) for 5, 20, 40 or 60 min. After the incubation period, cells were washed two times with 1 ml of the same buffer but containing 2 % BSA. Cells were then lysed in 0.1N NaOH and internalized radioactivity was evaluated by liquid scintillation. In order to compare the different mutant receptors, internalization levels at each time point were divided by the expression levels at 5 min (no internalization for the PAFR at this time point) to generate a net internalization ratio.
Immunoprecipitation of the PAF receptor
Forty eight hours after transfection, cells were washed and stimulation with PAF (10−6M) was performed for 15 min. Cells were harvested and samples were lysed in 0.9 ml of RIPA buffer (50 mM Tris, pH 7.5, 5 mM EDTA, 150 mM NaCl, 0.5 % sodium deoxycholate, 1 % IGEPAL, 0.1 % SDS, 2 μg/ml aprotinin, 1 μM/ml leupeptin, 10 μg/ml soybean trypsin inhibitor and 100 μg/ml AEBSF). The lysate was solubilized by incubation at 4 °C for 30 min, precleared with 50 μl of protein A-Sepharose beads at 4 °C for 1 h, and clarified by centrifugation at 14,000 rpm for 10 min. The pre-cleared lysate was incubated with an anti-c-Myc antibody for 30 min, then 50 μl of protein A-Sepharose beads were added and the mixture was incubated for 1 h. After extensive washing with RIPA buffer, the immunoprecipitated proteins were eluted from beads with 50 μl of SDS sample buffer, resolved by SDS-PAGE, transferred to nitrocellulose membrane. Western blots were exposed to Hyperfilm MP.
Results
Co-immunoprecipitation of EBP50 with PAFR
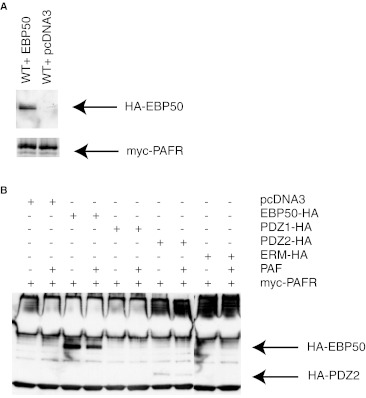
EBP50 has been found to interact with a variety of proteins implicated in G protein coupled receptor signalling such as G proteins, multiple effectors and the receptor themselves. The terminal C-tail portion of the PAFR is constituted by amino acids (NSLK) forming a putative PDZ interacting motif. We were interested to verify whether these residues are involved in the interaction between EBP50 and the PAFR. In Fig. 1a, we co-expressed the c-Myc-tagged WT PAFR with a construct encoding an HA-tagged EBP50 protein. Forty eight hours post-transfection, the receptors were immunoprecipitated with an anti-c-Myc antibody and analyzed by western blotting with an anti-HA antibody, revealing the tagged EBP50 construct. The co-immunoprecipitation of the PAFR and EBP50 was observed in unstimulated cells, in comparison to cells overexpressing solely the receptor.
Fig. 1.
Co-immunoprecipitation of EBP50 and PAFR. a 48 h post-transfection, HEK293 cells expressing cmyc-tagged PAFR and HA-tagged EBP50 or pcDNA3 cDNAs were immunoprecipitated in RIPA buffer using an anti-cmyc antibody, separated on SDS-PAGE, transferred to a nitrocellulose membranes. A Western blot analysis was done with antibodies for HA or cmyc epitopes. Results are representative of three independent experiments. b Results are showing the co-immunoprecipitation of cmyc-tagged PAFR and HA-tagged EBP50 PDZ1, PDZ2, ERM domains or pcDNA3 cDNAs in HEK293 cells. Stimulations were performed using PAF at a concentration of 10−6M for 15 min
Identification of EBP50 domain(s) interacting with PAFR
EBP50 contains two PDZ domains (PDZ1 and PDZ2) and an ERM domain. In Fig. 1b, we co-expressed constructions encoding the full length C-terminally HA-tagged EBP50, or portions of the protein (PDZ1-HA, PDZ2-HA or ER-HA domains) with the PAFR to identify the domain(s) responsible for EBP50 interaction with the receptor. Forty eight hours post-transfection, the receptors were immunoprecipitated with an anti-c-Myc antibody and analyzed by Western blotting with an anti-HA antibody, revealing the HA-tagged EBP50 constructs. The co-immunoprecipitation of the PAFR and WT EBP50 was observed in unstimulated cells with a decreased interaction (37.8 % decrease) following a 15 min stimulation with PAF (10−6M). No interaction was observed with the PDZ1 and ERM domains co-expressed alone with the PAFR, while the PDZ2 domain was co-immunoprecipitated with the receptor. Similarly to the full length EBP50 construct, stimulation with PAF resulted in a decrease by 56.4 % of EBP50 PDZ2 domain co-immunoprecipitating with PAFR.
EBP50 effect on PAFR response
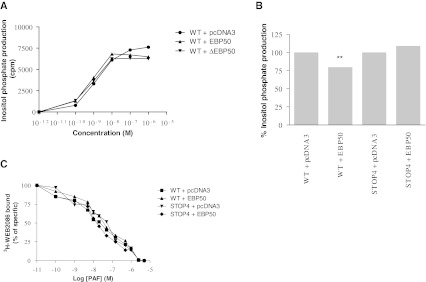
A concentration-dependent curve of PAF was also done with the WT PAFR in presence of WT EBP50 or dominant negative EBP50 (ΔEBP50; EBP50 lacking the last 61 amino acids and tagged HA (Cao et al. 1999)). Results illustrated in Fig. 2a show that EBP50 slightly reduces inositol phosphate production at high concentrations of PAF (between 5 × 10−7 and 10−6M) while similar IP production is observed at lower PAF concentrations. The use of ΔEBP50; (EBP50 lacking the last 61 amino acids and tagged HA (Cao et al. 1999)) suggests that the inhibitory effect is not mediated by the ERM domain, since this mutated EBP50 construct lacking part of the ERM domain can also inhibit the IP accumulation in a similarly to the WT EBP50.
Fig. 2.
EBP50 effect of PAFR response. HEK293 cells transiently expressing the WT or STOP4 mutant PAFR and EBP50 were used 48 h post-transfection for IP accumulation and binding reactions. a Effect of various concentrations of PAF on the response of PAFR in presence of various EBP50 constructs in HEK293 cells. The results are expressed as the means of three independent experiments, each done in triplicate. b The effect on inositol phosphate production of the co-expression of EBP50 on WT and STOP4 mutant PAFR was measured. Cells were stimulated for 15 min at 37 °C with PAF 10−6M. The results are expressed as the means ± s.e. of three independent experiments, each done in triplicate. T-test statistics were done using Graphpad Prism; where ** = p < 0.01. c Binding reactions were performed in presence of 3H-WEB2086 and PAF as described in the methods. The results are expressed as the means of three independent experiments, each done in duplicate
We then made a deletion mutant of PAFR in order to remove the last 9 amino acids thus, the putative C-terminal PDZ domain should be completely removed in the STOP4 (Q334STOP) mutant receptor. The activation of a response induced by PAF can be measured by the accumulation of inositol phosphates (IP). We evaluated IP production in HEK293 cells transfected with the cDNAs of WT PAFR or the STOP4 mutant receptor and EBP50. Figure 2b shows that co-expression of EBP50 blocked 20 ± 7 % of total inositol phosphate production with the WT PAFR while there was no effect of co-expressed EBP50 on IP production by the STOP4 mutant receptor. Figure 2c shows that the difference in IP production by the two receptors in the presence of EBP50 was not an effect on agonist binding since no major differences were found in the affinity for 3H-WEB2086, a PAF antagonist, for both WT PAFR or STOP4 with or without co-expression of EBP50.
Identification of PAFR interacting domain
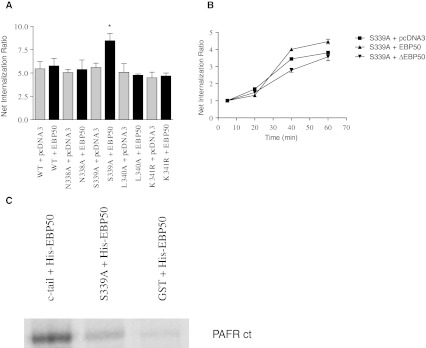
Although another receptor site might be involved in the binding of EBP50, we were still interested to fully characterize the distal C-terminal PDZ domain of the receptor to identify the key residues involved in the interaction. To do so, different mutations were performed in the PDZ domain: asparagine (N)338, serine (S)339 and leucine (L)340 were changed into an alanine (A) while the lysine (K)341 was substituted by an arginine (R). While all these mutants had a similar ligand affinity as the WT PAFR (data not shown), the effect of EBP50 on the internalization of the mutant receptors was relatively unaffected, except in one case, as depicted in Fig. 3a. The receptor internalization of the S339A mutant receptor was increased (8.7 ± 1.1) upon co-expression with EBP50, while all other substitution mutants showed no differences in internalization (approximately 5-fold increase over basal), with or without co-expression with EBP50. Internalization kinetics showed that EBP50 potentiated the internalization of the S339A mutant receptor at the later stages (8.3 ± 1.2) in comparison to receptor expressed alone (4.9 ± 0.9), while a decrease in internalization was seen with the ΔEBP50 (3.2 ± 1.4)(Fig. 3b).
Fig. 3.
Effect of EBP50 on the internalization of PDZ-binding motif PAFR mutants. a Effect of WT EBP50 on PAFR (WT, N338A, S339A, L340A, K341R) internalization, . Cells were stimulated 48 h post-transfection with PAF 10-6M. The results are expressed as the means ± s.e. of three independent experiments, each done in triplicate. T-test statistics were done using Graphpad Prism; where * = p < 0.05. b Kinetics were done for various time points ranging from 5 min (no internalization) to 60 min (maximal internalization). A ratio of internalization at each time point is represented over basal levels of internalization (5 min). The S339A mutant PAFR was co-expressed with pcDNA3, EBP50 or dominant negative EBP50. The results are expressed as the means of three independent experiments, each done in triplicate. c GST-fusion constructs of the WT and S339A mutant receptor c-tails were used for a histidine pull-down assay conducted between the GST-fusion protein cleaved by thrombin and 25 μg of histidine-tagged EBP50. Results are representative of three independent experiments
We verified whether the interaction of EBP50 with the PAFR was done via a direct interaction. GST-fusion constructs of the WT and S339A mutant receptor C-tails were purified and a pull-down was performed with a purified histidine-tagged EBP50 Fig. 3c shows that the WT PAFR C-tail can directly interact with the His-tagged EBP50 while the S339A mutant receptor C-tail demonstrates greatly impaired interaction. As a negative control, performed a histidine pulldown with the cleaved purified product from the vector PGEX 4T2. This GST control shows very limited binding to the His-tagged EBP50.
Effect of EBP50 and arrestin2 on PAFR internalization
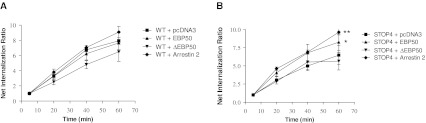
A decrease in the inositol phosphate response might result from an increased internalization rate of the cell-surface receptors. Since a role of EBP50 in degradation and activation-independent internalization of several receptors was previously demonstrated (Li et al. 2002), we ascertained whether EBP50 could play a role in the internalization of the PAFR. To evaluate the effect of EBP50 on receptor internalization, we co-expressed the PAFR with WT or ΔEBP50 constructs, as well as arrestin 2, which is known to regulate PAF receptor internalization (Chen et al. 2002). Receptor internalization was evaluated at time points ranging from 5 min (no PAFR internalization) to 60 min (maximal internalization). In Fig. 4a, Results show that the internalization rate of the PAFR is increased when co-expressed with the positive control arrestin 2 up to 9.0 ± 1-fold at 60 min, while EBP50 co-expression induced a similar internalization to co-expression with an empty vector (pcDNA3) with a 7.6 ± 0.2-fold internalization increase. The co-expression of ΔEBP50 reduced the levels of internalization at 60 min to 5.3 ± 0.5-fold increase over basal. We also evaluated the role of EBP50 in the internalization of the mutant STOP4, lacking the putative binding site for EBP50 in the C-terminal portion of PAFR C-tail. Figure 4b shows a significant increase of internalization with the co-expression of arrestin 2 (9.6 ± 0.6-fold over basal) with the PAFR. When the STOP4 mutant is coexpressed with EBP50, the internalization level is also increased significantly, in comparison to the STOP4 mutant receptor expressed alone (7.8 ± 1 vs 5.7 ± 0.5). Again, the co-expression of the ΔEBP50 construct with STOP4 mutant receptor was able to reduce the internalization levels to 5.0 ± 0.3. These results suggest that although the C-terminal PDZ domain of the receptor is involved in the interaction with EBP50, other domains of the receptor may also be involved.
Fig. 4.
Effect of EBP50 and arrestin2 on PAFR internalization. To evaluate the effect of EBP50 on receptor internalization, we co-expressed the PAFR, WT or dominant negative EBP50 constructs, as well as arrestin 2. Kinetics were done for time points between 5 min (no internalization) and 60 min (maximal internalization). A ratio of internalization at each time point is represented over basal levels of internalization (5 min). a Internalization of WT PAFR co-expressed with pcDNA3, EBP50, EBP50 dominant negative or arrestin 2. b Internalization of STOP4 PAFR mutant co-expressed with pcDNA3, EBP50, dominant negative EBP50 or arrestin 2. The results are expressed as the means of at least three independent experiments, each done in triplicate. T-test statistics were done using Graphpad Prism; where * = p < 0.05 and ** = p < 0.01
Internalization-deficient mutant receptor and EBP50
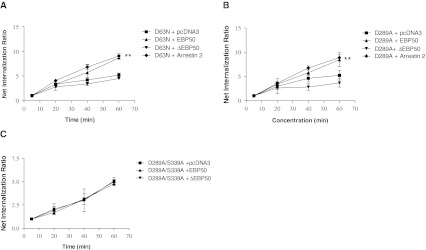
Rochdi et al. showed that G-alpha protein signaling can lead to internalization of GPCRs, with specificity in both the G-alpha proteins and GPCRs that are involved. Furthermore, a function has been described for EBP50 in its capacity to bind G-alpha proteins and to inhibit receptor endocytosis (Rochdi and Parent 2003). The PAF receptor is coupled to G alpha q protein and some mutants of this receptor (D63N and D289A) are functionally uncoupled from the G protein (Parent et al. 1996c; Le Gouill et al. 1997). Figure 4a shows the internalization levels of the WT receptor at various times. In comparison, the mutant receptor D63N shows 5.2 ± 0.4-fold internalization increase over basal at 60 min while co-expression of arrestin 2 or EBP50 generated a significant ratio of internalization over basal up to 9.1 ± 0.8-fold and 8.7 ± 0.2-fold respectively. The dominant negative EBP50 affect the levels of internalization at 60 min to levels comparable to D63N + pcDNA3 (4.4 ± 0.5-fold) (Fig. 5a). Figure 5b shows the results of another mutant that is unable to induce an inositol phosphate response, D289A. This mutant receptor displayed similar results to D63N with co-expression of the arrestin 2, EBP50, receptor expressed alone or dominant negative construct with 8.8 ± 1.0-fold, 8.4 ± 1.1-fold, 4.9 ± 1.0-fold and 4.0 ± 1.2-fold respectively. The response of arrestin 2 and EBP50 were significant, in comparison to pcDNA3.
Fig. 5.
Effect of EBP50 and arrestin2 on the internalization of uncoupled PAFR mutants. To evaluate the effect of EBP50 on PAFR mutant receptors internalization, D63N or D289A PAFR were co-expressed with WT or dominant negative EBP50 constructs, or arrestin 2. Kinetics were performed for time points between 5 min (no internalization) and 60 min (maximal internalization). A ratio of internalization at each time point is represented over basal levels of internalization (5 min). a Internalization of D63N mutant PAFR co-expressed with pcDNA3, EBP50, dominant negative EBP50 or arrestin 2. b Internalization of D289A mutant PAFR co-expressed with pcDNA3, EBP50, dominant negative EBP50 or arrestin 2. c Internalization of D289A/S339A mutant PAFR co-expressed with pcDNA3, EBP50, dominant negative EBP50 or arrestin 2. The results are expressed as the means of at least three independent experiments, each done in triplicate. T-test statistics were done using Graphpad Prism; where ** = p < 0.01
The previous results propose that two distinct sites could be important in the binding of EBP50 to the receptor; a C-tail terminal PDZ motif and a receptor conformation leading to an uncoupling from G-proteins. A double mutant receptor, D289A/S339A, was constructed in order to verify whether both the uncoupled receptor confirmation and the serine of the PDZ domain positions are required for the receptor function (internalization) rescue by EBP50. As demonstrated in Fig. 5c, EBP50 is no longer able to potentiate, nor the dominant negative EBP50 inhibit the internalization of this mutant PAFR receptor. These results suggest that removal of both sites inhibits EBP50’s capacity to affect internalization.
Receptor function rescue
Arrestins and GRKs can increase the internalization turnover of internalization-defective receptors (Ferguson et al. 1995, 1996; Menard et al. 1996, 1997; Zhang et al. 1998). Here, we wanted to evaluate the role of EBP50’s effect on internalization. We were interested to characterize a little further this property of EBP50 by comparing of the ability of EBP50, arrestins (2 and 3) and GRKs (2, 3, 5 and 6) to potentiate the internalization of the D63N internalization-defective mutant receptor. Figure 6a shows the ratio of internalization rescue over basal of the receptor expressed alone, coexpressed with EBP50, the arrestins or the GRKs. EBP50 displayed an increased level similar to GRK2, 3, 5 and arrestin 3 (approximately 7.5-fold increase) while arrestin 2 and GRK6 displayed the best potentiation levels with 11-fold and 9.9-fold, respectively.
Fig. 6.
Effect of EBP50, arrestins and GRKs on the internalization D63N mutant receptor. a Effect of WT EBP50, arrestin 2/3 and GRK2/3/5/6 on the internalization of D63N mutant PAFR. Cells were stimulated 48 h post-transfection with PAF 10−6M. Effect of WT EBP50, WT arrestin 2 or dominant negative isoforms (V53D and Arrestin2Δ318–419) expressed alone or co-expressed on the internalization of WT PAFR (b), D63N (c) or STOP4 PAFR (d). Cells were stimulated 48 h post-transfection with PAF 10−6M. The results are expressed as the means ± s.e. of three independent experiments, each done in triplicate. T-test statistics were done using Graphpad Prism; where * = p < 0.05; ** = p < 0.01; *** = p < 0.001
We previously reported that arrestins 2 and 3 are important in the internalization steps of the PAFR. Also, we identified the region important for the interaction of the PAFR with arrestins as the amino acids comprised between serine 318 and valine 330 (Chen et al. 2002). Since both arrestins and EBP50 appear to be involved in the internalization of the PAFR, we evaluated the effect of the combination of both regulators with different receptor constructs. We co-expressed EBP50 with the WT (Fig. 6b), D63N (Fig. 6c) or STOP4 (Fig. 6d) PAF receptors and established the ratios of internalization in presence of dominant negative arrestin2 V53D and Δarrestin2 (Δ318–419). The results show that EBP50, and arrestin 2 can induce an increase in the ratio of internalization of the mutant receptors, while only arrestin 2 increases the internalization ratio of the WT receptor at 60 min (Fig. 6b). When co-expressed with EBP50 and the dominant negative forms of arrestin2, the D63N and STOP4 ratios of internalization were greatly inhibited. Also, the arrestin dominant negative isoforms were able to reduce the internalization ratio of the different receptors. These results suggest an interdependence of the arrestin and EBP50 in the rescue of internalization, since dominant negative isoforms of arrestin can block EBP50 potentiation of internalization.
Discussion
The PAF receptor C-terminal tail contains a PDZ recognition motif constituted by an asparagine, a serine, a leucine and a lysine that allows EBP50 binding to the PAFR in its basal state. EBP50 interacts with the PAFR via its PDZ2 domain and regulates various functions of the PAFR, such as PAF-induced inositol phosphate production and receptor internalization. For example, EBP50 decreases WT PAFR inositol phosphate response and rescue internalization deficient mutant receptors, as previously described for two other major regulators: the arrestins and the GRKs.
EBP50, also known as NHERF1, has been previously shown to affect the function of GPCRs that contain PDZ-binding motifs. The platelet-activating factor receptor C-terminal tail PDZ recognition motif (NSLK) has not been evaluated for its potential role in PAFR trafficking; therefore, our work aimed at characterizing the potential interaction of EBP50 and PAFR and its role in PAFR trafficking. Up to now, most receptors have been shown to interact with EBP50 via the PDZ1 domain, as demonstrated for the β2-adrenergic receptor (Hall et al. 1998a), CFTR (Hall et al. 1998b), P2Y1R (Hall et al. 1998b) and PDGFR (Hall et al. 1998b). Other proteins implicated in GPCR trafficking regulation, such as GRK6A, also possess the ability to bind to the EBP50 PDZ1 domain and phosphorylate EBP50 (Hall et al. 1999). The PDZ2 domain binds to various proteins such as NHE3 (Weinman et al. 1998), YAP65 (Mohler et al. 1999), PTHR receptor (Sun and Mierke 2005) and more recently, it was found to interact with the CCR5 receptor (Hammad et al. 2010). Our results demonstrate that the PDZ2 domain of EBP50 is involved in the interaction of this protein with the PAFR.
To further characterize the EBP50-PAFR interaction, the PDZ binding motif of the PAFR was mutated to identify the residues involved in this interaction. The serine S339 of the PAFR PDZ binding motif was important but not exclusive, since its mutation to alanine (S339A) significantly reduced, but did not completely abolish the interaction. While the PAFR interacts with EBP50 basally, other studies have shown that EBP50 can regulate the internalization of Gαq-coupled receptors, and even interact with Gαq (Rochdi and Parent 2003; Rochdi et al. 2002). Our results show that upon activation of the receptor, the PAFR and EBP50 interaction is disrupted. When using mutant receptors with altered capacity to bind to G proteins and mediate signalling, we show that EBP50 overexpression can rescue the internalization of these receptors in HEK cells. Overexpression of EBP50 with a double mutant receptor (D289A/S339A) with an altered EBP50 binding site, does not promote the rescue of the internalization function of the receptor.
We then further characterized the effects of EBP50 on PAFR internalization and signaling. First, we showed that EBP50 overexpression diminished the levels of WT PAFR response, as measured by inositol phosphate production. Also, the EBP50 dominant negative mutant (lacking part of the ERM binding domain) was shown to inhibit the internalization of the WT PAFR. PAF can promote actin bundle formation after activation, It was shown that ERM domains are linked to actin bundle formation and endocytosis, suggesting a potential role of the ERM domain in this process (McLaughlin et al. 2006). When EBP50 was co-expressed with a deletion mutant of PAFR (STOP4 (Q334STOP); removing the PDZ C-terminal motif) that does not internalize as well as the WT PAFR (Fig. 4), we observed no effect of EBP50 on the mutant receptor inositol phosphate accumulation. However, there was a significant increase in the internalization rate. Such a phenomenon has previously been described for both arrestins and GRKs, two of the most important players in GPCR internalization regulation.
Overexpression of arrestins in HEK cells was shown to rescue the sequestration of β2-adrenergic mutant receptors defective in their ability to sequester, an effect enhanced by simultaneous overexpression of GRKs (Ferguson et al. 1996). Similar effects were seen for GRK2, which rescued a sequestration-defective mutant β2-adrenergic receptor upon overexpression (Ferguson et al. 1995). Since both arrestins (Chen et al. 2002) and EBP50 (seen in this study) have a role in the regulation and rescue of the PAFR, we were interested in determining how the combination of these proteins would affect the events involved in PAFR regulation. Upon expression of dominant negative arrestin2, EBP50 was unable to rescue the internalization of mutant PAFR receptors. It was suggested that EBP50 could act as a scaffold for GRKs and arrestins, helping for rapid and efficient phosphorylation of the receptor and arrestin binding. EBP50 can bind GRK6A by its PDZ1 domain, and was also recently shown to scaffold arrestin to the P2Y12 receptor (Nisar et al. 2012). In the case of the PAFR, a conformational change could occur upon activation that allows GRKs to phosphorylate the receptor, followed by the release of EBP50 from the receptor. Arrestin binds to a segment proximal to the PDZ binding motif of the PAFR (Chen et al. 2002). The release of EBP50 could free up the room required for arrestin binding. Translocated arrestins bound to the PAFR could then proceed to the mobilization of all adapters involved in the endocytic vesicle formation.
Our study shows that EBP50 interacts with the PAFR via its PDZ2 domain, while the PAFR PDZ motif docks EBP50 at the end of the cytoplasmic tail in the unstimulated state. Stimulation of the receptor promotes dissociation of EBP50 from the receptor. EBP50 plays a role in the regulation of PAF-induced inositol phosphate response and receptor internalization.
Acknowledgements
This work was supported by grants (MRP, JS) from the Canadian Institutes for Health Research and from the Natural Sciences and Engineering Research Council of Canada (NSERC RGPIN-355310-2008) (DJD). DJD holds a CIHR New Investigator Salary Award.
Glossary
- CFTR
Cystic fibrosis transmembrane conductance regulator
- EBP50
Ezrin-radixin-moesin binding protein of 50 kDa
- GPCR
G-protein coupled receptor
- GRK
GPCR-specific kinases
- NHERF1
Na+/H+ exchanger regulatory factor 1
- PAF
Platelet-activating factor
- WT
Wild-type
Footnotes
Concise 2–3 sentence summary
EBP50 has previously been shown to regulate several functions of G protein coupled receptors. Here, we show that EBP50 regulates several functions of the platelet-activating factor receptor, and acts in concert with arrestins and GRKs to regulate receptor internalization.
Contributor Information
Denis J. Dupré, Phone: +1-902-4942550, FAX: +1-902-4941388, Email: denis.dupre@dal.ca
Jana Stankova, Phone: +1-819-5645268, FAX: +1-819-5645215, Email: Jana.Stankova@USherbrooke.ca.
References
- Barak LS, Ferguson SS, Zhang J, Caron MG. A beta-arrestin/green fluorescent protein biosensor for detecting G protein-coupled receptor activation. J Biol Chem. 1997;272(44):27497–27500. doi: 10.1074/jbc.272.44.27497. [DOI] [PubMed] [Google Scholar]
- Braquet P, Rola-Pleszczynski M. The role of PAF in immunological responses: a review. Prostaglandins. 1987;34(2):143–148. doi: 10.1016/0090-6980(87)90190-0. [DOI] [PubMed] [Google Scholar]
- Brocheriou I, Stengel D, Mattsson-Hulten L, Stankova J, Rola-Pleszczynski M, Koskas F, Wiklund O, Charpentier Y, Ninio E. Expression of platelet-activating factor receptor in human carotid atherosclerotic plaques: relevance to progression of atherosclerosis. Circulation. 2000;102(21):2569–2575. doi: 10.1161/01.CIR.102.21.2569. [DOI] [PubMed] [Google Scholar]
- Cao TT, Deacon HW, Reczek D, Bretscher A, Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature. 1999;401(6750):286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- Chen Z, Dupre DJ, Gouill C, Rola-Pleszczynski M, Stankova J. Agonist-induced internalization of the platelet-activating factor receptor is dependent on arrestins but independent of G-protein activation. Role of the C terminus and the (D/N)PXXY motif. J Biol Chem. 2002;277(9):7356–7362. doi: 10.1074/jbc.M110058200. [DOI] [PubMed] [Google Scholar]
- Chen Z, Rola-Pleszczynski M, Stankova J. Activation of ERK1/2 by platelet-activating factor receptor is independent of receptor internalisation and G-protein activation. Cell Signal. 2003;15(9):843–850. doi: 10.1016/S0898-6568(03)00056-1. [DOI] [PubMed] [Google Scholar]
- Dupre DJ, Chen Z, Gouill C, Theriault C, Parent JL, Rola-Pleszczynski M, Stankova J. Trafficking, ubiquitination, and down-regulation of the human platelet-activating factor receptor. J Biol Chem. 2003;278(48):48228–48235. doi: 10.1074/jbc.M304082200. [DOI] [PubMed] [Google Scholar]
- Ferguson SS, Menard L, Barak LS, Koch WJ, Colapietro AM, Caron MG. Role of phosphorylation in agonist-promoted beta 2-adrenergic receptor sequestration. Rescue of a sequestration-defective mutant receptor by beta ARK1. J Biol Chem. 1995;270(42):24782–24789. doi: 10.1074/jbc.270.42.24782. [DOI] [PubMed] [Google Scholar]
- Ferguson SS, Downey WE, 3rd, Colapietro AM, Barak LS, Menard L, Caron MG. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271(5247):363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- Freedman NJ, Lefkowitz RJ. Desensitization of G protein-coupled receptors. Recent Prog Horm Res. 1996;51:319–351. [PubMed] [Google Scholar]
- Goldstein JL, Brown MS, Anderson RG, Russell DW, Schneider WJ. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Hall RA, Ostedgaard LS, Premont RT, Blitzer JT, Rahman N, Welsh MJ, Lefkowitz RJ. A C-terminal motif found in the beta2-adrenergic receptor, P2Y1 receptor and cystic fibrosis transmembrane conductance regulator determines binding to the Na+/H+ exchanger regulatory factor family of PDZ proteins. Proc Natl Acad Sci USA. 1998;95(15):8496–8501. doi: 10.1073/pnas.95.15.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RA, Premont RT, Chow CW, Blitzer JT, Pitcher JA, Claing A, Stoffel RH, Barak LS, Shenolikar S, Weinman EJ, Grinstein S, Lefkowitz RJ. The beta2-adrenergic receptor interacts with the Na+/H+ -exchanger regulatory factor to control Na+/H+ exchange. Nature. 1998;392(6676):626–630. doi: 10.1038/33458. [DOI] [PubMed] [Google Scholar]
- Hall RA, Spurney RF, Premont RT, Rahman N, Blitzer JT, Pitcher JA, Lefkowitz RJ. G protein-coupled receptor kinase 6A phosphorylates the Na(+)/H(+) exchanger regulatory factor via a PDZ domain-mediated interaction. J Biol Chem. 1999;274(34):24328–24334. doi: 10.1074/jbc.274.34.24328. [DOI] [PubMed] [Google Scholar]
- Hammad MM, Kuang YQ, Yan R, Allen H, Dupre DJ. Na+/H+ exchanger regulatory factor-1 is involved in chemokine receptor homodimer CCR5 internalization and signal transduction but does not affect CXCR4 homodimer or CXCR4-CCR5 heterodimer. J Biol Chem. 2010;285(45):34653–34664. doi: 10.1074/jbc.M110.106591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff WP, Caron MG, Lefkowitz RJ. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990;4(11):2881–2889. [PubMed] [Google Scholar]
- Hwang JI, Heo K, Shin KJ, Kim E, Yun C, Ryu SH, Shin HS, Suh PG. Regulation of phospholipase C-beta 3 activity by Na+/H+ exchanger regulatory factor 2. J Biol Chem. 2000;275(22):16632–16637. doi: 10.1074/jbc.M001410200. [DOI] [PubMed] [Google Scholar]
- Ishii S, Shimizu T. Platelet-activating factor (PAF) receptor and genetically engineered PAF receptor mutant mice. Prog Lipid Res. 2000;39(1):41–82. doi: 10.1016/S0163-7827(99)00016-8. [DOI] [PubMed] [Google Scholar]
- Ishii I, Saito E, Izumi T, Ui M, Shimizu T. Agonist-induced sequestration, recycling, and resensitization of platelet-activating factor receptor. Role of cytoplasmic tail phosphorylation in each process. J Biol Chem. 1998;273(16):9878–9885. doi: 10.1074/jbc.273.16.9878. [DOI] [PubMed] [Google Scholar]
- Krueger KM, Daaka Y, Pitcher JA, Lefkowitz RJ. The role of sequestration in G protein-coupled receptor resensitization. Regulation of beta2-adrenergic receptor dephosphorylation by vesicular acidification. J Biol Chem. 1997;272(1):5–8. doi: 10.1074/jbc.272.1.5. [DOI] [PubMed] [Google Scholar]
- Laporte SA, Oakley RH, Zhang J, Holt JA, Ferguson SS, Caron MG, Barak LS. The beta2-adrenergic receptor/betaarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci USA. 1999;96(7):3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte SA, Oakley RH, Holt JA, Barak LS, Caron MG. The interaction of beta-arrestin with the AP-2 adaptor is required for the clustering of beta 2-adrenergic receptor into clathrin-coated pits. J Biol Chem. 2000;275(30):23120–23126. doi: 10.1074/jbc.M002581200. [DOI] [PubMed] [Google Scholar]
- Gouill C, Parent JL, Rola-Pleszczynski M, Stankova J. Structural and functional requirements for agonist-induced internalization of the human platelet-activating factor receptor. J Biol Chem. 1997;272(34):21289–21295. doi: 10.1074/jbc.272.34.21289. [DOI] [PubMed] [Google Scholar]
- Li JG, Chen C, Liu-Chen LY. Ezrin-radixin-moesin-binding phosphoprotein-50/Na+/H+ exchanger regulatory factor (EBP50/NHERF) blocks U50,488H-induced down-regulation of the human kappa opioid receptor by enhancing its recycling rate. J Biol Chem. 2002;277(30):27545–27552. doi: 10.1074/jbc.M200058200. [DOI] [PubMed] [Google Scholar]
- Lin FT, Krueger KM, Kendall HE, Daaka Y, Fredericks ZL, Pitcher JA, Lefkowitz RJ. Clathrin-mediated endocytosis of the beta-adrenergic receptor is regulated by phosphorylation/dephosphorylation of beta-arrestin1. J Biol Chem. 1997;272(49):31051–31057. doi: 10.1074/jbc.272.49.31051. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283(5402):655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- Maudsley S, Zamah AM, Rahman N, Blitzer JT, Luttrell LM, Lefkowitz RJ, Hall RA. Platelet-derived growth factor receptor association with Na(+)/H(+) exchanger regulatory factor potentiates receptor activity. Mol Cell Biol. 2000;20(22):8352–8363. doi: 10.1128/MCB.20.22.8352-8363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin NJ, Banerjee A, Kelher MR, Gamboni-Robertson F, Hamiel C, Sheppard FR, Moore EE, Silliman CC. Platelet-activating factor-induced clathrin-mediated endocytosis requires beta-arrestin-1 recruitment and activation of the p38 MAPK signalosome at the plasma membrane for actin bundle formation. J Immunol. 2006;176(11):7039–7050. doi: 10.4049/jimmunol.176.11.7039. [DOI] [PubMed] [Google Scholar]
- Menard L, Ferguson SS, Barak LS, Bertrand L, Premont RT, Colapietro AM, Lefkowitz RJ, Caron MG. Members of the G protein-coupled receptor kinase family that phosphorylate the beta2-adrenergic receptor facilitate sequestration. Biochemistry. 1996;35(13):4155–4160. doi: 10.1021/bi952961+. [DOI] [PubMed] [Google Scholar]
- Menard L, Ferguson SS, Zhang J, Lin FT, Lefkowitz RJ, Caron MG, Barak LS. Synergistic regulation of beta2-adrenergic receptor sequestration: intracellular complement of beta-adrenergic receptor kinase and beta-arrestin determine kinetics of internalization. Mol Pharmacol. 1997;51(5):800–808. [PubMed] [Google Scholar]
- Mohler PJ, Kreda SM, Boucher RC, Sudol M, Stutts MJ, Milgram SL. Yes-associated protein 65 localizes p62(c-Yes) to the apical compartment of airway epithelia by association with EBP50. J Cell Biol. 1999;147(4):879–890. doi: 10.1083/jcb.147.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer BD, Duhaime M, Shaw C, Denton J, Reynolds D, Karlson KH, Pfeiffer J, Wang S, Mickle JE, Milewski M, Cutting GR, Guggino WB, Li M, Stanton BA. The PDZ-interacting domain of cystic fibrosis transmembrane conductance regulator is required for functional expression in the apical plasma membrane. J Biol Chem. 2000;275(35):27069–27074. doi: 10.1074/jbc.M004951200. [DOI] [PubMed] [Google Scholar]
- Murthy A, Gonzalez-Agosti C, Cordero E, Pinney D, Candia C, Solomon F, Gusella J, Ramesh V. NHE-RF, a regulatory cofactor for Na(+)-H+ exchange, is a common interactor for merlin and ERM (MERM) proteins. J Biol Chem. 1998;273(3):1273–1276. doi: 10.1074/jbc.273.3.1273. [DOI] [PubMed] [Google Scholar]
- Nisar SP, Cunningham M, Saxena K, Pope RJ, Kelly E, Mundell SJ. Arrestin Scaffolds NHERF1 to the P2Y12 Receptor to Regulate Receptor Internalization. J Biol Chem. 2012;287(29):24505–24515. doi: 10.1074/jbc.M112.347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem. 1999;274(45):32248–32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- Parent JL, Gouill CL, Escher E, Rola-Pleszczynski M, Stakova J. Identification of transmembrane domain residues determinant in the structure-function relationship of the human platelet-activating factor receptor by site-directed mutagenesis. J Biol Chem. 1996;271(38):23298–23303. doi: 10.1074/jbc.271.38.23298. [DOI] [PubMed] [Google Scholar]
- Parent JL, Gouill C, Brum-Fernandes AJ, Rola-Pleszczynski M, Stankova J. Mutations of two adjacent amino acids generate inactive and constitutively active forms of the human platelet-activating factor receptor. J Biol Chem. 1996;271(14):7949–7955. doi: 10.1074/jbc.271.14.7949. [DOI] [PubMed] [Google Scholar]
- Parent JL, Gouill C, Rola-Pleszczynski M, Stankova J. Mutation of an aspartate at position 63 in the human platelet-activating factor receptor augments binding affinity but abolishes G-protein-coupling and inositol phosphate production. Biochem Biophys Res Commun. 1996;219(3):968–975. doi: 10.1006/bbrc.1996.0341. [DOI] [PubMed] [Google Scholar]
- Pearse BM, Robinson MS. Clathrin, adaptors, and sorting. Annu Rev Cell Biol. 1990;6:151–171. doi: 10.1146/annurev.cb.06.110190.001055. [DOI] [PubMed] [Google Scholar]
- Perron A, Chen ZG, Gingras D, Dupre DJ, Stankova J, Rola-Pleszczynski M. Agonist-independent desensitization and internalization of the human platelet-activating factor receptor by coumermycin-gyrase B-induced dimerization. J Biol Chem. 2003;278(30):27956–27965. doi: 10.1074/jbc.M212302200. [DOI] [PubMed] [Google Scholar]
- Pippig S, Andexinger S, Lohse MJ. Sequestration and recycling of beta 2-adrenergic receptors permit receptor resensitization. Mol Pharmacol. 1995;47(4):666–676. [PubMed] [Google Scholar]
- Poulat F, Barbara PS, Desclozeaux M, Soullier S, Moniot B, Bonneaud N, Boizet B, Berta P. The human testis determining factor SRY binds a nuclear factor containing PDZ protein interaction domains. J Biol Chem. 1997;272(11):7167–7172. doi: 10.1074/jbc.272.11.7167. [DOI] [PubMed] [Google Scholar]
- Reczek D, Berryman M, Bretscher A. Identification of EBP50: A PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J Cell Biol. 1997;139(1):169–179. doi: 10.1083/jcb.139.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochdi MD, Parent JL. Galphaq-coupled receptor internalization specifically induced by Galphaq signaling. Regulation by EBP50. J Biol Chem. 2003;278(20):17827–17837. doi: 10.1074/jbc.M210319200. [DOI] [PubMed] [Google Scholar]
- Rochdi MD, Watier V, Madeleine C, Nakata H, Kozasa T, Parent JL. Regulation of GTP-binding protein alpha q (Galpha q) signaling by the ezrin-radixin-moesin-binding phosphoprotein-50 (EBP50) J Biol Chem. 2002;277(43):40751–40759. doi: 10.1074/jbc.M207910200. [DOI] [PubMed] [Google Scholar]
- Shenolikar S, Weinman EJ. NHERF: targeting and trafficking membrane proteins. Am J Physiol Renal Physiol. 2001;280(3):F389–F395. doi: 10.1152/ajprenal.2001.280.3.F389. [DOI] [PubMed] [Google Scholar]
- Smythe E, Warren G. The mechanism of receptor-mediated endocytosis. Eur J Biochem. 1991;202(3):689–699. doi: 10.1111/j.1432-1033.1991.tb16424.x. [DOI] [PubMed] [Google Scholar]
- Sneddon WB, Syme CA, Bisello A, Magyar CE, Rochdi MD, Parent JL, Weinman EJ, Abou-Samra AB, Friedman PA. Activation-independent parathyroid hormone receptor internalization is regulated by NHERF1 (EBP50) J Biol Chem. 2003;278(44):43787–43796. doi: 10.1074/jbc.M306019200. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Fanning AS, Fu C, Xu J, Marfatia SM, Chishti AH, Crompton A, Chan AC, Anderson JM, Cantley LC. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science. 1997;275(5296):73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- Sun C, Mierke DF. Characterization of interactions of Na+/H+ exchanger regulatory factor-1 with the parathyroid hormone receptor and phospholipase C. J Pept Res. 2005;65(3):411–417. doi: 10.1111/j.1399-3011.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- Tang Y, Tang J, Chen Z, Trost C, Flockerzi V, Li M, Ramesh V, Zhu MX. Association of mammalian trp4 and phospholipase C isozymes with a PDZ domain-containing protein, NHERF. J Biol Chem. 2000;275(48):37559–37564. doi: 10.1074/jbc.M006635200. [DOI] [PubMed] [Google Scholar]
- Trowbridge IS. Endocytosis and signals for internalization. Curr Opin Cell Biol. 1991;3(4):634–641. doi: 10.1016/0955-0674(91)90034-V. [DOI] [PubMed] [Google Scholar]
- Vogler O, Bogatkewitsch GS, Wriske C, Krummenerl P, Jakobs KH, Koppen CJ. Receptor subtype-specific regulation of muscarinic acetylcholine receptor sequestration by dynamin. Distinct sequestration of m2 receptors. J Biol Chem. 1998;273(20):12155–12160. doi: 10.1074/jbc.273.20.12155. [DOI] [PubMed] [Google Scholar]
- Wang B, Yang Y, Abou-Samra AB, Friedman PA. NHERF1 regulates parathyroid hormone receptor desensitization: interference with beta-arrestin binding. Mol Pharmacol. 2009;75(5):1189–1197. doi: 10.1124/mol.108.054486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinman EJ, Steplock D, Wang Y, Shenolikar S. Characterization of a protein cofactor that mediates protein kinase A regulation of the renal brush border membrane Na(+)-H+ exchanger. J Clin Invest. 1995;95(5):2143–2149. doi: 10.1172/JCI117903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinman EJ, Steplock D, Tate K, Hall RA, Spurney RF, Shenolikar S. Structure-function of recombinant Na/H exchanger regulatory factor (NHE-RF) J Clin Invest. 1998;101(10):2199–2206. doi: 10.1172/JCI204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye RD, Prossnitz ER, Zou AH, Cochrane CG. Characterization of a human cDNA that encodes a functional receptor for platelet activating factor. Biochem Biophys Res Commun. 1991;180(1):105–111. doi: 10.1016/S0006-291X(05)81261-6. [DOI] [PubMed] [Google Scholar]
- Yun CH, Oh S, Zizak M, Steplock D, Tsao S, Tse CM, Weinman EJ, Donowitz M. cAMP-mediated inhibition of the epithelial brush border Na+/H+ exchanger, NHE3, requires an associated regulatory protein. Proc Natl Acad Sci USA. 1997;94(7):3010–3015. doi: 10.1073/pnas.94.7.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Barak LS, Winkler KE, Caron MG, Ferguson SS. A central role for beta-arrestins and clathrin-coated vesicle-mediated endocytosis in beta2-adrenergic receptor resensitization. Differential regulation of receptor resensitization in two distinct cell types. J Biol Chem. 1997;272(43):27005–27014. doi: 10.1074/jbc.272.43.27005. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, Caron MG. Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc Natl Acad Sci USA. 1998;95(12):7157–7162. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]