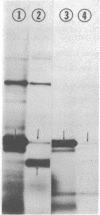
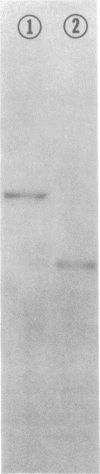
Abstract
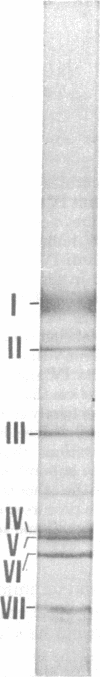
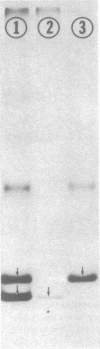
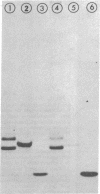
Subunit-specific antisera prepared against each of the four cytoplasmically made subunits (IV, V, VI, and VII) of yeast mitochondrial cytochrome c oxidase (EC 1.9.3.1) were used to precipitate immunoreactive polypeptides that were synthesized either in vitro, in a cell-free protein-synthesizing system programmed with total yeast mRNA, or in vivo, in intact cells and in spheroplasts, under conditions of pulse labeling, pulse-chase labeling, and continuous labeling. Using N-formyl-[35S]Met-rTNA as the only radioactively labeled component in the cell-free system, we demonstrated (i) that each of the four cytoplasmically made subunits is synthesized as a separate entity and not as part of a polyprotein as was claimed by others; (ii) that subunits IV, V, and VI are synthesized as precursors, larger by 1500-3000 daltons than their mature counterparts; in contrast, subunit VII is not synthesized as a larger precursor. Precursor forms of subunits IV, V, and VI identical to those synthesized in vitro were also detected in vivo by pulse-labeling of spheroplasts. The observed disappearance of these larger forms after a chase is compatible with the notion that they represent short-lived precursors that are rapidly converted to their mature counterparts during or shortly after import into mitochondria. Furthermore, using N-formyl-[35S]Met-tRNA, we provide definitive evidence that two of the cytoplasmically made subunits (beta and gamma) of another oligomeric inner mitochondrial membrane protein (F1-ATPase, EC 3.6.1.3) are not synthesized as part of a polyprotein but as individual precursors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boime I., Szczesna E., Smith D. Membrane-dependent cleavage of the human placental lactogen precursor to its native form in ascites cell-free extracts. Eur J Biochem. 1977 Mar 1;73(2):515–520. doi: 10.1111/j.1432-1033.1977.tb11345.x. [DOI] [PubMed] [Google Scholar]
- Bonatti S., Blobel G. Absence of a cleavable signal sequence in Sindbis virus glycoprotein PE2. J Biol Chem. 1979 Dec 25;254(24):12261–12264. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chang C. N., Blobel G., Model P. Detection of prokaryotic signal peptidase in an Escherichia coli membrane fraction: endoproteolytic cleavage of nascent f1 pre-coat protein. Proc Natl Acad Sci U S A. 1978 Jan;75(1):361–365. doi: 10.1073/pnas.75.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy J. G., Kalousek F., Rosenberg L. E. In vitro synthesis of a putative precursor of mitochondrial ornithine transcarbamoylase. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5724–5727. doi: 10.1073/pnas.76.11.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerman H. W., Steers E., Jr, Redfield B. G., Weissbach H. Methionyl soluble ribonucleic acid transformylase. I. Purification and partial characterization. J Biol Chem. 1967 Apr 10;242(7):1522–1525. [PubMed] [Google Scholar]
- Goldman B. M., Blobel G. Biogenesis of peroxisomes: intracellular site of synthesis of catalase and uricase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5066–5070. doi: 10.1073/pnas.75.10.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housman D., Jacobs-Lorena M., Rajbhandary U. L., Lodish H. F. Initiation of haemoglobin synthesis by methionyl-tRNA. Nature. 1970 Aug 29;227(5261):913–918. doi: 10.1038/227913a0. [DOI] [PubMed] [Google Scholar]
- Kellems R. E., Butow R. A. Cytoplasmic-type 80 S ribosomes associated with yeast mitochondria. I. Evidence for ribosome binding sites on yeast mitochondria. J Biol Chem. 1972 Dec 25;247(24):8043–8050. [PubMed] [Google Scholar]
- Korb H., Neupert W. Biogenesis of cytochrome c in Neurospora crassa. Synthesis of apocytochrome c, transfer to mitochondria and conversion to Holocytochrome c. Eur J Biochem. 1978 Nov 15;91(2):609–620. doi: 10.1111/j.1432-1033.1978.tb12714.x. [DOI] [PubMed] [Google Scholar]
- Lin J. J., Kanazawa H., Ozols J., Wu H. C. An Escherichia coli mutant with an amino acid alteration within the signal sequence of outer membrane prolipoprotein. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4891–4895. doi: 10.1073/pnas.75.10.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa V. R., Devillers-Thiery A., Blobel G. Nascent prehormones are intermediates in the biosynthesis of authentic bovine pituitary growth hormone and prolactin. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2432–2436. doi: 10.1073/pnas.74.6.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa V. R., Lingappa J. R., Blobel G. Chicken ovalbumin contains an internal signal sequence. Nature. 1979 Sep 13;281(5727):117–121. doi: 10.1038/281117a0. [DOI] [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Blobel G., Schatz G. Import of proteins into mitochondria: precursor forms of the extramitochondrially made F1-ATPase subunits in yeast. Proc Natl Acad Sci U S A. 1979 Jan;76(1):343–347. doi: 10.1073/pnas.76.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Schatz G. Transport of proteins across the mitochondrial outer membrane. A precursor form of the cytoplasmically made intermembrane enzyme cytochrome c peroxidase. J Biol Chem. 1979 Aug 25;254(16):7468–7471. [PubMed] [Google Scholar]
- Mason T. L., Poyton R. O., Wharton D. C., Schatz G. Cytochrome c oxidase from bakers' yeast. I. Isolation and properties. J Biol Chem. 1973 Feb 25;248(4):1346–1354. [PubMed] [Google Scholar]
- Mori M., Miura S., Tatibana M., Cohen P. P. Cell-free synthesis and processing of a putative precursor for mitochondrial carbamyl phosphate synthetase I of rat liver. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5071–5075. doi: 10.1073/pnas.76.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N., Schatz G. Energy-dependent processing of cytoplasmically made precursors to mitochondrial proteins. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4365–4369. doi: 10.1073/pnas.76.9.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono B. I., Fink G., Schatz G. Mitochondrial assembly in respiration-deficient mutants of Saccharomyces cerevisiae. IV. Effects of nuclear amber suppressors on the accumulation of a mitochondrially made subunit of cytochrome c oxidase. J Biol Chem. 1975 Jan 25;250(2):775–782. [PubMed] [Google Scholar]
- Poyton R. O., Kavanagh J. Regulation of mitochondrial protein synthesis by cytoplasmic proteins. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3947–3951. doi: 10.1073/pnas.73.11.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyton R. O., McKemmie E. A polyprotein precursor to all four cytoplasmically translated subunits of cytochrome c oxidase from Saccharomyces cerevisiae. J Biol Chem. 1979 Jul 25;254(14):6763–6771. [PubMed] [Google Scholar]
- Poyton R. O., McKemmie E. Post-translational processing and transport of the polyprotein precursor to subunits IV to VII of yeast cytochrome c oxidase. J Biol Chem. 1979 Jul 25;254(14):6772–6780. [PubMed] [Google Scholar]
- Poyton R. O., Schatz G. Cytochrome c oxidase from bakers' yeast. III. Physical characterization of isolated subunits and chemical evidence for two different classes of polypeptides. J Biol Chem. 1975 Jan 25;250(2):752–761. [PubMed] [Google Scholar]
- Rosén S. Purification of beef-heart cytochrome c oxidase by hydrophobic interaction chromatography on octyl-Sepharose CL-4B. Biochim Biophys Acta. 1978 Apr 12;523(2):314–320. doi: 10.1016/0005-2744(78)90034-7. [DOI] [PubMed] [Google Scholar]
- Schechter I., Burstein Y., Zemell R., Ziv E., Kantor F., Papermaster D. S. Messenger RNA of opsin from bovine retina: isolation and partial sequence of the in vitro translation product. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2654–2658. doi: 10.1073/pnas.76.6.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields D., Blobel G. Efficient cleavage and segregation of nascent presecretory proteins in a reticulocyte lysate supplemented with microsomal membranes. J Biol Chem. 1978 Jun 10;253(11):3753–3756. [PubMed] [Google Scholar]
- Shore G. C., Carignan P., Raymond Y. In vitro synthesis of a putative precursor to the mitochondrial enzyme, carbamyl phosphate synthetase. J Biol Chem. 1979 May 10;254(9):3141–3144. [PubMed] [Google Scholar]
- Stanley W. M., Jr Specific aminoacylation of the methionine-specific tRNA's of eukaryotes. Methods Enzymol. 1974;29:530–547. doi: 10.1016/0076-6879(74)29049-9. [DOI] [PubMed] [Google Scholar]
- Takeshige K., Hess B., Böhm M., Zimmermann-Telschow H. Mitochondrial adenosine triphosphatase from yeast, Saccharomyces cerevisiae. Purification, subunit structure and kinetics. Hoppe Seylers Z Physiol Chem. 1976 Nov;357(11):1605–1622. doi: 10.1515/bchm2.1976.357.2.1605. [DOI] [PubMed] [Google Scholar]
- Thomas P. E., Korzeniowski D., Ryan D., Levin W. Preparation of monospecific antibodies against two forms of rat liver cytochrome P-450 and quantitation of these antigens in microsomes. Arch Biochem Biophys. 1979 Feb;192(2):524–532. doi: 10.1016/0003-9861(79)90122-x. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A. Assembly of the mitochondrial membrane system. I. Characterization of some enzymes of the inner membrane of yeast mitochondria. J Biol Chem. 1969 Sep 25;244(18):5020–5026. [PubMed] [Google Scholar]
- Zimmerman R., Paluch U., Sprinzl M., Neupert W. Cell-free synthesis of the mitochondrial ADP/ATP carrier protein of Neurospora crassa. Eur J Biochem. 1979 Sep;99(2):247–252. doi: 10.1111/j.1432-1033.1979.tb13251.x. [DOI] [PubMed] [Google Scholar]