Abstract
Background: Complex interplays of diet and metabolism influence circulating fatty acids (FAs), possibly constituting FA patterns related to cardiovascular disease (CVD) risk.
Objectives: We aimed to derive FA patterns from circulating FAs, relate the patterns to CVD incidence, and extend the derived patterns to atherosclerosis progression in another independent cohort.
Design: We used principal component analysis (PCA) to derive FA patterns from 38 plasma phospholipid FAs in 2972 older adults in the Cardiovascular Health Study (CHS). Identified patterns were evaluated for prospective associations with 14-y incidence of CVD [ischemic heart disease (IHD) or stroke]. In another independent cohort of postmenopausal women with IHD, we evaluated associations of the CHS-derived patterns with 3.2-y progression of angiographically defined coronary atherosclerosis.
Results: Three distinct patterns were identified, characterized by higher proportions of trans FAs, de novo lipogenesis (DNL) FAs, and long-chain MUFAs (LCMUFAs). During 32,265 person-years, 780 incident CVD events occurred. The trans FA pattern was associated with higher CVD risk (multivariable-adjusted HR for the highest compared with the lowest quintiles = 1.58; 95% CI: 1.17, 2.12; P-trend = 0.006), primarily attributable to higher risk of stroke (HR: 2.46; 95% CI: 1.54, 3.92; P-trend = 0.005). The DNL and LCMUFA patterns were not associated with CVD incidence or with IHD or stroke (P-trend > 0.11 each). In the second cohort, the trans FA pattern, but not the other 2 patterns, was positively associated with progression of coronary atherosclerosis (P-trend < 0.05).
Conclusions: PCA appears to provide informative circulating FA patterns. A pattern driven mainly by trans FA levels related to greater CVD risk in older adults and coronary atherosclerosis progression in women with IHD.
INTRODUCTION
Several individual fatty acid (FA)4 biomarkers in tissues and blood, eg, biomarkers of PUFAs, trans FAs, or de novo lipogenesis (DNL), have been associated with the risk of cardiovascular disease (CVD) (1–9). Although these studies have contributed substantially to knowledge on etiologic roles of FAs, nearly all such studies have focused on selected individual FAs. However, there are numerous trace or minor circulating FAs (<1 mol%), the concentrations of which are interrelated and reflect complex interplays of dietary consumption and endogenous metabolism. Thus, a conventional approach separately examining major individual FAs may not identify potentially important interplays among different FAs.
Principal component analysis (PCA) is a powerful data-reduction technique to discover novel informative patterns in complex data. For example, PCA has been used to identify novel genetic and dietary patterns (10, 11). By maximizing the information from both major and minor FA and also accounting for previously unrecognized relationships between different dietary and endogenously synthesized FA, PCA may provide novel insights into the effects of circulating FAs on human health and disease (12–14). To identify FA patterns, including evaluation of many trace FAs and isomers of trans FAs that have been relatively understudied, and to determine the relations of such patterns to the 14-y incidence of CVD, we applied PCA to 38 different plasma phospholipid FAs measured in 2972 US adults in the Cardiovascular Health Study (CHS). We validated the findings internally in the CHS by means of split-sample cross-validation. We also extended the investigation to a separate cohort, the Estrogen Replacement and Atherosclerosis trial (ERA), to assess whether the CHS-derived FA patterns related to the progression of coronary atherosclerosis measured by serial quantitative coronary angiography.
SUBJECTS AND METHODS
Design and population
The CHS is a prospective cohort study among older adults (15, 16). Briefly, 5201 ambulatory, noninstitutionalized adults older than 65 y of age were randomly selected and enrolled in 1989–1990 and an additional 687 African American adults in 1992 from Medicare eligibility lists in 4 US communities (Forsyth County, NC; Sacramento County, CA; Washington County, MD; Allegheny County, PA). Of all eligible adults contacted, 53% agreed to enroll. Participants received clinical examinations annually for 10 y with interim 6-mo telephone contacts and subsequent 6-mo telephone contacts thereafter. Each center's institutional review committee approved the study; all participants provided informed written consent.
Stored blood was available for FA measurements from the 1992 study-clinic visit, considered baseline for all current analyses. Of 5565 participants alive in 1992, plasma phospholipid FAs were measured in 3941 participants (71%), including 656 participants from a prior nested case-control study (2, 3) and 3285 participants randomly selected from those with available blood samples. These individuals were not randomly sampled from all CHS participants; thus, our analyses accounted for the sampling probability by inverse probability weighting. For the current analysis, we evaluated the 2972 participants after the exclusion of 969 participants with prevalent ischemic heart disease (IHD) or stroke at baseline.
Phospholipid FA analysis
Blood samples were drawn after the subjects had fasted for ≥8 h, stored at −70°C, and shipped to the central CHS laboratory for long-term storage at −80°C. Our studies and others have documented the reliability of FA assessments after long-term storage (2, 3, 17–19). Methods for the assessment of plasma phospholipid FAs have been described previously in detail (2, 3). Briefly, plasma lipids were extracted according to Folch et al (20). The phospholipid fractions were isolated by thin-layer chromatography (silica, solvent of hexane:ether:acetate = 67.5:15:0.75 with 0.005% dibutyl-hydroxy-toluene). Then, phospholipid FA moieties were transmethylated and separated by gas chromatography (100 m × 0.25 mm silica column, model 6890; Agilent Technologies Inc). After the separation, 42 known peaks were identified. Of those, we excluded 3 FAs (trans,trans-18:2n−6, cis-14:1n−5, and cis-18:1n−8) because of identified laboratory drift and one trace FA (22:5n−6) that was only measured in the latter half of the FA samples. In total, 38 FAs were quantified as a percentage of total phospholipid FAs and evaluated in this study. Laboratory CVs were <3% for major FAs, including 16:0, 18:1n−9, 18:2n−6, 20:4n−6, and 22:6n−3 (DHA) and 2–10% for minor FAs. We also performed serial measurements of FA levels in a subset of 100 participants, by using stored blood samples drawn in 1992–1993, 1998–1999, and 2005–2006.
Derivation of fatty acid patterns
We used PCA to identify a finite number of novel FA patterns that maximized the variation in the levels and interrelations of 38 individual FAs. PCA is a dimension reduction technique that generates new variables as principal components (patterns) to explain as much variability as possible of numerous original variables (21). We determined the number of patterns to generate according to a scree plot that indicates the additional variability explained by derivation of each additional pattern. When deriving FA patterns, we applied oblique rotation, which is commonly done to obtain pattern structures with greater interpretability than crude nonrotated pattern structures (21). A score for each pattern was calculated for each participant, characterizing the consistency of their FA profile with that pattern, based on a linear combination of the PCA-derived weights for each FA multiplied by the levels of that FA. Factor loading was used as a measure of contribution of each FA to each pattern. We named each pattern based on the major contributing FA, taking into account known dietary sources and the FA metabolism of various individual FAs, including trans, omega-3 (n−3), dairy products, and DNL-related FAs (1, 7, 9, 22–27). In addition, we examined the variability of each individual FA explained by the FA patterns. We calculated the R2 value for each FA by linear regression analysis, regressing each individual FA on all of the FA patterns. Similarly, we calculated R2 for each FA explained by each FA pattern.
To examine the reproducibility of the FA patterns over the follow-up, we evaluated 6-y and 13-y reproducibilities of the baseline FA patterns by using serial measures of FA levels in a subset of participants (n = 100). We assigned scoring weights of the baseline FA patterns to FA levels assessed repeatedly during the follow-up to determine the pattern scores at each follow-up time point and then calculated the intraclass correlations of the pattern scores over time as a measure of reproducibility.
Assessment of risk factors and covariates
At the baseline and annual follow-up examinations, participants completed standardized questionnaires and interviews to assess demographic characteristics, CVD risk factors, lifestyle risk factors, and medications and underwent detailed in-clinic examinations (16, 28, 29). Fasting concentrations of total cholesterol, HDL cholesterol, and triglycerides were measured by standardized methods (30). Concentrations of LDL cholesterol were derived by using the Friedewald formula (31). Leisure-time activity (kcal/wk) was assessed by using a modified Minnesota Leisure-Time Activities questionnaire (32). Dietary habits were assessed with validated food-frequency questionnaires in 1989–1990 (33, 34).
Ascertainment of cardiovascular disease outcomes
We evaluated CVD (IHD death, fatal or nonfatal myocardial infarction, or stroke) as the primary outcome, and IHD and stroke were evaluated separately as secondary outcomes. Events were identified from annual examinations through 1999, interim 6-mo phone interviews through 2006, hospital discharge records (obtained for every hospitalization), death certificates, and medical examiner forms (28). All identified incident CVD events were adjudicated by the CHS morbidity and mortality committee by review of all available records, including available electrocardiogram, computed tomographic scans, or magnetic resonance images. Myocardial infarction was based on typical chest pain with abnormal cardiac enzyme concentrations or serial electrocardiogram changes. Fatal IHD additionally included deaths not meeting criteria for definite myocardial infarction when participants had chest pain <72 h before death or documented IHD history. Stroke was defined as a neurologic deficit of rapid onset lasting for >24 h unless death supervened or as a subarachnoid hemorrhage. Strokes were classified as 1) ischemic if there was evidence of focal brain deficit without evidence of primary hemorrhage; 2) hemorrhagic if there was bloody spinal fluid on lumbar puncture or evidence of blood in the subarachnoid space, ventricles, or parenchyma on cerebral imaging or at surgery or autopsy that did not appear consistent with hemorrhage into an infarction; or 3) unknown type if information was insufficient for classification (35).
Extension of the CHS-derived FA patterns to a second independent cohort
Because generalizability is a potential limitation of PCA, we extended our investigation of the CHS-derived FA patterns to a separate independent cohort. The study design of the ERA was described previously (5, 36, 37). Briefly, serial quantitative coronary angiography was performed between 1996 and 2000 in 1912 major epicardial coronary arteries (8.3 segments per person) in 228 postmenopausal women with established IHD in whom FA biomarkers were measured at baseline. These measurements allow quantitative evaluation of coronary atherosclerosis progression, including changes in mean minimal arterial diameter and percentage of coronary artery stenosis (36–41). This design incorporates measures from multiple coronary segments to provide substantial statistical power to detect atherosclerosis progression.
In the ERA, 27 individual FAs were measured in 3 different lipid compartments: phospholipids, cholesteryl esters, and triglycerides (5). We primarily evaluated phospholipid FAs because 1) CHS also assessed phospholipid FAs, 2) circulating phospholipid FAs reflect tissue membrane concentrations, and 3) phospholipid FAs have been frequently used in long-term epidemiologic research (22). We also evaluated cholesteryl ester and triglyceride fractions as additionally available samples to assess the generalizability of the findings for phospholipid FAs in the ERA to these other circulating lipid compartments.
Statistical analyses
Cox proportional hazard models were used to estimate HRs and their 95% CIs for longitudinal relations of each FA pattern with incident CVD. In the model, a time-scale variable was the duration between FA measurement and the first clinical CVD events or censoring. When age was used as the variable (42), the results were similar (data not shown). FA patterns were evaluated categorically by indicator variables of quartile groups and continuously by a single continuous term and by restricted cubic splines. We tested the trend across the quartile categories by assigning each participant the median value in their quartile group of FA pattern score and then analyzing this as a continuous variable. Potential confounders were included in the models based on biological relevance as factors that might influence exposures and outcomes, previously published associations with CVD, or associations between exposures and outcomes in the current data set. Identified FA patterns were also included in the model simultaneously to determine independent associations. For parsimony, other covariates not materially altering the relations of FA patterns with CVD outcomes (<5% of the HR by including each covariate) were excluded from the final model, eg, including aspirin use, lipid-lowering drugs, and estrogen use. We also evaluated multivariable-adjusted partial Pearson correlations of FA patterns with concentrations of HDL cholesterol, LDL cholesterol, and triglycerides and whether further adjustment for these blood lipids altered the associations of the FA patterns with incident CVD.
Changes over time in both exposures and time-varying covariates can cause regression dilution bias and residual confounding. To account for misclassification and bias as a result of temporal variations in FA patterns and major covariates (including dietary factors and physical activity), we performed multivariate regression calibration using serial measures of each of these variables in a subset of the participants (43, 44). Calculated regression dilution ratios were used to estimate the multivariable-adjusted HRs according to “usual” levels of FA patterns and covariates.
Potential effect modification was evaluated in subgroups of age (≥ or < median), sex, race (white or nonwhite), BMI (≥ or < median), and diabetes (yes or no). To evaluate statistical significance, we constructed a Wald test comparing the 2 regression β coefficients and their variances separately estimated from stratified analyses by each of these subgroups.
We performed several sensitivity analyses to assess the reproducibility of our findings. First, we separately derived the FA patterns in specific subgroups to determine whether similar patterns would be derived and whether similar longitudinal associations with disease would be observed in different subgroups. Second, we performed cross-validation within the CHS, in which we first derived the FA patterns in one subgroup (derivation subgroup) and then applied these derived patterns to another subgroup (validation subgroup) to determine the relations with disease, which provides evidence for reproducibility of findings when FA patterns were derived from independent individuals. Third, we assessed whether our findings were sensitive to the number of FA patterns constructed from PCA, testing between 4 and 10 patterns. Finally, we rederived FA patterns by PCA after first aggregating highly correlated FAs (r > 0.8) to ensure that the main findings were not driven by a handful of highly intercorrelated individual FAs (45).
To extend the FA patterns identified in the CHS to the ERA, we assigned the CHS-derived PCA scoring weights to each individual measured FA of each lipid compartment in the ERA to calculate each participant's pattern scores. Because only 27 FAs were assessed in the ERA, compared with 38 in the CHS, the missing data could cause misclassification of the FA patterns in the ERA, attenuating the results toward the null. We estimated the potential magnitude of this bias by applying the FA patterns derived from 38 FAs back to the CHS cohort but using only the same 27 FAs available in the ERA. The full (38 FAs) and reduced (27 FAs) patterns were highly intercorrelated in the CHS (r = 0.81–0.97) and each similarly predicted incident CVD in the CHS (data not shown). To examine the prospective associations of the CHS-derived FA patterns with progression of coronary atherosclerosis in the ERA, we used multivariable-adjusted mixed models, modeling within-individual correlations between atherosclerosis progression of multiple coronary segments. Interactions by age, race, BMI, and diabetes were assessed by using a likelihood-ratio test for nested regression models with and without a cross-product term of each FA pattern and the potential effect-modifier. Statistical analyses were performed by using STATA 10.0 (StataCorp) and SAS 9.1 (2-tailed α = 0.05; SAS Institute).
RESULTS
At baseline, CHS participants were aged 72.1 ± 5.1 y (mean ± SD), and slightly more than one-half were women (Table 1). More than one-half were overweight or obese (mean BMI: 26.7), and about one-half were medically treated for high blood pressure. The ERA participants were all women with established IHD. Compared with the ERA participants, the CHS participants were less likely to be current smokers (8% compared with 22%), patients with diabetes (15% compared with 57%), or users of antihypertensive drugs (48% compared with 96%) or cholesterol-lowering drugs (6% compared with 64%) and more likely to have higher HDL-cholesterol (1.4 compared with 1.1 mmol/L) and lower fasting glucose (5.8 compared with 6.6 mmol/L) and lower triglyceride (1.6 compared with 2.2 mmol/L) concentrations.
TABLE 1.
Baseline characteristics of participants in the Cardiovascular Health Study and the Estrogen Replacement and Atherosclerosis trial
| Characteristics | Cardiovascular Health Study (n = 2972) | Estrogen Replacement and Atherosclerosis trial (n = 1912) |
| Age (y) | 72.1 ± 5.11 | 65.4 ± 7.2 |
| White (%) | 88 | 83 |
| Male sex (%) | 40 | 0 |
| Education, some college or higher (%) | 45 | 21 |
| Income ≥$25,000/y (%) | 42 | —2 |
| Current smokers (%) | 8 | 22 |
| Former smokers (%) | 42 | 19 |
| Estrogen ever-users, women only (%) | 35 | 03 |
| Aspirin use (%) | 39 | 26 |
| Hypertension medication use (%) | 48 | 96 |
| Cholesterol-lowering drug use (%) | 6 | 64 |
| Type 2 diabetes (%) | 15 | 57 |
| Ischemic heart disease (%) | 03 | 1003 |
| Mean of coronary stenosis (%)4 | —2 | 28.2 ± 8.2 |
| Mean of coronary minimal diameters (mm)4 | —2 | 1.9 ± 0.3 |
| BMI (kg/m2) | 26.7 ± 4.6 | 29.9 ± 7.9 |
| Waist circumference (cm) | 97.0 ± 13.0 | 93.4 ± 15.4 |
| Systolic blood pressure (mm Hg) | 136 ± 21 | 134 ± 18 |
| Diastolic blood pressure (mm Hg) | 71 ± 11 | 74 ± 8 |
| Total cholesterol (mmol/L) | 5.4 ± 1.0 | 5.3 ± 1.0 |
| HDL cholesterol (mmol/L) | 1.4 ± 0.4 | 1.1 ± 0.3 |
| Triglycerides (mmol/L) | 1.6 ± 0.9 | 2.2 ± 1.2 |
| Glucose (mmol/L) | 5.8 ± 1.6 | 6.6 ± 2.4 |
| Leisure-time physical activity (kcal/wk) | 1063 ± 1450 | —2 |
| Walking (frequency/wk) | —2 | 3.0 ± 2.1 |
| Alcohol drinkers (%) | 46 | 18 |
| Total energy intake (kcal/d) | 2024 ± 648 | 1649 ± 575 |
| Fiber intake (g/1000 kcal/d) | 7.4 ± 2.7 | 12.4 ± 6.2 |
| Total fat intake (% of energy) | 33.6 ± 7.2 | 27.5 ± 6.7 |
| SFA intake (% of energy) | 11.8 ± 3.1 | 9.1 ± 2.6 |
| trans Fatty acid intake (% of energy) | 1.2 ± 0.4 | 1.1 ± 0.5 |
Mean ± SD (all such values).
Not assessed.
In the Cardiovascular Health Study, participants free of cardiovascular disease were identified to examine incident cardiovascular disease. In the Estrogen Replacement and Atherosclerosis trial, postmenopausal women with established heart disease and without use of estrogen replacement therapy were recruited to examine their atherosclerosis progression of multiple coronary segments.
Coronary stenosis (% of narrowing) and minimal diameters of multiple coronary segments (8.3 on average) were assessed by coronary angiography. Between-individual means (±SDs) were calculated from multiple coronary segments.
Through use of PCA for 38 individual circulating FAs in the CHS, we identified 3 novel FA patterns; greater numbers of patterns added progressively less information (see Supplementary Figure 1 under “Supplemental data” in the online issue). On the basis of the major contributors to each pattern (Table 2), we characterized the first FA pattern as a trans FA pattern, with high factor loading for trans-isomers of 18:1 (factor loading >0.86), trans-isomers of 18:2n−6 (0.61 for cis,trans-18:2n−6 and 0.60 for trans,cis-18:2n−6), and trans-16:1n−9 (0.82). We characterized the second FA pattern as a DNL pattern, with high factor loading (0.55–0.85) for 14:0, 16:0, cis-16:1n−7, cis-16:1n−9, and cis-18:1n−9. This pattern also had negative factor loading (−0.75 to −0.40) for long-chain SFAs, including 18:0, 20:0, 22:0, and 24:0. We characterized the third pattern as a long-chain MUFA (LCMUFA) pattern, with high factor loading for cis-16:1n−9, cis-17:1n−9, cis-18:1n−7, 20:1, 22:1, and 24:1 (0.43–0.75). This pattern also had moderately high factor loading for dairy FAs (0.35–0.37 for 15:0, 17:0, and trans-16:1n−7). Major dietary omega-3 PUFAs were not strongly contributory to any of the 3 patterns, with only modest factor loading in the trans FA pattern (−0.24 for 20:5n−3 and −0.25 for 22:6n−3), DNL pattern (0.17 for 18:3n−3), and LCMUFA pattern (0.36 for 22:6n−3). The 3 FA patterns captured 6–89% of the variability of each individual FA (Table 2), including, for example, a total R2 of 16–26% for long-chain n−3 FAs, 86% for 18:2n–6 (linoleic acid), and 23–89% for individual trans FAs.
TABLE 2.
Associations between 38 fatty acids and 3 principal components identified in the Cardiovascular Health Study (n = 2972)1
| Component 1: trans fatty acidfactor loading (R2)2 | Component 2: de novo lipogenesis factor loading (R2)2 | Component 3: LCMUFAfactor loading (R2)2 | Total variation explained by all 3 patterns (R2)3 | |
| 14:0 | −0.17 (0.02) | 0.55 (0.30)* | 0.16 (0.00) | 0.32 |
| 15:0 | 0.01 (0.00) | 0.17 (0.01) | 0.35 (0.08) | 0.11 |
| 16:0 | −0.51 (0.23)* | 0.60 (0.42)* | −0.10 (0.02) | 0.64 |
| 17:0 | 0.02 (0.00) | −0.40 (0.20)* | 0.37 (0.24) | 0.66 |
| 18:0 | −0.13 (0.02) | −0.40 (0.15)* | −0.31 (0.05) | 0.20 |
| cis-16:1n−7 | −0.18 (0.04) | 0.85 (0.74)* | −0.02 (0.02) | 0.29 |
| cis-16:1n−9 | 0.08 (0.00) | 0.46 (0.14)* | 0.43 (0.08)* | 0.78 |
| cis-17:1n−9 | 0.02 (0.01) | −0.11 (0.05) | 0.46 (0.21)* | 0.37 |
| cis-18:1n−5 | 0.87 (0.76)* | 0.04 (0.00) | −0.02 (0.02) | 0.23 |
| cis-18:1n−7 | 0.30 (0.05) | 0.34 (0.06) | 0.45 (0.21)* | 0.28 |
| cis-18:1n−9 | 0.05 (0.00) | 0.68 (0.45)* | 0.10 (0.00) | 0.84 |
| cis, cis-18:2n−6 | 0.13 (0.13) | −0.35 (0.09) | −0.12 (0.01) | 0.86 |
| 18:3n−6 | 0.05 (0.00) | 0.36 (0.18) | −0.13 (0.09) | 0.86 |
| 18:3n−3 | −0.15 (0.00) | 0.17 (0.07) | −0.09 (0.00) | 0.89 |
| trans-16:1n−7 | 0.14 (0.02) | −0.21 (0.07) | 0.35 (0.14) | 0.89 |
| trans-16:1n−9 | 0.82 (0.60)* | −0.01 (0.01) | 0.26 (0.02) | 0.46 |
| trans-18:1n−6 | 0.94 (0.87)* | −0.14 (0.03) | 0.00 (0.01) | 0.42 |
| trans-18:1n−7 | 0.87 (0.81)* | −0.30 (0.10) | −0.06 (0.01) | 0.76 |
| trans-18:1n−8 | 0.93 (0.82)* | −0.15 (0.04) | 0.08 (0.00) | 0.68 |
| trans-18:1n−9 | 0.92 (0.86)* | −0.03 (0.00) | −0.08 (0.02) | 0.54 |
| trans-18:1n−10,12 | 0.93 (0.84)* | 0.01 (0.00) | 0.01 (0.02) | 0.23 |
| cis,trans-18:2n−6 | 0.61 (0.54)* | 0.21 (0.08) | −0.30 (0.13) | 0.46 |
| trans,cis-18:2n−6 | 0.60 (0.42)* | 0.27 (0.08) | −0.17 (0.09) | 0.23 |
| 20:0 | −0.09 (0.01) | −0.60 (0.40)* | 0.21 (0.13) | 0.27 |
| 22:0 | 0.09 (0.02) | −0.75 (0.55)* | −0.04 (0.00) | 0.07 |
| 24:0 | −0.07 (0.00) | −0.67 (0.44)* | −0.04 (0.00) | 0.11 |
| 20:1n−9 | 0.06 (0.01) | 0.05 (0.00) | 0.50 (0.25)* | 0.58 |
| 22:1n−9 | 0.04 (0.02) | 0.09 (0.01) | 0.75 (0.50)* | 0.28 |
| 24:1n−9 | −0.20 (0.07) | −0.19 (0.09) | 0.59 (0.50)* | 0.50 |
| 20:2n−6 | 0.05 (0.05) | 0.17 (0.05) | −0.09 (0.01) | 0.35 |
| 20:3n−6 | −0.16 (0.01) | 0.35 (0.16) | −0.30 (0.15) | 0.09 |
| 20:4n−6 | −0.04 (0.08) | −0.06 (0.01) | −0.03 (0.00) | 0.18 |
| 22:2n−6 | −0.01 (0.00) | −0.15 (0.05) | 0.25 (0.16) | 0.44 |
| 22:4n−6 | 0.19 (0.00) | 0.21 (0.07) | −0.34 (0.12) | 0.18 |
| 20:3n−3 | 0.01 (0.00) | 0.03 (0.01) | 0.58 (0.34)* | 0.57 |
| 20:5n−3 | −0.24 (0.09) | 0.17 (0.01) | 0.28 (0.08) | 0.16 |
| 22:5n−3 | 0.01 (0.03) | 0.12 (0.00) | 0.22 (0.03) | 0.06 |
| 22:6n−3 | −0.25 (0.09) | −0.10 (0.02) | 0.36 (0.19) | 0.26 |
Values are factor loading (R2) as measures of associations between each fatty acid pattern and each fatty acid. *Higher or lower factor loading (>0.4 or <−0.4, as commonly used), indicating fatty acids driving the pattern. LCMUFA, long-chain MUFA.
The derived components were labeled on the basis of our interpretation of the factor loading values. R2 in parentheses is interpreted as the proportion of variance of each fatty acid explained by each principal component.
Total R2 is interpreted as the proportion of variance of each fatty acid explained by all 3 principal components. In total, the 3 principal components explained 43.7% of total fatty acid variances.
On the basis of serial FA measurements in a subset of participants, we examined the reproducibility of the 3 FA patterns over time. The intraclass correlations at 6 and 13 y, respectively, were 0.65 and 0.56 for the trans FA pattern, 0.71 and 0.58 for the DNL pattern, and 0.53 and 0.41 for the LCMUFA pattern.
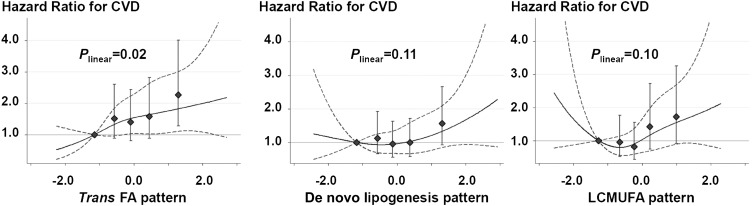
After multivariable adjustment for potential confounders, the trans FA pattern (P = 0.02), but not the other 2 FA patterns (P ≥ 0.1), was positively associated with risk of incident CVD (Figure 1). Compared with participants in the lowest quintile, participants in the highest quintile of the trans FA pattern score experienced a 53% higher risk of CVD (HR: 1.53; 95% CI: 1.14, 2.06; P-trend = 0.010). In similar analyses, a significantly higher risk was not seen with the DNL pattern (HR: 1.27; 95% CI: 0.96, 1.69; P-trend = 0.11) or the LCMUFA pattern (HR: 1.27; 95% CI: 0.96, 1.70; P-trend = 0.09). When we evaluated continuously across the interdecile range (90th to 10th percentiles), we observed similar findings for the trans FA pattern (HR: 1.29; 95% CI: 1.06, 1.57), DNL pattern (HR: 1.22; 95% CI: 0.96, 1.56), and LCMUFA pattern (HR: 1.18; 95% CI: 0.93, 1.48). Evaluated semiparametrically, the evidence of nonlinear associations between the 3 FA patterns and CVD risk was not significant (Figure 1).
FIGURE 1.
Multivariable-adjusted associations of plasma phospholipid FA patterns with risk of CVD in the Cardiovascular Health Study (n = 2972). During the 32,265 person-years, 780 CVD events occurred (incident rate = 2417/100,000). Each panel shows results from 2 separate Cox proportional hazard models by using the restrictive cubic spline (solid line) or using quintile categories (◆); dotted lines and error bars represent 95% CIs. P values for linear associations are presented. None of the patterns showed significant nonlinear associations (P-nonlinearity > 0.05). Cox proportional hazard model was adjusted for sex, race (white or nonwhite), education (<high school, high school graduate, college graduate, or >college), smoking status (never, former, or current), exercise intensity (no exercise, low, moderate, or high), leisure-time physical activity (kcal/wk), 3-y weight change (kg), prevalent diabetes (yes or no), treated hypertension (yes or no), alcohol intake (tertile categories), total energy intake (kcal/d), and food consumptions (fish, high-fat dairy products, margarine, sweets, nuts, fried products, meats, and processed meats; servings/wk each). The 3 patterns were also mutually adjusted. Estimates were corrected for regression dilution bias and consequently strengthened from the estimates (see the main text) without the bias correction. CVD, cardiovascular disease; FA, fatty acid; LCMUFA, long-chain MUFA.
The trans FA pattern was not correlated with LDL cholesterol or HDL cholesterol concentrations [partial Pearson correlation (r) = −0.01 and 0.00, respectively] and weakly correlated with lower triglyceride concentrations (r = −0.06, P < 0.001). The DNL and LCMUFA patterns were each modestly correlated with lower LDL cholesterol (r = −0.27 and −0.10, respectively) and higher HDL cholesterol (r = 0.11 for both) but correlated in different directions with triglycerides (r = 0.25 and −0.35, respectively; P < 0.001 each). Further adjustment for LDL cholesterol, HDL cholesterol, and triglycerides in the multivariable model had no appreciable effect on the relation of each FA pattern with incident CVD (data not shown).
After multivariable-correction for temporal changes in FA and time-varying covariates such as diet and physical activity, the magnitude of the association of the trans FA pattern with CVD was strengthened, with an HR of 2.26 (95% CI: 1.26, 4.05) compared with 1.53 (95% CI: 1.14, 2.06) without such correction in a comparison between participants in quintiles 5 and 1.
When we evaluated IHD and stroke separately, the positive association of the trans FA pattern with incident CVD was primarily a result of a higher risk of incident stroke (Table 3). After adjustment for potential confounders, the trans FA pattern was positively associated with incident stroke (HR: 2.22; 95% CI: 1.41, 3.48) in a comparison between participants in quintiles 5 and 1 (P-trend = 0.001). In contrast, the trans FA was not associated with incident IHD (HR: 1.04; 95% CI: 0.72, 1.50; P-trend = 0.93). Neither the DNL nor the LCMUFA pattern was associated with incident IHD or stroke evaluated separately (Table 3). In restricted cubic spline analyses, we found no significant evidence of nonlinear association between each FA pattern and IHD or stroke (P-nonlinearity > 0.05).
TABLE 3.
Multivariable-adjusted associations of fatty acid patterns with 14-y risks of coronary heart disease and stroke in the Cardiovascular Health Study (n = 2972)1
| Quintile of each fatty acid pattern score |
||||||
| 1 | 2 | 3 | 4 | 5 | P-trend | |
| Ischemic heart disease incidence | ||||||
| trans Fatty acid pattern | ||||||
| Cases (rate/1000 person-years) | 98 (15.1) | 112 (17) | 105 (15.6) | 96 (14.3) | 101 (15.4) | |
| Multivariable-adjusted HR (95% CI)2 | 1.0 (reference) | 1.14 (0.81, 1.58) | 1.02 (0.71, 1.46) | 1.02 (0.70, 1.48) | 1.04 (0.72, 1.50) | 0.93 |
| + corrected for regression-dilution bias3 | 1.0 (reference) | 1.04 (0.54, 2.00) | 1.00 (0.49, 2.05) | 1.01 (0.49, 2.08) | 1.01 (0.49, 2.08) | 0.99 |
| De novo lipogenesis pattern | ||||||
| Cases (rate/1000 person-years) | 89 (13.6) | 100 (14.6) | 106 (16.1) | 122 (18.5) | 95 (14.5) | |
| Multivariable-adjusted HR (95% CI)2 | 1.0 (reference) | 0.99 (0.70, 1.41) | 1.07 (0.77, 1.50) | 1.19 (0.85, 1.66) | 1.08 (0.76, 1.53) | 0.48 |
| + Corrected for regression-dilution bias3 | 1.0 (reference) | 0.98 (0.53, 1.84) | 1.14 (0.63, 2.09) | 1.40 (0.76, 2.57) | 1.16 (0.62, 2.16) | 0.43 |
| LCMUFA pattern | ||||||
| Cases (rate/1000 person-years) | 87 (12.5) | 92 (13.4) | 98 (14.6) | 97 (15.2) | 138 (22.3) | |
| Multivariable-adjusted HR (95% CI)2 | 1.0 (reference) | 0.98 (0.69, 1.37) | 0.89 (0.61, 1.28) | 1.08 (0.75, 1.57) | 1.34 (0.93, 1.93) | 0.06 |
| + Corrected for regression-dilution bias3 | 1.0 (reference) | 0.94 (0.42, 2.12) | 0.75 (0.31, 1.78) | 1.21 (0.51, 2.89) | 1.99 (0.84, 4.74) | 0.06 |
| Stroke incidence | ||||||
| trans Fatty acid pattern | ||||||
| Cases (rate/1000 person-years) | 56 (8.6) | 61 (9.0) | 60 (8.8) | 75 (11.2) | 94 (14.4) | |
| Multivariable-adjusted HR (95% CI)2 | 1.0 (reference) | 1.52 (0.97, 2.40) | 1.39 (0.90, 2.16) | 1.70 (1.08, 2.67) | 2.22 (1.41, 3.48) | 0.001 |
| + Corrected for regression-dilution bias3 | 1.0 (reference) | 2.28 (0.94, 5.54) | 1.91 (0.81, 4.51) | 2.81 (1.16, 6.82) | 4.74 (1.95, 11.5) | 0.001 |
| De novo lipogenesis pattern | ||||||
| Cases (rate/1000 person-years) | 67 (10.2) | 70 (10.3) | 66 (9.9) | 66 (9.8) | 77 (11.7) | |
| Multivariable-adjusted HR (95% CI)2 | 1.0 (reference) | 1.11 (0.72, 1.71) | 0.77 (0.48, 1.23) | 0.80 (0.51, 1.26) | 1.31 (0.85, 2.01) | 0.33 |
| + Corrected for regression-dilution bias3 | 1.0 (reference) | 1.19 (0.55, 2.58) | 0.64 (0.28, 1.46) | 0.68 (0.31, 1.53) | 1.59 (0.74, 3.42) | 0.34 |
| LCMUFA pattern | ||||||
| Cases (rate/1000 person-years) | 74 (10.7) | 56 (8.2) | 64 (9.5) | 90 (14.2) | 62 (9.6) | |
| Multivariable-adjusted HR (95% CI)2 | 1.0 (reference) | 0.93 (0.62, 1.39) | 0.91 (0.60, 1.39) | 1.27 (0.84, 1.90) | 1.08 (0.70, 1.68) | 0.41 |
| + Corrected for regression-dilution bias3 | 1.0 (reference) | 0.90 (0.35, 2.32) | 0.88 (0.33, 2.34) | 1.41 (0.54, 3.69) | 1.12 (0.40, 3.15) | 0.61 |
LCMUFA, long-chain MUFA.
Values were derived by Cox regression analysis. The model was adjusted for age, sex, race (white or nonwhite), education (<high school, high school graduate, college graduate, or >college), smoking status (never, former, or current), exercise intensity (no exercise, low, moderate, or high), leisure-time physical activity (kcal/wk), 3-y weight change (kg), prevalent diabetes (yes or no), treated hypertension (yes or no), alcohol intake (tertile categories), total energy intake (kcal/d), and food consumptions (fish, high-fat dairy products, margarine, sweets, nuts, fried products, meats, and processed meats; servings/wk each). The 3 patterns were also mutually adjusted.
Regression calibration was applied to correct the estimates for bias as a result of temporal variation of the exposures (3 fatty acid patterns) and selected covariates (physical activity and dietary consumptions) after no significant nonlinear association was confirmed. The assumption of a linear association was met for each association (P-nonlinearity > 0.10) according to restricted cubic spline analyses that allowed a test for a nonlinear association.
Interactions for the prespecified factors for the associations of FA patterns with incident CVD were not significant (P > 0.10); the associations did not vary across subgroups of age (≥ or < median), sex, race (white or nonwhite), BMI (≥ or < median), or diabetes (yes or no) (see Supplementary Table 1 under “Supplemental data” in the online issue), although power may have been limited to confirm statistical interactions. The DNL pattern was associated with significantly higher CVD risk among white participants (HR for the interdecile range: 1.38; 95% CI: 1.06, 1.79), and the LCMUFA pattern was among oldest participants (1.45; 1.04, 2.02), women (1.38; 1.04, 1.84), white participants (1.36; 1.04, 1.77), and those with lower BMI (1.57; 1.12, 2.20), but none of these exploratory interactions were statistically significant.
When PCA was separately derived and evaluated in relation to incident CVD in subgroups of age, sex, race, BMI, and diabetes, similar FA patterns were derived (r > 0.95 with the original patterns), and similar associations with CVD were observed (data not shown). In cross-validation analyses, we derived FA patterns in one subgroup (eg, younger participants) and applied it to the other subgroup (eg, older participants) and vice versa (see Supplementary Table 2 under “Supplemental data” in the online issue). Each subgroup-derived pattern, which was then applied to the other subgroup, was still highly correlated (r ≥ 0.90) with the original pattern derived in the full cohort, and the associations of these cross-validation patterns with CVD were also broadly consistent with the results from the full cohort.
If we constructed up to 10 FA patterns, the first 3 major FA patterns were consistently derived (r > 0.85 with the original patterns) and similarly associated with incident CVD (data not shown). When we aggregated FAs that were highly correlated (pairwise correlation > 0.8, which resulted in 33 rather than 38 FAs), the derived FA patterns were correlated with the original 3 patterns (r > 0.85) and were similarly associated with incident CVD (data not shown).
When we extended the CHS-derived FA patterns to the ERA cohort, the trans FA pattern in both phospholipids and cholesteryl esters was associated with significantly greater progression of coronary stenosis (Table 4). Neither the DNL nor LCMUFA pattern was significantly associated with progression of atherosclerosis (see Supplementary Table 3 under “Supplemental data” in the online issue). These associations in the ERA did not significantly vary across subgroups of age, race, BMI, or diabetes (P-interaction > 0.10 each; see Supplementary Table 3 under “Supplemental data” in the online issue). In these exploratory subgroup analyses, the DNL pattern was positively associated with atherosclerosis progression among diabetic women, and the LCMUFA pattern was inversely associated with atherosclerosis progression among nonwhite women.
TABLE 4.
Multivariable-adjusted association of trans fatty acid pattern with progression of coronary atherosclerosis in the Estrogen Replacement and Atherosclerosis trial1
| Quartiles of the trans fatty acid pattern score2 | P-trend | ||||
| 1 | 2 | 3 | 4 | ||
| Phospholipid fraction | |||||
| Minimal diameter change (mm3) | −0.15 ± 0.04 | −0.12 ± 0.04 | −0.24 ± 0.04 | −0.19 ± 0.04 | 0.15 |
| Stenosis change (%)3 | 4.1 ± 1.5 | 2.7 ± 1.7 | 8.3 ± 1.6 | 5.5 ± 1.7 | 0.04 |
| Cholesteryl ester fraction | |||||
| Minimal diameter change (mm3) | −0.11 ± 0.04 | −0.1 ± 0.04 | −0.12 ± 0.04 | −0.18 ± 0.04 | 0.07 |
| Stenosis change (%)3 | 4.2 ± 1.5 | 4.1 ± 1.5 | 5.1 ± 1.6 | 7.8 ± 1.6 | 0.01 |
| Triglyceride fraction | |||||
| Minimal diameter change (mm3) | −0.15 ± 0.04 | −0.15 ± 0.04 | −0.17 ± 0.04 | −0.17 ± 0.04 | 0.55 |
| Stenosis change (%)3 | 6.0 ± 1.5 | 6.9 ± 1.6 | 7.9 ± 1.6 | 7.8 ± 1.7 | 0.19 |
All values are mean ± SE changes in minimal coronary artery diameter and percentage stenosis of 10 coronary segments, based on 1912 coronary segments among 228 women as defined by serial quantitative coronary angiography over a mean 3.2 y of follow-up. CHS, Cardiovascular Health Study; ERA, Estrogen Replacement and Atherosclerosis trial.
Each fatty acid pattern score was calculated based on scoring weights derived in the CHS. Fewer individual fatty acids were measured in the ERA (n = 27) compared with the CHS (n = 38); thus, scoring weights were simplified for the ERA, excluding fatty acids not available in the ERA (eg, cis-18:1n−8 and trans-16:1n−9) and aggregating scoring weights for fatty acids in the ERA (eg, total trans-18:1) compared with the CHS (isomers of trans-18:1). When the CHS-derived fatty acid patterns based on 38 fatty acids were applied back to the CHS cohort but using only the same 27 fatty acids available in ERA, the trans fatty acid pattern based on 27 fatty acids in the CHS was strongly correlated with the original trans fatty acid pattern (r = 0.81) and predicted incident cardiovascular disease and stroke (data not shown).
For minimal diameter changes (mm) of coronary arteries, negative values represent a greater progression of coronary atherosclerosis. For stenosis change (%) of coronary arteries, positive values represent a greater progression of coronary atherosclerosis. Values were adjusted for age, study sites, education status, race, experimental arms (estrogen, estrogen plus progestin, or placebo), coronary segments (10 locations), duration of follow-up, physical activity level, smoking status, prevalent diabetes, antihypertensive drug use, aspirin use, alcohol drinking, caloric intake, consumption of foods, and the other 2 fatty acid patterns.
DISCUSSION
In our study, PCA enabled us to identify 3 novel FA patterns from 38 individual circulating FAs, including patterns characterized by, among other FA differences, relatively higher proportions of trans FA, FA derived from DNL, and LCMUFAs. We found the trans FA pattern to be associated with significantly higher risk of incident CVD in a cohort of older adults and with greater progression of coronary atherosclerosis in an independent cohort of postmenopausal women with IHD. To our knowledge, no prior studies have used PCA to derive and relate circulating FA patterns to incident CVD or atherosclerosis progression.
The strength of pattern analysis is the combining of numerous interrelated variables into common overall patterns, providing novel information on potential biologic patterns. A necessary caveat is that observed associations for a pattern cannot be attributed to any single constituent, which could have different effects in isolation. For example, the trans FA pattern was characterized by higher proportions of trans FA isomers, but also a higher proportion of cis,cis-18:2n−6 and lower proportions of 16:0, 20:4n−6, 20:5n−3, and 22:6n−3. Future work should evaluate potential roles of these specific FAs and their combinations in the development of CVD, especially stroke. To our knowledge, no prior studies have evaluated trans FA biomarkers and incident stroke.
When we evaluated the trans FA pattern and subtypes of CVD separately in the CHS, significant relations were seen only with stroke and not IHD. However, the trans FA pattern was associated in the ERA with greater progression of coronary atherosclerosis, which is a risk factor for both IHD and stroke events (38). Reasons for the absence of association with IHD in the CHS are unknown. In the CHS, the upper CI for IHD included the possibility of 50% higher risk in the highest quintile of the trans FA pattern. In addition to the possibility of false-negative findings for IHD, some differences were observed between the CHS and ERA cohorts, such as inclusion of only women and a lower average baseline age in ERA (72.1 compared with 65.4 y, respectively). However, subgroup analyses in the CHS provided little evidence that the trans FA pattern was less strongly associated with CVD in men or at older ages. Whereas many risk factors for stroke, clinical IHD events, and atherosclerosis progression are at least partly shared, the trans FA pattern or its correlates could have stronger effects on general risk factors for stroke and for atherosclerosis progression, eg, thrombosis (46), rather than for acute plaque rupture that is a major determinant of clinical IHD events, eg, bursts of shear stress. Given the older age of the CHS participants who were also free of prevalent clinical CVD, the absence of association with IHD could also represent a survivor effect, in that susceptible individuals were already depleted from the population. Overall, our findings suggest an association of this particular circulating FA pattern with higher CVD risk in 2 different cohorts, which supports the need for future investigation of potential mechanisms of the association and confirmation of and reasons for potential differences for event subtypes.
Neither the DNL nor LCMUFA pattern was significantly associated with clinical CVD events or atherosclerosis progression. Exploratory and cross-validation analyses suggested potential associations of the 2 patterns with incident CVD in certain CHS subgroups, but these findings were not replicated in the ERA and should be interpreted cautiously. Our findings suggest a need for future research on how demographic and cardiometabolic profiles influence FA patterns and their relations to the development of CVD. Interestingly, the null findings for the DNL and LCMUFA patterns could also partly reflect competing (opposing) CVD effects of different individual FAs in these patterns. In a prior CHS analysis and other studies on individual FAs, positive associations have been seen between higher concentrations of certain individual DNL FA and IHD risk (7–9), whereas long-chain n−6 PUFAs, which also contributed positively to the DNL pattern, may be protective against incident CVD (47). Similarly, LCMUFA exposure may be cardiotoxic according to animal experiments (48), whereas dairy FAs, which also contributed to the LCMUFA pattern, may be protective against incident CVD (49).
A few prior studies have used PCA to construct FA patterns, based on cholesteryl ester, total plasma, or adipose tissue FAs and in predominantly middle-aged adults (12–14). Warensjö et al (12) identified 3 FA patterns from 11 cholesteryl ester FAs and 4 FA-desaturation indexes (eg, ratio of 18:3n−6 to 18:2n−6). Of these, one pattern was characterized by higher DNL FA and was partially consistent with our DNL pattern. This pattern was associated with a higher risk of the metabolic syndrome in both cross-sectional and prospective analyses (12). Anderson et al (13) applied PCA to 10 plasma FAs. Of 5 FA patterns that they identified, they focused on 2 FA patterns: one partly consistent with our DNL pattern and another was characterized by higher proportions of 20:4n−6, 20:5n−3, and 22:6n−3. The latter pattern appeared to be associated with lower all-cause mortality, but few cases (n = 58) occurred in this cohort. Neither of these prior studies measured any trans FA. Dahm et al (14) used 34 individual adipose tissue FAs, including trans FAs, to construct 7 sex-specific FA patterns. One pattern characterized by higher trans FAs was associated with higher increases in weight and waist circumference in men. Our findings extend these previous results by measuring 38 individual FAs; by evaluating phospholipid FAs, which best reflect tissue-membrane concentrations; by prospectively evaluating incident CVD and atherosclerosis progression in 2 separate cohorts; and by evaluating the reproducibility of FA patterns over time and under a variety of PCA assumptions. Our cross-validation analyses and extension to a second cohort are particularly important during any application of PCA to identify new patterns in complex data.
Potential limitations should be considered. The CHS included older US adults. Because of increases in both competing causes and underlying rates of CVD, the relative effects of many CVD risk factors decrease with aging (50). Conversely, absolute effects of CVD risk factors often remain similar or even increase with aging. Whereas we did not identify significantly stronger associations among younger participants in this cohort, we cannot exclude the possibility that the DNL and LCMUFA patterns might be associated with CVD risk in younger adults. This study was observational, and, whereas we adjusted for a range of major CVD risk factors, residual confounding from imprecisely measured or unmeasured factors may be present. FAs were measured at baseline, and changes in FA patterns over time would cause exposure misclassification and attenuation toward the null. Because laboratory CVs for individual FA were low (most <3%), changes in FA patterns over time would be largely a result of true biologic variation, ie, related to dietary and/or metabolic changes. We partly accounted for such misclassification using multivariate-regression calibration. Our endpoint in the ERA, atherosclerosis progression, is not the same as clinical CVD events. Nonetheless, atherosclerosis progression represents a key pathological process for clinical vascular events and predicts future clinical coronary events (39–41).
In conclusion, by applying PCA to circulating FA biomarkers, we identified novel FA patterns, including a trans FA pattern that was prospectively associated with a higher incidence of CVD (particularly stroke) and progression of coronary atherosclerosis in 2 independent cohorts. Our results support the utility of PCA to evaluate FA biomarkers and indicate a need for future detailed investigation of the key lifestyle, environmental, and genetic determinants of circulating FA patterns and the biologic mechanisms whereby such patterns may influence health.
Acknowledgments
We are grateful to the Cardiovascular Health Study (CHS) investigators and to Geogia Saylor, Susan M Jalbert, and Nancy A Resteghini for fatty acid measurements in the Estrogen Replacement and Atherosclerosis trial (ERA) and to each of the CHS and ERA participants.
The authors’ responsibilities were as follows—FI and DM: study concept, study design, and draft of the manuscript; FI: statistical analysis; IBK, XS, and NRM: laboratory measurements; DM, RNL, and DSS: funding for the CHS; AHL and DSS: funding for the ERA; and DMH and DSS: participant recruitment. All authors participated in the data interpretation and critical review and revision of the manuscript and approved the final manuscript. The funders had no role in the study design or conduct; collection, management, analysis, or interpretation of the data; or manuscript preparation, review, or approval. Potential conflict of interest: DM received research support from GlaxoSmithKline, Sigma Tau, and Pronova for a trial of fish oil and postsurgical complications; ad hoc travel reimbursement and/or honoraria for research presentations from Aramark, Unilever, SPRIM, and Nutrition Impact; ad hoc consulting fees from Foodminds and McKinsey Health Systems Institute; and royalties from UpToDate. None of the other authors declared a conflict of interest.
Footnotes
Abbreviations used: CHS, Cardiovascular Health Study; CVD, cardiovascular disease; DNL, de novo lipogenesis; ERA, Estrogen Replacement and Atherosclerosis trial; FA, fatty acid; IHD, ischemic heart disease; LCMUFA, long-chain MUFA; PCA, principal component analysis.
REFERENCES
- 1.Baylin A, Campos H. The use of fatty acid biomarkers to reflect dietary intake. Curr Opin Lipidol 2006;17:22–7 [DOI] [PubMed] [Google Scholar]
- 2.Lemaitre RN, King IB, Mozaffarian D, Kuller LH, Tracy RP, Siscovick DS. n−3 Polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: the Cardiovascular Health Study. Am J Clin Nutr 2003;77:319–25 [DOI] [PubMed] [Google Scholar]
- 3.Lemaitre RN, King IB, Mozaffarian D, Sotoodehnia N, Rea TD, Kuller LH, Tracy RP, Siscovick DS. Plasma phospholipid trans fatty acids, fatal ischemic heart disease, and sudden cardiac death in older adults: the cardiovascular health study. Circulation 2006;114:209–15 [DOI] [PubMed] [Google Scholar]
- 4.Zheng ZJ, Folsom AR, Ma J, Arnett DK, McGovern PG, Eckfeldt JH. Plasma fatty acid composition and 6-year incidence of hypertension in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol 1999;150:492–500 [DOI] [PubMed] [Google Scholar]
- 5.Erkkilä AT, Matthan NR, Herrington DM, Lichtenstein AH. Higher plasma docosahexaenoic acid is associated with reduced progression of coronary atherosclerosis in women with CAD. J Lipid Res 2006;47:2814–9 [DOI] [PubMed] [Google Scholar]
- 6.Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, Hotamisligil GS. Circulating palmitoleic acid and risk of metabolic abnormalities and new-onset diabetes. Am J Clin Nutr 2010;92:1350–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemaitre RN, King IB, Sotoodehnia N, Knopp RH, Mozaffarian D, McKnight B, Rea TD, Rice K, Friedlander Y, Lumley TS, et al. Endogenous red blood cell membrane fatty acids and sudden cardiac arrest. Metabolism 2010;59:1029–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warensjö E, Sundstrom J, Vessby B, Cederholm T, Riserus U. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: a population-based prospective study. Am J Clin Nutr 2008;88:203–9 [DOI] [PubMed] [Google Scholar]
- 9.Wu JHY, Lemaitre RN, Imamura F, King IB, Song X, Spiegelman D, Siscovick DS, Mozaffarian D. Fatty acids in the de novo lipogenesis pathway and risk of coronary heart disease: the Cardiovascular Health Study. Am J Clin Nutr 2011;94:431–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imamura F, Jacques PF. Invited commentary: dietary pattern analysis. Am J Epidemiol 2011;173:1105–8 [DOI] [PubMed] [Google Scholar]
- 11.Newby PK, Tucker KL. Empirically derived eating patterns using factor or cluster analysis: a review. Nutr Rev 2004;62:177–203 [DOI] [PubMed] [Google Scholar]
- 12.Warensjö E, Sundstrom J, Lind L, Vessby B. Factor analysis of fatty acids in serum lipids as a measure of dietary fat quality in relation to the metabolic syndrome in men. Am J Clin Nutr 2006;84:442–8 [DOI] [PubMed] [Google Scholar]
- 13.Anderson SG, Sanders TAB, Cruickshank JK. Plasma fatty acid composition as a predictor of arterial stiffness and mortality. Hypertension 2009;53:839–45 [DOI] [PubMed] [Google Scholar]
- 14.Dahm CC, Gorst-Rasmussen A, Jakobsen MU, Schmidt EB, Tjønneland A, Sørensen TIA, Overvad K. Adipose tissue fatty acid patterns and changes in anthropometry: a cohort study. PLoS ONE 2011;6:e22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol 1993;3:358–66 [DOI] [PubMed] [Google Scholar]
- 16.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1:263–76 [DOI] [PubMed] [Google Scholar]
- 17.Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long-chaing n−3 fatty acids and the risk of sudden death. N Engl J Med 2002;346:1113–8 [DOI] [PubMed] [Google Scholar]
- 18.Stanford JL, King I, Kristal AR. Long-term storage of red blood cells and correlations between red cell and dietary fatty acids: results from a pilot study. Nutr Cancer 1991;16:183–8 [DOI] [PubMed] [Google Scholar]
- 19.Matthan NR, Ip B, Resteghini N, Ausman LM, Lichtenstein AH. Long-term fatty acid stability in human serum cholesteryl ester, triglyceride, and phospholipid fractions. J Lipid Res 2010;51:2826–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509 [PubMed] [Google Scholar]
- 21.Cureton EE, D'Agostino RB. Factor analysis: an applied approach. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc, 1983 [Google Scholar]
- 22.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008;47:348–80 [DOI] [PubMed] [Google Scholar]
- 23.Micha R, King IB, Lemaitre RN, Rimm EB, Sacks F, Song X, Siscovick DS, Mozaffarian D. Food sources of individual plasma phospholipid trans fatty acid isomers: the Cardiovascular Health Study. Am J Clin Nutr 2010;91:883–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med 2006;354:1601–13 [DOI] [PubMed] [Google Scholar]
- 25.Willett W, Mozaffarian D. Ruminant or industrial sources of trans fatty acids: public health issue or food label skirmish? Am J Clin Nutr 2008;87:515–6 [DOI] [PubMed] [Google Scholar]
- 26.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health. JAMA 2006;296:1885–99 [DOI] [PubMed] [Google Scholar]
- 27.Baylin A, Kabagambe EK, Siles X, Campos H. Adipose tissue biomarkers of fatty acid intake. Am J Clin Nutr 2002;76:750–7 [DOI] [PubMed] [Google Scholar]
- 28.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol 1995;5:278–85 [DOI] [PubMed] [Google Scholar]
- 29.Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. J Clin Epidemiol 1992;45:683–92 [DOI] [PubMed] [Google Scholar]
- 30.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem 1995;41:264–70 [PubMed] [Google Scholar]
- 31.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502 [PubMed] [Google Scholar]
- 32.Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, Tracy RP. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol 2001;153:242–50 [DOI] [PubMed] [Google Scholar]
- 33.Kumanyika S, Tell GS, Fried L, Martel JK, Chinchilli VM. Picture-sort method for administering a food frequency questionnaire to older adults. J Am Diet Assoc 1996;96:137–44 [DOI] [PubMed] [Google Scholar]
- 34.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–6 [DOI] [PubMed] [Google Scholar]
- 35.Longstreth WT, Jr, Bernick C, Fitzpatrick A, Cushman M, Knepper L, Lima J, Furberg CD. Frequency and predictors of stroke death in 5,888 participants in the Cardiovascular Health Study. Neurology 2001;56:368–75 [DOI] [PubMed] [Google Scholar]
- 36.Herrington DM, Reboussin DM, Brosnihan KB, Sharp PC, Shumaker SA, Snyder TE, Furberg CD, Kowalchuk GJ, Stuckey TD, Rogers WJ, et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med 2000;343:522–9 [DOI] [PubMed] [Google Scholar]
- 37.Herrington DM, Reboussin DM, Klein KP, Sharp PC, Shumaker SA, Snyder TE, Geisinger KR. The Estrogen Replacement and Atherosclerosis (ERA) Study: study design and baseline characteristics of the cohort. Control Clin Trials 2000;21:257–85 [DOI] [PubMed] [Google Scholar]
- 38.Bots ML, Baldassarre D, Simon A, de Groot E, O'Leary DH, Riley W, Kastelein JJ, Grobbee DE. Carotid intima-media thickness and coronary atherosclerosis: weak or strong relations? Eur Heart J 2007;28:398–406 [DOI] [PubMed] [Google Scholar]
- 39.Azen SP, Mack WJ, Cashin-Hemphill L, LaBree L, Shircore AM, Selzer RH, Blankenhorn DH, Hodis HN. Progression of coronary artery disease predicts clinical coronary events: long-term follow-up from the Cholesterol Lowering Atherosclerosis Study. Circulation 1996;93:34–41 [DOI] [PubMed] [Google Scholar]
- 40.Mack WJ, Xiang M, Selzer RH, Hodis HN. Serial quantitative coronary angiography and coronary events. Am Heart J 2000;139:993–9 [DOI] [PubMed] [Google Scholar]
- 41.Zhao X-Q, Krasuski RA, Baer J, Whitney EJ, Neradilek B, Chait A, Marcovina S, Albers JJ, Brown BG. Effects of combination lipid therapy on coronary stenosis progression and clinical cardiovascular events in coronary disease patients with metabolic syndrome: a combined analysis of the Familial Atherosclerosis Treatment Study (FATS), the HDL-Atherosclerosis Treatment Study (HATS), and the Armed Forces Regression Study (AFREGS). Am J Cardiol 2009;104:1457–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cologne J, Hsu W-L, Abbott RD, Ohishi W, Grant EJ, Fujiwara S, Cullings HM. Proportional hazards regression in epidemiologic follow-up studies: an intuitive consideration of primary time scale. Epidemiology 2012;23:565–73 [DOI] [PubMed] [Google Scholar]
- 43.Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, Peto R. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol 1999;150:341–53 [DOI] [PubMed] [Google Scholar]
- 44.Carroll RJ, Ruppert D, Stefanski LA, Crainiceanu CM. Measurement error in nonlinear models: a modern perspective. 2nd ed. Boca Raton, FL: Chapman and Hall/CRC, 2006 [Google Scholar]
- 45.Lawlor DA, Ebrahim S, May M, Davey Smith G. (Mis)use of factor analysis in the study of insulin resistance syndrome. Am J Epidemiol 2004;159:1013–8 [DOI] [PubMed] [Google Scholar]
- 46.Mizurini DM, Maia IC, Sardinha FL, Monteiro RQ,Ortiz-Costa S, do Carmo MG. Venous thrombosis risk: effects of palm oil and hydrogenated fat diet in rats. Nutrition 2011;27:233–8 [DOI] [PubMed] [Google Scholar]
- 47.Fan YY, Chapkin RS. Importance of dietary γ-linolenic acid in human health and nutrition. J Nutr 1998;128:1411–4 [DOI] [PubMed] [Google Scholar]
- 48.Van Vleet JF, Ferrans VJ. Myocardial diseases of animals. Am J Pathol 1986;124:98–178 [PMC free article] [PubMed] [Google Scholar]
- 49.Soedamah-Muthu SS, Ding EL, Al-Delaimy WK, Hu FB, Engberink MF, Willett WC, Geleijnse JM. Milk and dairy consumption and incidence of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Am J Clin Nutr 2011;93:158–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903–13 [DOI] [PubMed] [Google Scholar]