Abstract
Background: Multiple micronutrients (vitamin B complex and vitamins C and E) were effective at reducing infectious disease morbidity, HIV disease progression, and poor pregnancy outcomes in HIV-infected women.
Objective: The objective was to evaluate whether direct supplementation of these micronutrients to HIV-exposed infants reduces mortality and morbidity.
Design: Infants born to HIV-infected women from Dar es Salaam, Tanzania, were randomly assigned to receive daily oral supplementation of multiple multivitamins (vitamin B complex and vitamins C and E) or placebo from age 6 wk for 24 mo. All-cause mortality, hospitalizations, and unscheduled clinic visits were recorded. Morbidities were recorded during monthly follow-up visits. All mothers received multiple micronutrients throughout the study.
Results: A total of 1193 infants were randomly assigned to receive micronutrients and 1194 to receive placebo. There were 138 child deaths in the multivitamin group and 124 deaths in the placebo group (HR: 1.13; 95% CI: 0.88, 1.44; P = 0.33). Hospitalizations (RR: 0.83; 95% CI: 0.62, 1.13; P = 0.23), unscheduled clinic visits (RR: 0.97; 95% CI: 0.85, 1.10; P = 0.59), and maternal reports of diarrhea (RR: 0.97; 0.87, 1.10; P = 0.64) were not significantly different between the 2 groups. Fever (P = 0.02) and vomiting (P = 0.007) were significantly lower in the multivitamin group. Among 429 children whose mothers received antiretroviral (ARV) therapy, multivitamin use had no effect on mortality but was associated with a significant reduction in hospitalizations (P = 0.035), episodes of fever (P = 0.005), and episodes of fever and cough (P = 0.019).
Conclusions: In the setting of maternal micronutrient supplementation, supplementation of HIV-exposed infants with vitamin B and vitamins C and E does not reduce mortality. Studies of nutrition supplementation in ARV-exposed infants may be warranted. This trial was registered at clinicaltrials.gov as NCT00197730.
INTRODUCTION
The most significant public health problems for children on a global scale continue to be infectious diseases (including lower respiratory tract infections and diarrheal diseases) and diseases of the perinatal period (1). Diarrheal diseases, lower respiratory diseases, perinatal disorders, malaria, measles, and other illnesses claim nearly 8 million lives of children younger than 5 y annually (2). In addition, HIV infection, especially in sub-Saharan Africa, is a major cause of morbidity and mortality among children (3).
Micronutrient supplementation may benefit children living in poor countries, where dietary intake and/or bioavailability of micronutrients are low. Randomized trials have confirmed that supplementation is effective at reducing mortality (vitamin A) and infectious disease morbidity (vitamin A and zinc) (4–7). Children born to HIV-infected women may be at higher risk of infectious morbidities and nutritional problems, including protein-energy malnutrition, food insecurity, micronutrient deficiencies, diarrhea, and respiratory infections (8, 9). We previously observed that multiple micronutrients (B vitamins, vitamin C, and vitamin E) were effective at reducing infectious disease morbidity and HIV progression and improving pregnancy outcomes, in a cohort of HIV-infected women (10, 11). In addition, children born to mothers who had received this regimen had fewer episodes of diarrhea (12), better growth (13), and higher micronutrient blood concentrations (14) than did infants born to HIV-infected women who did not receive this supplement. To evaluate the efficacy of direct child supplementation with a similar mix of micronutrients, we performed a randomized trial of micronutrient supplementation (B vitamins, vitamin C, and vitamin E) among infants born to HIV-infected women living in Dar es Salaam, Tanzania. Our aims were to determine whether daily administration of multiple micronutrients reduced the risk of mortality and infectious disease morbidity, in comparison with placebo.
SUBJECTS AND METHODS
The study was a randomized, double-blind, placebo-controlled trial. Women aged ≥18 y presenting for prenatal care at the 32nd week of gestation or earlier in 1 of 8 antenatal clinics in Dar es Salaam were offered HIV screening with pre- and posttest counseling. Women who tested HIV positive were further screened for eligibility, including the intention to reside in Dar es Salaam for the duration of follow-up. Maternal HIV-1 serostatus was determined by 2 sequential enzyme-linked immunosorbent assays with the use of Murex HIV antigen/antibody (Abbott Murex) followed by the Enzygnost anti-HIV-1/2 Plus (Dade Behring) (15); discordant results were resolved by a Western blot test (Bio-Rad Laboratories). Written informed consent was obtained from women for participation in the trial while still pregnant. Eligibility for infant participation in the trial included singleton birth and age between 5 and 7 wk at randomization. Excluded were infants born of multiple gestation or those with serious congenital anomalies or other conditions that would interfere with study procedures, including the ability to consume a daily micronutrient supplement. Hemoglobin concentrations were measured by using the AcT5 Diff AL hematology analyzer (Beckman Coulter). Weight was measured to the nearest 10 g with a digital infant balance (TANITA), and length was measured to the nearest 1 mm with a rigid length board with a movable foot piece. Anthropometric equipment was calibrated on a regular basis.
Institutional approval was granted by the Harvard School of Public Health Human Subjects Committee, the Muhimbili University of Health and Allied Sciences Committee of Research and Publications, the Tanzanian National Institute of Medical Research, and the Tanzanian Food and Drugs Authority. A Data Safety and Monitoring Board (DSMB)5 met twice annually during the course of the study.
Treatment allocation
Infants were randomly assigned to receive a daily oral dose of multivitamins or placebo from enrollment at age 6 wk for 24 mo. A randomization list from 1 to 2400 was prepared by the study biostatistician in Boston with the use of permuted blocks of size 20 and provided to the pharmacy Department in Dar es Salaam, with each number corresponding to a code denoting 1 of the 2 treatment arms. On-site study pharmacists stored the coded randomization list in a locked file cabinet and concealed allocation by covering the numeric regimen code on each blister pack with a sticker. Infants enrolled at the study clinic were provided the next consecutive number in series. Study physicians, research nurses, and participants were unaware of the treatment groups.
From age 6 wk to 6 mo, infants in the multivitamin (active) arm received one capsule containing 60 mg vitamin C, 8 mg vitamin E, 0.5 mg thiamine, 0.6 mg riboflavin, 4 mg niacin, 0.6 mg vitamin B-6, 130 μg folate, and 1 mg vitamin B-12. From age 7 mo to the end of follow-up, 2 capsules were given daily. These dosages represent between 150% and 600% of the US Adequate Intake for children aged 0–6 mo and 200% and 400% for children aged 7–12 mo (16–20). The supplement used was a powder encapsulated in an opaque gelatinous capsule manufactured by Nutriset. Mothers were instructed how to push the capsule through the back of the blister pack, open it, and decant the powder into a small plastic cup. Sterile water (5 mL) supplied with the supplement was added to the powder, and the dose was given to the child orally. A pilot phase of open-label vitamin use in 12 infants and mothers confirmed that this supplement preparation and use was well accepted by the mothers and infants. Both the placebo and active capsules contained an orange powder of identical taste and appearance. All study personnel and participants were blinded to treatment assignment for the duration of the study. Only the study statisticians and the DSMB saw unblinded data, but none had any contact with the study participants.
Provision of standard of care and follow-up
Mothers and children were asked to return monthly to a central clinic site on the campus of Muhimbili University of Health and Allied Sciences for research visits and standard clinical care. As part of standard medical care, all children received growth monitoring, immunizations, and routine medical care for illnesses. All children enrolled in the trial received periodic high-dose vitamin A supplementation, as per Tanzanian Ministry of Health guidelines (100,000 IU at 9 mo and 200,000 IU at 15 and 21 mo). Fortified complementary foods were not universally available, and universal iron supplementation was not provided to the children. Children found to be anemic were treated with iron supplementation.
Mothers were counseled on the risks and benefits of exclusive breastfeeding. The provision of the study regimen was in keeping with WHO recommendation concerning exclusive breastfeeding, in that drops, syrups, or oral rehydration solutions are allowed. Women were instructed not to give any additional foods or fluids while they were exclusively breastfeeding, in keeping with WHO and Tanzanian Ministry of Health guidelines supporting exclusive breastfeeding.
All mothers were provided with oral multivitamins, in keeping with our prior findings (10), from enrollment until study end postpartum. Maternal multivitamin doses were generally several times the Recommended Dietary Allowance (RDA) for B-complex vitamins, vitamin C, and vitamin E, but women who were started on antiretroviral therapy (ARV) were changed to single RDA multivitamin dosages.
When the study was proposed, routine medical care for pregnant women with HIV infection included malaria prophylaxis, iron and folate supplementation, diagnosis and treatment of sexually transmitted infections and prophylaxis, and diagnosis and treatment of opportunistic infections. ARV medication was limited to nevirapine prophylaxis for maternal to child transmission (one dose given to the mother at the onset of labor and one dose given to the infant within 72 h of birth) (21). As the study progressed, the availability of ARVs increased substantially through programs such as the President's Plan for AIDS Relief and other governmental and nongovernmental programs. Beginning in July 2005, women and children in the study were screened for ARV eligibility and treated according to Tanzanian Ministry of Health guidelines. For adults, eligibility was based on WHO stage IV HIV disease, or CD4 cell count <200 cells/μL, or WHO stage III and CD4 cell count <350 cells/μL. For children aged <18 mo, eligibility was based on CD4% <20 or Pediatric WHO Stage III; for children aged ≥18 mo, eligibility was based on Pediatric WHO Stage III or CD4% <15%. The standard first-line regimen was stavudine, lamivudine, and nevirapine for adults and zidovudine, lamivudine, and nevirapine for children; alternative drugs were available for specific circumstances. All children were tested for HIV infection at 6 wk of age by using the Amplicor HIV-1 DNA assay version 1.5 (Roche Molecular Systems Inc). Tests at 18 mo of age were performed by using HIV ELISAs followed by Enzygnost anti-HIV-1/2 Plus (Dade Behring); discordant results were resolved by using a Western blot test. Samples from children who tested positive at 18 mo were then back tested via polymerase chain reaction to estimate time of transmission. Time of transmission was estimated as the date halfway between the last negative and first positive HIV test result.
During the monthly follow-up visits, mothers underwent standardized assessment of child morbidity with the prompting of symptom diaries that had been distributed at the previous visit. By using pictorials of illness symptoms (eg, diarrhea, vomiting, and fever), mothers were asked to check off days when their children suffered these symptoms. These diaries were reviewed by trained research nurses during the monthly visits to the clinic to document the occurrence of infectious morbidities, including the need for unscheduled clinic visits or hospitalizations. Diarrhea was defined as ≥3 loose or watery stools within a 24-h period. Rapid respiratory rate was defined as >60 breaths/min in infants younger than 2 mo, >50 breaths/min in 2–11-mo-olds, and >40 breaths/min in those aged ≥12 mo. Every 3 mo, and/or when acute complaints of illness were noted, children underwent a physical examination and medical treatment by study physicians. Standardized diagnostic criteria were used to make physician diagnoses.
Compliance with the daily regimen was measured by research nurses who counted the number of unused pills. Mothers who were traveling out of Dar es Salaam temporarily were provided with extra regimen and water to suffice until the next visit to the research clinic. Mothers were reimbursed for their travel expenses to the study clinic, but received no other payment.
Children who missed their monthly follow-up appointment were visited at home, and their vital status was confirmed through contact with immediate family members. A verbal autopsy was performed in cases of child death to determine the cause of death. Coding of the cause of death from the verbal autopsies was performed independently by 2 pediatricians (KPM and CD), and differences were adjudicated by a third pediatrician.
Data management and analysis
The primary outcome was all-cause mortality. We initially estimated that the mortality rate in the placebo arm would be 25% and powered the trial to detect a 30% reduction in mortality. During the course of the trial, the mortality rate was substantially lower than expected. On the basis of a 2-sided test for a comparison of proportions with 80% power, we increased our sample size from 1600 to 2360 to detect a 30% reduction in child mortality, assuming a placebo arm mortality rate of 12.5%. Because of both this increased sample size and budgetary limits, follow-up time was truncated to before 24 mo for some of the infants.
Data were double entered by using Microsoft Access software and converted to SAS software (version 9.1; SAS Institute) for analysis. Final data sets were uploaded onto a UNIX-based server in Boston, Massachusetts. Intention-to-treat analyses were carried out according to a preestablished analysis plan. To evaluate the effect of supplements on mortality, we created Kaplan-Meier curves and evaluated the effect of multivitamins with the use of the Cox proportional hazard test. For morbidity outcomes, the probability of a reported event at a visit was compared between arms by using generalized estimating equations, adjusted for the child's age (<6, 6–18, or >18 mo). For physician diagnoses, which were made both during routine and additional sick visits, the rate per year of follow-up was compared between arms by using Poisson regression. Stratified analyses by prespecified (child sex, HIV status, low birth weight, maternal age, and parity) and post hoc (maternal ARV use) factors were also performed. All analyses were performed by using SAS software (version 9.2; SAS Institute).
RESULTS
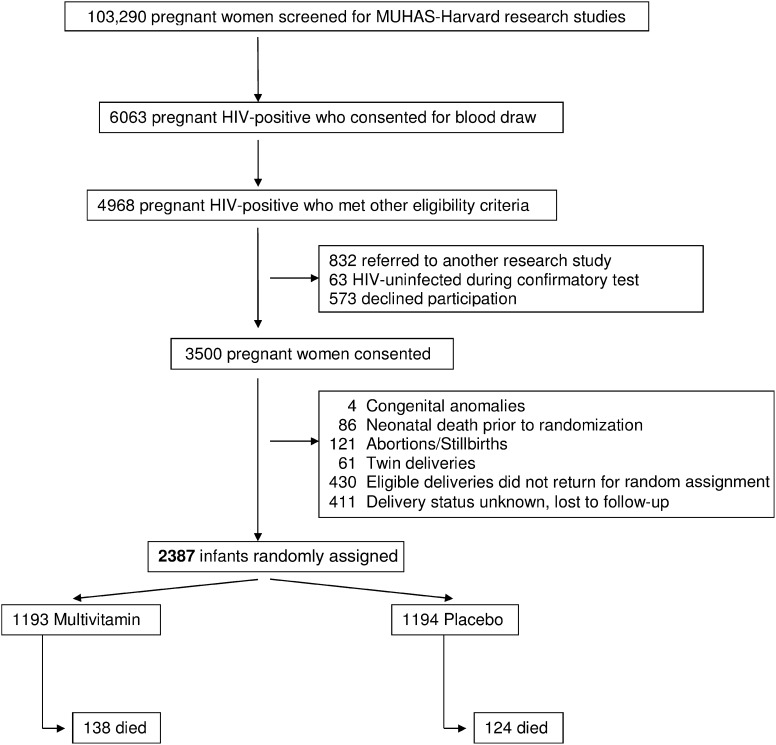
The study profile is shown in Figure 1. From August 2004 until November 2007, 2387 infants were randomly assigned; follow-up ended 31 May 2008. Median (25th, 75th percentiles) duration of follow-up was 22.4 (12.7, 25.2) mo. The baseline maternal and child characteristics are shown in Table 1, which confirm that measures of age, socioeconomic characteristics, and WHO disease stage were comparable between the 2 groups. Thirty percent of women were anemic at presentation, and 20% received ARVs during pregnancy. Infants in the 2 study arms had similar characteristics, including rates of low birth weight, HIV infection at age 6 wk, and anemia at 6 wk. Rates of exclusive breastfeeding were comparable between the 2 study arms. Eleven infants were enrolled slightly outside of the age criteria (10 between 8 and 10 wk of age and 1 at 4 wk of age); after review by the DSMB and the Institutional Review Board, we included these infants in the trial.
FIGURE 1.
Study profile of randomized trial of micronutrient supplementation to infants born to HIV-infected women in Dar es Salaam, Tanzania. MUHAS, Muhimbili University of Health and Allied Sciences.
TABLE 1.
Characteristics of HIV-infected mothers and their children enrolled in a clinical trial of micronutrient supplementation in Tanzania1
| Multivitamin (n = 1193) | Placebo (n = 1194) | |
| Maternal characteristics | ||
| Age (y) | 28.2 ± 5.02 | 28.2 ± 4.9 |
| Education [n (%)] | ||
| None | 68 (5.8) | 90 (7.6) |
| 1–7 y | 856 (72.5) | 844 (71.3) |
| ≥8 y | 257 (21.8) | 250 (21.1) |
| Employment [n (%)] | ||
| Housewife without income | 780 (67.8) | 748 (65.2) |
| Housewife with income | 148 (12.9) | 182 (15.9) |
| Other | 223 (19.4) | 217 (18.9) |
| Marital status [n (%)] | ||
| Married/living with partner | 1031 (87.5) | 1020 (86.2) |
| Prior pregnancies [n (%)] | ||
| None | 270 (22.9) | 269 (22.8) |
| 1–3 | 820 (69.4) | 812 (68.7) |
| ≥4 | 91 (7.7) | 101 (8.5) |
| Daily food expenditure per person <500 T shillings [n (%)]3 | 598 (53.5) | 565 (50.3) |
| Midupper arm circumference (cm)4 | 25.9 ± 3.3 | 26.0 ± 3.2 |
| Hemoglobin4 | ||
| <11 g/dL [n (%)] | 320 (30.4) | 328 (30.6) |
| Concentration | 11.6 ± 1.4 | 11.4 ± 1.4 |
| BMI group [n (%)]4 | ||
| <18.5 kg/m2 | 53 (4.5) | 51 (4.3) |
| 18.5 to <25 kg/m2 | 675 (57.0) | 656 (55.9) |
| 25.0 to <30 kg/m2 | 346 (29.2) | 353 (30.1) |
| ≥30 kg/m2 | 110 (9.3) | 114 (9.7) |
| Received ARV during pregnancy [n (%)] | 214 (20.4) | 215 (20.1) |
| WHO disease stage I/II [n (%)] | 629 (88.6) | 623 (87.1) |
| CD4 count [n (%)]4 | ||
| <200 cells/mm4 | 98 (9.4) | 100 (9.4) |
| 200–349 cells/mm4 | 232 (22.3) | 189 (17.8) |
| ≥350 cells/mm4 | 711 (68.3) | 771 (72.7) |
| Gestational age at enrollment (wk) | 24.6 ± 5.5 | 24.6 ± 5.6 |
| Child characteristics | ||
| Age (wk) | 5.8 ± 0.5 | 5.8 ± 0.6 |
| Male sex [n (%)] | 651 (54.6) | 638 (53.4) |
| Low birth weight, <2500 g [n (%)] | 79 (6.9) | 82 (7.2) |
| Prematurity, <37 wk [n (%)] | 172 (14.6) | 185 (15.7) |
| HIV positive [n (%)] | 125 (10.7) | 139 (11.8) |
| Weight at baseline (kg) | 4.5 ± 0.7 | 4.4 ± 0.7 |
| LAZ | −0.36 ± 1.4 | −0.43 ± 1.3 |
| WHZ | 0.21 ± 1.3 | −0.25 ± 1.3 |
| WAZ | −0.48 ± 1.1 | −0.57 ± 1.2 |
| Duration of breastfeeding (mo) | 4.4 ± 2.6 | 4.3 ± 2.5 |
| Hemoglobin at baseline | ||
| ≤10 g/dL [n (%)] | 463 (40.8) | 490 (42.9) |
| Concentration (g/dL) | 10.3 ± 1.5 | 10.3 ± 1.6 |
ARV, antiretroviral therapy; LAZ, length-for-age z score; T, Tanzanian; WAZ, weight-for-age z score; WHZ, weight-for-height z score.
Mean ± SD (all such values).
1250 T shillings = ∼1 US$ at the time of the study.
Maternal characteristics measured at 6 wk postpartum.
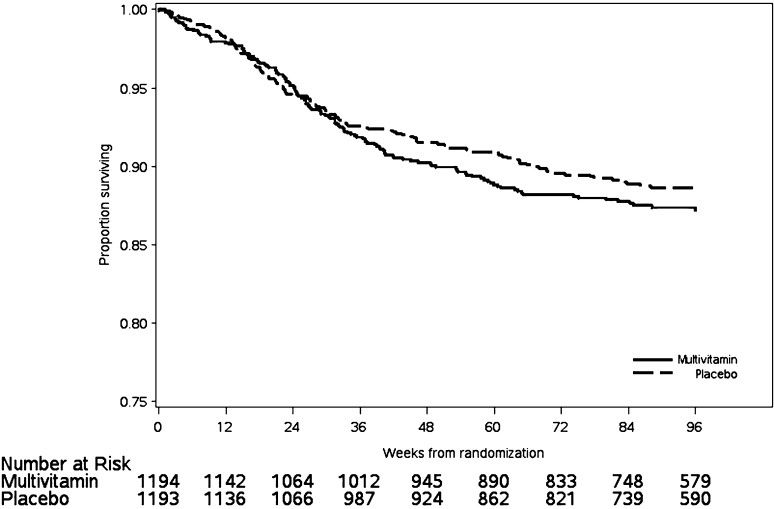
After a median (25th, 75th percentiles) of 22.4 (12.7, 25.2) mo of follow-up, there were 138 child deaths in the multivitamin group and 124 deaths in the placebo group (RR: 1.13; 95% CI: 0.88, 1.44; P = 0.33). The survival curves for the 2 arms are shown in Figure 2. The most common causes of death were lower respiratory infections, septicemia, malaria, and diarrheal diseases, and these did not differ between the 2 study arms. We examined mortality rates stratified by infant sex, HIV status at baseline, low birth weight (<2500 g), maternal age (< median 28 y), and parity and observed no modification of the vitamin supplement by these factors (Table 2).
FIGURE 2.
Kaplan-Meier plot for all-cause mortality among 2387 HIV-exposed Tanzanian infants.
TABLE 2.
All-cause mortality rates among HIV-exposed Tanzanian children randomly assigned to receive daily multivitamins or placebo, stratified by baseline characteristics1
| Multivitamin (n = 1193) | Placebo (n = 1194) | HR (95% CI) | P | |
| n (%) | n (%) | |||
| Overall (n = 2387) | 138 (11.6) | 124 (10.4) | 1.13 (0.88, 1.44) | 0.33 |
| Subgroups | ||||
| Sex | ||||
| Male (n = 1289) | 78 (12.0) | 67 (10.5) | 1.14 (0.83, 1.59) | 0.42 |
| Female (n = 1098) | 60 (11.1) | 57 (10.3) | 1.11 (0.77, 1.59) | 0.59 |
| HIV positive at 6 wk | ||||
| Positive (n = 264) | 64 (51.2) | 55 (39.6) | 1.38 (0.96, 1.98) | 0.08 |
| Negative (n = 2081) | 68 (6.5) | 63 (6.1) | 1.09 (0.77, 1.53) | 0.64 |
| Low birth weight (g) | ||||
| ≥2500 (n = 2128) | 106 (9.9) | 97 (9.2) | 1.08 (0.82, 1.43) | 0.57 |
| <2500 (n = 161) | 18 (22.8) | 17 (20.7) | 1.07 (0.55, 2.08) | 0.84 |
| Maternal age | ||||
| >28 y (n = 1074) | 56 (10.7) | 58 (10.6) | 1.00 (0.69, 1.45) | 0.99 |
| ≤28 y (n = 1257) | 77 (12.1) | 63 (10.2) | 1.22 (0.87, 1.70) | 0.24 |
| Prior pregnancies | ||||
| 0 or 1 (n = 1326) | 84 (12.4) | 62 (9.5) | 1.33 (0.95, 1.84) | 0.09 |
| ≥2 (n = 1036) | 51 (10.1) | 59 (11.1) | 0.91 (0.63, 1.33) | 0.64 |
HRs, 95% CIs, and corresponding P values were obtained from Cox proportional hazards models. There were no significant interactions between groups.
The effect of multivitamin supplementation on the incidence of hospitalizations, unscheduled clinic visits, and common infectious morbidities is shown in Table 3. As measured by the proportion of monthly clinic visits at which daily diaries noted the occurrence of various symptoms, no effect of supplementation was observed on the occurrence of diarrhea (RR: 0.97; 95% CI: 0.87, 1.10), unscheduled outpatient visits (RR: 0.97; 95% CI: 0.85, 1.10), or hospitalizations (RR: 0.83; 95% CI: 0.62, 1.13). Significant reductions in the occurrence of vomiting and fever were noted, and vitamin supplementation was associated with an 8% reduction in the occurrence of fever (RR: 0.92; 95% CI: 0.85, 0.99) and a 22% reduction in the occurrence of vomiting (RR: 0.78; 95% CI: 0.65, 0.93). Physician diagnoses of diarrhea (RR: 1.10; 95% CI: 0.98, 1.24) and respiratory tract infections (RR: 1.03; 95% CI: 0.98, 1.09) were not significantly lower in the supplemented group, nor were diagnoses of malaria (RR: 0.99; 95% CI: 0.89, 1.10).
TABLE 3.
Rates of hospitalizations, unscheduled clinic visits, and symptoms of common infectious illnesses among HIV-exposed Tanzanian children randomly assigned to receive daily multivitamins or placebo
| Morbidity | Multivitamin | Placebo | RR (95% CI) | 1 |
| 2 | 2 | |||
| Hospitalizations | 0.62 (127/20,533) | 0.80 (166/20,775) | 0.83 (0.62, 1.13) | 0.23 |
| Unscheduled outpatient visits | 3.01 (619/2,0533) | 3.15 (655/20,775) | 0.97 (0.85, 1.10) | 0.59 |
| Diarrhea | 4.31 (885/20,531) | 4.47 (929/20,774) | 0.97 (0.87, 1.10) | 0.64 |
| Cold | 25.67 (5261/20,493) | 25.94 (5373/20,716) | 1.00 (0.95, 1.05) | 0.95 |
| Cough | 30.79 (6310/20,492) | 30.45 (6308/20,715) | 1.02 (0.9, 1.07) | 0.51 |
| Difficulty breathing | 1.43 (293/20,493) | 1.31 (272/20,719) | 1.06 (0.85, 1.33) | 0.60 |
| Fever | 11.46 (2349/20,493) | 12.60 (2611/20,720) | 0.92 (0.85, 0.99) | 0.02 |
| Refusal to eat, drink, or breastfeed | 2.72 (557/20,493) | 2.95 (611/20,721) | 0.93 (0.80, 1.07) | 0.30 |
| Pus draining from ears | 0.82 (168/20,493) | 0.97 (201/20,721) | 0.81 (0.58, 1.12) | 0.20 |
| Vomiting | 1.46 (299/20,493) | 1.91 (395/20,721) | 0.78 (0.65, 0.93) | 0.007 |
| Cough and fever | 6.65 (1362/20,493) | 7.28 (1508/20,721) | 0.92 (0.84, 1.01) | 0.09 |
| Cough plus3 | 2.46 (505/20,493) | 2.45 (508/20,721) | 1.02 (0.87, 1.20) | 0.80 |
| Cough with rapid respiratory rate4 | 0.02 (4/20,493) | 0.01 (1/20,721) | 4.05 (0.45, 36.1) | 0.34 |
value was obtained from generalized estimating equations with the binomial distribution, log link, and exchangeable correlation structure. The analyses were adjusted for the age of the children (<6, 6–18, and >18 mo).
Total number of events occurring during follow-up, defined as being reported in the 28 d (4 wk) before the visit.
Defined as cough with one or more of the following events: difficulty breathing, chest retractions, and refusal to eat, drink, or breastfeed.
Defined as a respiratory rate >60/min in infants aged <2 mo, >50/min in those aged 2 mo, and >40/min in those aged 12–59 mo.
Results of analyses to determine whether the effect of multivitamin supplementation on the occurrence of diarrhea, cough, fever, and combined cough and fever were modified by sex, HIV infection, or low birth weight are shown in Table 4. No significant effect modification was found for the first 2 morbidities, but multivitamins were associated with a significantly higher risk of fever among low-birth-weight infants (RR: 1.28; 95% CI: 0.97, 1.70) than among infants with a birth weight ≥2500 g (RR: 0.89; 95% CI: 0.82, 0.96; P-interaction = 0.013) and with a significantly higher risk of fever and cough among low-birth-weight infants (RR: 1.46; 95% CI: 1.04, 2.05) than among infants with a birth weight ≥2500 g (RR: 0.88; 95% CI: 0.80, 0.98; P-interaction = 0.006). Multivitamins were associated with a significantly lower risk of having a physician-diagnosis of diarrhea among low-birth-weight infants (RR: 0.65; 95% CI: 0.42, 0.99) than among infants with a birth weight ≥2500 g (RR: 1.14; 95% CI: 1.01, 1.29; P-interaction = 0.016).
TABLE 4.
Effect of multivitamin supplementation on common infectious morbidities among HIV-exposed Tanzanian children, stratified by baseline characteristics
| Effect modifier | Multivitamin | Placebo | RR (95% CI)1 | P value1 | 2 |
| Diarrhea [no. events/visits (%)]3 | |||||
| Sex | |||||
| Male | 510/11,295 (4.5) | 513/10,950 (4.7) | 0.97 (0.83, 1.14) | 0.69 | 0.96 |
| Female | 375/9236 (4.1) | 416/9824 (4.2) | 0.97 (0.82, 1.15) | 0.76 | |
| HIV status at 6 wk [no. events/visits (%)] | 0.42 | ||||
| Positive | 80/1411 (5.7) | 90/1803 (5.0) | 1.12 (0.80, 1.57) | 0.50 | |
| Negative | 800/18,852 (4.2) | 830/18,777 (4.4) | 0.97 (0.86, 1.10) | 0.64 | |
| Low birth weight [no. events/visits (%)] | 0.39 | ||||
| ≥2500 g | 815/18,961 (4.3) | 837/18,809 (4.5) | 0.80 (0.53, 1.21) | 0.29 | |
| <2500 g | 50/1186 (4.2) | 68/1257 (5.4) | 0.97 (0.86, 1.10) | 0.61 | |
| Cough [no. events/visits (%)] | |||||
| Sex | 0.28 | ||||
| Male | 3456/11,272 (30.7) | 3396/10,909 (31.1) | 0.99 (0.93, 1.06) | 0.80 | |
| Female | 2854/9220 (31.0) | 2921/9806 (29.7) | 1.05 (0.98, 1.12) | 0.22 | |
| HIV status at 6 wk [no. events/visits (%)] | 0.33 | ||||
| Positive | 536/1409 (38.0) | 624/1797 (34.7) | 1.10 (0.95, 1.27) | 0.21 | |
| Negative | 5707/18,816 (30.3) | 5611/18,724 (30.0) | 1.02 (0.97, 1.07) | 0.51 | |
| Low birth weight [no. events/visits (%)] | 0.65 | ||||
| ≥2500 g | 5792/18,928 (30.6) | 5707/18,758 (30.4) | 1.01 (0.96, 1.06) | 0.70 | |
| <2500 g | 389/1180 (33.0) | 395/1251 (31.6) | 1.05 (0.88, 1.26) | 0.56 | |
| Fever [no. events/visits (%)] | |||||
| Sex | 0.49 | ||||
| Male | 1337/11,273 (11.9) | 1450/10,914 (13.3) | 0.90 (0.81, 0.99) | 0.025 | |
| Female | 1012/9220 (11.0) | 1161/9806 (11.8) | 0.94 (0.84, 1.06) | 0.32 | |
| HIV status at 6 wk [no. events/visits (%)] | 0.08 | ||||
| Positive | 294/1409 (20.9) | 336/1797 (18.7) | 1.10 (0.91, 1.34) | 0.32 | |
| Negative | 2029/18,817 (10.8) | 2236/18,729 (11.9) | 0.91 (0.84, 0.99) | 0.019 | |
| Low birth weight [no. events/visits (%)] | 0.013 | ||||
| ≥2500 g | 2092/18,829 (11.1) | 2358/18,763 (12.6) | 0.89 (0.82, 0.96) | 0.002 | |
| <2500 g | 205/1180 (17.4) | 163/1251 (13.0) | 1.28 (0.97, 1.70) | 0.08 | |
| Respiratory infections, cough and fever [no. events/visits (%)] | |||||
| Sex | 0.41 | ||||
| Male | 766/11,273 (6.8) | 843/10,915 (7.7) | 0.89 (0.78, 1.01) | 0.07 | |
| Female | 596/9220 (6.5) | 665/9806 (6.8) | 0.96 (0.83, 1.11) | 0.58 | |
| HIV status at 6 wk [no. events/visits (%)] | 0.07 | ||||
| Positive | 181/1409 (12.9) | 192/1797 (10.7) | 1.18 (0.91, 1.54) | 0.21 | |
| Negative | 1164/18,817 (6.2) | 1290/18,730 (6.9) | 0.90 (0.82, 1.00) | 0.05 | |
| Low birth weight [no. events/visits (%)] | 0.006 | ||||
| ≥2500 g | 1210/18,929 (6.4) | 1367/18,764 (7.3) | 0.88 (0.80, 0.98) | 0.027 | |
| <2500 g | 129/1180 (10.9) | 91/1251 (7.3) | 1.46 (1.04, 2.05) | 0.029 | |
| Physician diagnoses of diarrhea4 | |||||
| Sex | |||||
| Male | 0.86 ± 1.25 | 0.85 ± 1.3 | 1.09 (0.93, 1.28) | 0.28 | 0.94 |
| Female | 0.94 ± 1.3 | 0.79 ± 1.2 | 1.10 (0.93, 1.31) | 0.27 | |
| HIV status at 6 wk4 | |||||
| Positive | 0.78 ± 1.2 | 0.86 ± 1.3 | 1.03 (0.71, 1.49) | 0.11 | 0.73 |
| Negative | 0.92 ± 1.3 | 0.83 ± 1.3 | 1.11 (0.71, 1.49) | 0.87 | |
| Low birth weight4 | |||||
| ≥2500 g | 0.91 ± 1.3 | 0.80 ± 1.2 | 1.14 (1.01, 1.29) | 0.036 | 0.016 |
| <2500 g | 0.93 ± 1.4 | 1.42 ± 1.8 | 0.65 (0.42, 0.99) | 0.047 |
RRs, 95% CIs, and corresponding P values were obtained from generalized estimating equations with binomial distribution, log link, and exchangeable correlation structure. The analyses were adjusted for the age of the children (<6, 6–18, and >18 mo).
value for interaction effect.
Total number of events occurring during follow-up, defined as being reported in the 28 d (4 wk) before the visit.
RRs, 95% CIs, and corresponding P values were obtained from generalized estimating equations with the Poisson distribution and log link and by using follow-up time as the offset variable.
Mean ± SD (all such values).
Median (25th, 75th percentiles) regimen compliance among children was 96 (91, 99) of the allocated regimen. Of 1157 total children with compliance greater than the median, there were 49 deaths (8.3%) in the multivitamin group and 61 deaths (10.7%) in the placebo group [HR: 0.77 (0.53, 1.12), P = 0.17]. Median (25th, 75th percentiles) multivitamin compliance among mothers was 64 (56, 71) of the allocated regimen. Of 1231 total mothers with compliance greater than the median, there were 86 child deaths (13.7%) in the multivitamin group and 72 deaths (12.0%) in the placebo group [HR: 1.17 (0.85, 1.60), P = 0.34].
Of 429 children whose mothers received ARVs during pregnancy, there were 21 child deaths in the multivitamin group compared with 25 deaths in the placebo group (RR: 0.81; 95% CI: 0.46, 1.45; P = 0.49) and 13 unscheduled hospitalizations in the multivitamin group compared with 28 in the placebo group (RR: 0.48; 95% CI: 0.24, 0.95; P = 0.035). Maternal reports of fever and cough with fever were significantly lower in the multivitamin group in this subset (Table 5).
TABLE 5.
Rates of hospitalizations, unscheduled clinic visits, and symptoms of common infectious illnesses among 429 HIV-exposed Tanzanian children randomly assigned to receive daily multivitamins or placebo whose mothers received antiretrovirals during pregnancy
| Morbidity | Multivitamin | Placebo | RR (95% CI) | P value1 |
| 2 | 2 | |||
| Hospitalizations | 0.30 (13/4295) | 0.69 (28/4057) | 0.48 (0.24, 0.95) | 0.035 |
| Unscheduled outpatient visits | 3.31 (142/4295) | 3.18 (129/4057) | 1.05 (0.80, 1.38) | 0.72 |
| Diarrhea | 3.42 (147/4295) | 3.45 (140/4057) | 0.99 (0.77, 1.28) | 0.94 |
| Cold | 24.96 (1069/4295) | 26.96 (1090/4057) | 0.92 (0.83, 1.02) | 0.12 |
| Cough | 33.08 (1417/4295) | 33.12 (1339/4057) | 0.98 (0.89, 1.09) | 0.71 |
| Difficulty breathing | 0.93 (40/4295) | 0.96 (39/4057) | 0.95 (0.59, 1.55) | 0.85 |
| Fever | 10.11 (433/4295) | 12.27 (496/4057) | 0.79 (0.67, 0.93) | 0.005 |
| Refusal to eat, drink, or breastfeed | 2.47 (106/4295) | 2.70 (109/4057) | 0.93 (0.69, 1.25) | 0.61 |
| Pus draining from ears | 0.63 (27/4295) | 0.74 (30/4057) | 0.78 (0.40, 1.53) | 0.47 |
| Vomiting | 1.21 (52/4295) | 1.34 (54/4057) | 0.91 (0.60, 1.39) | 0.66 |
| Cough and fever | 5.98 (256/4295) | 7.35 (297/4057) | 0.79 (0.65, 0.96) | 0.019 |
| Cough plus3 | 1.84 (79/4295) | 2.33 (94/4057) | 0.78 (0.57, 1.08) | 0.13 |
Obtained from generalized estimating equations with the binomial distribution, log link, and exchangeable correlation structure. The analyses were adjusted for the age of the child (<6, 6–18, and >18 mo).
Total number of events occurring during follow-up, defined as being reported in the 28 d (4 wk) before the visit.
Defined as cough with one or more of the following events: difficulty breathing, chest retractions, and refusal to eat, drink, or breastfeed.
DISCUSSION
The metabolic and nutritional demands of infants born to HIV-infected women in resource-poor countries may differ from those of healthy infants, and much evidence suggests that their diets may be low or deficient in several micronutrients. Among breastfeeding women in South Africa, low blood concentrations of folate, zinc, vitamin B-12, and vitamins E and A were noted (22), which suggests that infant micronutrient status might also be impaired. Dietary requirements of micronutrients may also be increased in the settings of frequent infectious or inflammatory stress (23). We performed a randomized, double-blinded, placebo-controlled clinical trial of multivitamin supplements among 2387 HIV-exposed Tanzanian infants. We detected no difference in all-cause mortality with micronutrient supplementation or substantive declines in important morbidities (eg, hospitalizations, unscheduled clinic visits, and frequency of diarrheal symptoms). The frequencies of vomiting and fever were reduced in the multivitamin group.
The role of multiple micronutrient supplements in reducing child mortality has not been widely evaluated. In a randomized trial of 847 children with HIV infection in Uganda, the provision of twice the RDA of 14 micronutrients (thiamine, riboflavin, niacin, folate, and vitamins A, B-6, B-12, C, D, and E and zinc, copper, iodine, and selenium) was not associated with reduced mortality (5.9% at 12 mo) compared with children who received a single RDA of 6 vitamins (thiamine, riboflavin, and niacin and vitamins A, C, and D) (6.7%; RR: 0.9; 95% CI: 0.5,1.5) (24). Among 373 rural South African children (32 of whom were HIV-infected and 154 of whom were born to HIV-infected mothers), supplementation with micronutrients (thiamine, riboflavin, niacin and vitamins A, C, D, E, and K and zinc, copper, iodine, and iron) was not associated with lower mortality than was vitamin A or vitamin A plus zinc (25). Respiratory and gastrointestinal infections were also not reduced with multivitamin supplementation (26). Compared with these studies, our trial was larger, began micronutrient supplementation earlier in life (6 wk compared with 6–12 mo), followed the cohort longer (24 mo compared with 12–18 mo), and observed higher mortality rates.
Subgroup analyses resulted in some potentially interesting findings in the trial. Among low-birth-weight infants, multivitamin supplementation was associated with a significantly higher incidence of maternally reported fever and cough and fever, but a lower incidence of physician-diagnosed diarrhea. Among children whose mothers received ARVs, multivitamins significantly reduced unscheduled hospitalizations and reports of fever and cough with fever. These findings deserve further study and indicate the importance of considering nutritional status and common morbidities when evaluating the effect of ARV use in women and children in resource-poor areas (27).
Our results contrast with previous data documenting reductions in child mortality or diarrhea among children whose mothers received multivitamins during pregnancy and lactation—findings that supported the rationale for our intervention directly to children. For example, in Indonesia, infants of women provided with multiple micronutrients showed an 18% reduction in early infant mortality compared with those of women given iron and folate alone (35.5 deaths/1000 live births compared with 43/1000; RR: 0.82; 95% CI: 0.70, 0.95; P = 0.010) (28). Among Tanzanian infants born to HIV-infected mothers, children born to women who were assigned to receive multivitamins (vitamin B-complex and vitamins C and E) had a 17% significantly lower risk of diarrhea compared with children whose mothers received no multivitamins (RR: 0.83; 95% CI: 0.71, 0.98; P < 0.03) (12). Owing to these latter findings, routine maternal micronutrient supplementation was the standard of care in our trial—an intervention that may have limited any possible benefits of direct child supplementation.
Several other aspects of our trial deserve comment. As noted, the availability of antiretroviral therapy increased substantially over the course of the study, which may have influenced the occurrence of our primary and secondary outcomes. We did, however, take into consideration the lower observed rates of infant and child mortality (29) by increasing the sample size. Other possible explanations for our lack of positive findings include poor compliance with the regimen, replete baseline nutritional status of the infants, and inadequate dosing/composition of the micronutrient regimen selected. In addition, all children received high-dose vitamin A supplementation, a practice that itself reduces child mortality (5). Compliance was measured by pill count and monthly review of dosing practices and generally seemed adequate. High rates of baseline anemia and undernutrition (30) in our cohort suggest an underlying risk of micronutrient deficiencies. We chose micronutrient doses that were safe for young infants and whose components had previously been shown to be effective among HIV-infected mothers.
Perhaps the most important factor to consider in the trial was our provision of high-dose micronutrient supplements to all mothers enrolled. Micronutrient supplementation may positively affect quality and quantity of life among pregnant HIV-infected women, which may have implications for quality of caregiving behavior to infants and young children that may secondarily reduce morbidity and mortality. In addition, maternal micronutrients may improve breast-milk nutrient quantity (14, 31), thereby indirectly increasing infant micronutrient intakes. Our results, therefore, do not exclude the possibility that direct infant supplementation with micronutrients in areas of the world where maternal micronutrient supplementation is not the standard of care could improve child health outcomes.
In summary, daily micronutrients provided to infants born to HIV-positive Tanzanian mothers did not reduce all-cause mortality, hospitalizations, or unscheduled clinic visits. Reports of fever and vomiting were less frequent in supplemented children. Infants whose mothers received ARVs had fewer unscheduled hospitalizations and reports of fever and cough with fever, but unchanged mortality. In the setting of high-dose maternal micronutrient supplementation, HIV-exposed infants may not benefit substantially from routine multivitamin supplementation. Studies of alternative nutrient supplementation regimens that include vitamin A and zinc, and studies in the setting of more widespread maternal ARV use, should be considered.
Acknowledgments
We thank the mothers and children and field teams, including physicians, nurses, midwives, supervisors, laboratory staff, and administrative staff, who made this study possible; Muhimbili National Hospital, Muhimbili University of Health and Allied Sciences, and the National AIDS Control Program in Dar es Salaam for their institutional support; the field and study staff for their tireless efforts (Rehema Mtonga, Illuminata Ballonzi, Godwin Njiro, Frank Killa, Edgar Basheka, Susie Welty, Rachel Steinfeld, Anne Marie Darling, Angela Jardin, Lana Corrales, Sibtain Moledina, Faheem Sheriff, Mohamed Manji, Frank Lazaro, Mohamed Aloo, and Elizabeth Long); and members of the DSMB (Marcello Pagano – Chair, Nicholas Horton, Andrew Kitua, and Charles Mgone).
The authors’ responsibilities were as follows—CD, WWF, RJB, and KPM: designed the research; R Kupka, R Kisenge, SA, and KPM: conducted the research; JO and RJB: analyzed the data; and CD: wrote the manuscript and had primary responsibility for its final content. All authors contributed to and approved the final manuscript. None of the authors had any conflicts of interest.
Footnotes
Abbreviations used: ARV, antiretroviral therapy; DSMB, Data Safety and Monitoring Board; RDA, Recommended Dietary Allowance.
REFERENCES
- 1.WHO The global burden of disease: 2004 update. Geneva, Switzerland: World Health Organization, 2008 [Google Scholar]
- 2.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012;379:2151–61 [DOI] [PubMed] [Google Scholar]
- 3.Joint United Nations Program on HIV/AIDS/World Health Organization (UNAIDS/WHO) AIDS epidemic update 2009. Geneva, Switzerland: UNAIDS/WHO, 2009 [Google Scholar]
- 4.Yakoob MY, Theodoratou E, Jabeen A, Imdad A, Eisele TP, Ferguson J, Jhass A, Rudan I, Campbell H, Black RE, et al. Preventive zinc supplementation in developing countries: impact on mortality and morbidity due to diarrhea, pneumonia and malaria. BMC Public Health 2011;11(suppl 3):S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ 2011;343:d5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haider BA, Bhutta ZA. The effect of therapeutic zinc supplementation among young children with selected infections: a review of the evidence. Food Nutr Bull 2009;30(Suppl):S41–59 [DOI] [PubMed] [Google Scholar]
- 7.Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, Haider BA, Kirkwood B, Morris SS, Sachdev HP, et al. What works? Interventions for maternal and child undernutrition and survival. Lancet 2008;371:417–40 [DOI] [PubMed] [Google Scholar]
- 8.Duggan C, Fawzi W. Micronutrients and child health: studies in international nutrition and HIV infection. Nutr Rev 2001;59:358–69 [DOI] [PubMed] [Google Scholar]
- 9.Lunney KM, Jenkins AL, Tavengwa NV, Majo F, Chidhanguro D, Iliff P, Strickland GT, Piwoz E, Iannotti L, Humphrey JH. HIV-positive poor women may stop breast-feeding early to protect their infants from HIV infection although available replacement diets are grossly inadequate. J Nutr 2008;138:351–7 [DOI] [PubMed] [Google Scholar]
- 10.Fawzi WW, Msamanga GI, Spiegelman D, Urassa EJ, McGrath N, Mwakagile D, Antelman G, Mbise R, Herrera G, Kapiga S, et al. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1-infected women in Tanzania. Lancet 1998;351:1477–82 [DOI] [PubMed] [Google Scholar]
- 11.Fawzi WW, Msamanga GI, Spiegelman D, Wei R, Kapiga S, Villamor E, Mwakagile D, Mugusi F, Essex M, Hunter D. A randomized trial of multivitamin supplements and HIV disease progression and mortality. N Engl J Med 2004;351:23–32 [DOI] [PubMed] [Google Scholar]
- 12.Fawzi WW, Msamanga GI, Wei R, Spiegelman D, Antelman G, Villamor E, Manji K, Hunter D. Effect of providing vitamin supplements to human immunodeficiency virus-infected, lactating mothers on the child's morbidity and CD4+ cell counts. Clin Infect Dis 2003;36:1053–62 [DOI] [PubMed] [Google Scholar]
- 13.Villamor E, Saathoff E, Bosch RJ, Hertzmark E, Baylin A, Manji K, Msamanga G, Hunter DJ, Fawzi WW. Vitamin supplementation of HIV-infected women improves postnatal child growth. Am J Clin Nutr 2005;81:880–8 [DOI] [PubMed] [Google Scholar]
- 14.Baylin A, Villamor E, Rifai N, Msamanga G, Fawzi WW. Effect of vitamin supplementation to HIV-infected pregnant women on the micronutrient status of their infants. Eur J Clin Nutr 2005;59:960–8 [DOI] [PubMed] [Google Scholar]
- 15.Aboud S, Urassa W, Lyamuya E, Mhalu F, Biberfeld G. Evaluation of HIV antibody and antigen/antibody combination ELISAs for use in an alternative confirmatory HIV testing strategy in Dar es Salaam, Tanzania. J Virol Methods 2006;135:192–6 [DOI] [PubMed] [Google Scholar]
- 16.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: National Academy Press, 1997 [Google Scholar]
- 17.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: National Academy Press, 2000 [Google Scholar]
- 18.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academy Press, 2000 [Google Scholar]
- 19.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academy Press, 2002 [Google Scholar]
- 20.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Washington, DC: National Academy Press, 2002 [Google Scholar]
- 21.Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, Sherman J, Bakaki P, Ducar C, Deseyve M, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 1999;354:795–802 [DOI] [PubMed] [Google Scholar]
- 22.Papathakis PC, Rollins NC, Chantry CJ, Bennish ML, Brown KH. Micronutrient status during lactation in HIV-infected and HIV-uninfected South African women during the first 6 mo after delivery. Am J Clin Nutr 2007;85:182–92 [DOI] [PubMed] [Google Scholar]
- 23.Scrimshaw NS. Historical concepts of interactions, synergism and antagonism between nutrition and infection. J Nutr 2003;133:316S–21S [DOI] [PubMed] [Google Scholar]
- 24.Ndeezi G, Tylleskar T, Ndugwa CM, Tumwine JK. Effect of multiple micronutrient supplementation on survival of HIV-infected children in Uganda: a randomized, controlled trial. J Int AIDS Soc 2010;13:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luabeya KK, Mpontshane N, Mackay M, Ward H, Elson I, Chhagan M, Tomkins A, Van den Broeck J, Bennish ML. Zinc or multiple micronutrient supplementation to reduce diarrhea and respiratory disease in South African children: a randomized controlled trial. PLoS ONE 2007;2:e541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chhagan MK, Van den Broeck J, Luabeya KK, Mpontshane N, Tucker KL, Bennish ML. Effect of micronutrient supplementation on diarrhoeal disease among stunted children in rural South Africa. Eur J Clin Nutr 2009;63:850–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nielsen-Saines K, Komarow L, Cu-Uvin S, Jourdain G, Klingman KL, Shapiro DE, Mofenson L, Moran L, Campbell TB, Hitti J, et al. Infant outcomes after maternal antiretroviral exposure in resource-limited settings. Pediatrics 2012;129(6):e1525–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shankar AH, Jahari AB, Sebayang SK, Aditiawarman , Apriatni M, Harefa B, Muadz H, Soesbandoro SD, Tjiong R, Fachry A, et al. Effect of maternal multiple micronutrient supplementation on fetal loss and infant death in Indonesia: a double-blind cluster-randomised trial. Lancet 2008;371:215–27 [DOI] [PubMed] [Google Scholar]
- 29.Masanja H, de Savigny D, Smithson P, Schellenberg J, John T, Mbuya C, Upunda G, Boerma T, Victora C, Smith T, et al. Child survival gains in Tanzania: analysis of data from demographic and health surveys. Lancet 2008;371:1276–83 [DOI] [PubMed] [Google Scholar]
- 30.McDonald C, Kupka R, Manji KP, Okuma J, Bosch RJ, Fawzi WW, Duggan C. Predictors of stunting, wasting, and underweight among infants born to HIV-infected women in Tanzania. FASEB J 2011;25:216.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olafsdottir AS, Wagner K-H, Thorsdottir I, Elmadfa I. Fat-soluble vitamins in the maternal diet, influence of cod liver oil supplementation and impact of the maternal diet on human milk composition. Ann Nutr Metab 2001;45:265–72 [DOI] [PubMed] [Google Scholar]