Abstract
Background: Choline is essential for fetal brain development, and it is not known whether a typical American diet contains enough choline to ensure optimal brain development.
Objective: The study was undertaken to determine whether supplementing pregnant women with phosphatidylcholine (the main dietary source of choline) improves the cognitive abilities of their offspring.
Design: In a double-blind, randomized controlled trial, 140 pregnant women were randomly assigned to receive supplemental phosphatidylcholine (750 mg) or a placebo (corn oil) from 18 wk gestation through 90 d postpartum. Their infants (n = 99) were tested for short-term visuospatial memory, long-term episodic memory, language development, and global development at 10 and 12 mo of age.
Results: The women studied ate diets that delivered ∼360 mg choline/d in foods (∼80% of the recommended intake for pregnant women, 65% of the recommended intake for lactating women). The phosphatidylcholine supplements were well tolerated. Groups did not differ significantly in global development, language development, short-term visuospatial memory, or long-term episodic memory.
Conclusions: Phosphatidylcholine supplementation of pregnant women eating diets containing moderate amounts of choline did not enhance their infants’ brain function. It is possible that a longer follow-up period would reveal late-emerging effects. Moreover, future studies should determine whether supplementing mothers eating diets much lower in choline content, such as those consumed in several low-income countries, would enhance infant brain development. This trial was registered at clinicaltrials.gov as NCT00678925.
INTRODUCTION
Choline is an essential nutrient found in foods such as milk, meat, and eggs; it has important functions during the fetal and neonatal periods, when the brain is rapidly developing. Attesting to the importance of choline for development, large amounts of choline are delivered to the fetus from the mother across the placenta (1, 2), exposing the fetus to very high choline concentrations (3, 4) and thereby presumably ensuring enhanced availability of choline to tissues. In mammals, the choline concentration in amniotic fluid is 10-fold greater than that in maternal blood (5). Human and rodent milk provide large amounts of choline to the neonate (6, 7), because mechanisms in the mammary glands uptake choline at high rates (8).
Nonetheless, we do not know the amount of dietary choline that women need to ensure optimal infant brain development. There is a wide variation in choline intake in the diet; in several US cohorts the average daily choline intake has been estimated to be ∼300 mg (9–12), but intake of choline is estimated to be half of this amount in low-income countries (13). The recommended choline intake (Adequate Intake) is 450 mg/d for pregnant women and 550 mg/d for lactating women (14). Normal dietary intake of betaine (derived from choline) is 100 mg/d in the United States (11). There is no recommended intake for betaine.
In rodents, high maternal dietary intake of choline enhances hippocampal function (15–17) and related cognitive abilities (18–23) in offspring pups. More specifically, in rodent studies choline supplementation and/or choline deficiency during late pregnancy is associated with significant and irreversible changes in hippocampal function in the adult animal, including altered long-term potentiation, an electrical property of brain plasticity that is active in memory processing (15, 16, 24–26) and altered memory (18–23). It is not yet known whether the effects on brain development and subsequent cognition that are seen in animal models translate to effects in humans. It is known that low maternal dietary intake of choline is associated with an increase in birth defects in rodents (27, 28) and in humans (29, 30).
In humans, the behavioral evidence indicates that once the hippocampal system reaches peak synaptic development [between 12 and 15 mo of age (31)] it is functionally mature. In the months before this (7–12 mo of age), we would expect, and do indeed find, variation in long-term episodic memory (memory across a delay for events in the environment) (32) and short-term visuospatial memory (memory for location of objects in visual space) (33, 34). Given the effects of choline on the hippocampus, we explored its effects on hippocampal-related cognition. To our knowledge, the effects of maternal choline supplementation on cognitive development in humans have not been explored. This study was undertaken to determine whether supplementing pregnant and lactating women with phosphatidylcholine in addition to normal dietary intake of choline would enhance the cognitive abilities of their infants.
SUBJECTS AND METHODS
Subjects
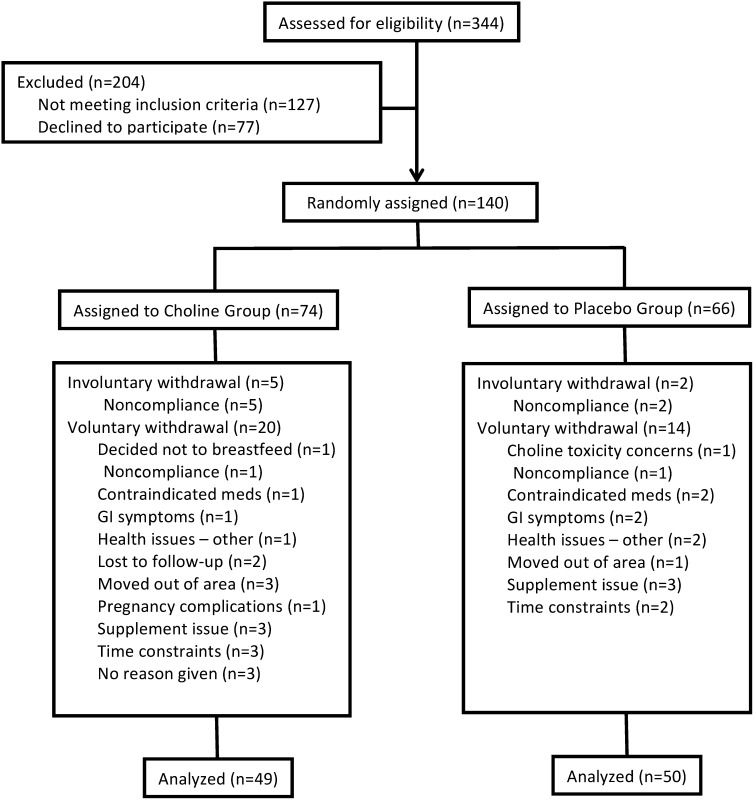
Resources and limitations in the availability of participants restricted sample size. Nonetheless, a power analysis using data from a pilot study showed that a sample of 128 would enable us to detect a medium effect size (f = 0.25) at a power level of 0.8. It was estimated that dropouts or complications at birth would result in a 10% loss. Thus, between December 2004 and July 2006, 140 pregnant, healthy women were enrolled from the Raleigh-Durham–Chapel Hill, North Carolina, metropolitan area in a double-blind study approved by the Institutional Review Board at the University of North Carolina at Chapel Hill. Neither participants nor researchers were aware of any individual participant's group membership. Details on the enrollment, randomization, withdrawal, and follow-up of participants are shown in Figure 1: 99 participants completed the intervention (n = 49 treatment, 50 placebo), with a total of 41 either dropping out voluntarily (n = 34) or being discharged for noncompliance (n = 7). The age range of the mothers was 21–41 y. Pregnant women who were healthy, who had a typically proceeding pregnancy, expressed an intention to breastfeed for ≥90 d, were taking a prenatal vitamin, were receiving regular prenatal care, and were fluent in English were eligible to participate. Women who were pregnant with multiple fetuses or were using tobacco products, alcohol, or illicit drugs were deemed ineligible. In addition, women were excluded if they had a history of chronic illness, were allergic to soy or corn, or had a prepregnancy BMI (in kg/m2) <18 or ≥35.
FIGURE 1.
Flow diagram of the enrollment, random assignment, voluntary and involuntary withdrawals, and follow-up of the study participants. GI, gastrointestinal; meds, medications.
All 99 infants (58 females, 41 males) were born healthy between June 2005 and December 2006. Baseline characteristics of the 99 mothers and their infants are provided in Table 1. Women in the control group weighed more and had a higher BMI on average at conception than did women in the supplemented group. The ethnic composition of the final sample was as follows: white (89%), African American (3%), Asian (6%), Native American (1%), and other (1%), which approximates the diversity of the catchment area. The study ended as planned when the infants had all completed the 12-mo assessments.
TABLE 1.
Characteristics of the mothers and infants at baseline1
| Choline group(n = 49) | Control group(n = 50) | |
| Maternal characteristics | ||
| Age at conception (y) | 30.2 ± 3.8 | 30.8 ± 4.9 |
| Weight at conception (kg) | 59.5 ± 11.8 | 65.3 ± 15.1* |
| BMI at conception (kg/m2) | 22.1 ± 2.2 | 24.0 ± 4.1* |
| Weight gain during pregnancy (kg) | 15.3 ± 4.9 | 15.6 ± 4.6 |
| Maternal education (y) | 17.3 ± 1.7 | 17.0 ± 1.6 |
| Paternal education (y) | 17.0 ± 2.2 | 16.7 ± 2.2 |
| Annual income ($ thousands) | 62.6 ± 31.4 | 68.2 ± 42.7 |
| Infant characteristics | ||
| Length of gestation (wk) | 39.5 ± 1.6 | 39.3 ± 1.4 |
| Weight at birth (kg) | 3.4 ± 0.5 | 3.5 ± 0.5 |
| Length at birth (cm) | 51.6 ± 2.5 | 51.8 ± 2.5 |
| Occipitofrontal circumference (cm) | 34.6 ± 1.4 | 34.4 ± 1.6 |
All values are means ± SDs. *Significantly different from the supplemented group, P < 0.05.
Study design
Intervention
Pregnant women were randomly assigned to consume either choline or placebo capsules from 18 wk of gestation to 90 d postpartum according to a table generated by the study biostatistician. The randomization was done in 25 blocks of 4, which ensured that every 4 participants were assigned equally between the 2 groups.
Staff personnel randomly assigned participants (n = 74) to consume supplemental choline (750 mg choline/d; PhosChol, Nutrasal) as 6 gel caps. These capsules contain 833 mg phosphatidylcholine/gel cap; phosphatidylcholine is ∼15% choline molecule by weight. The other participants (n = 66) were assigned to consume 6 corn oil placebo gel caps per day. Choline and placebo capsules were identical in color, shape, and size. Researchers and mothers were blind to group assignment. Phosphatidylcholine was used as the dietary supplement because it is not converted by gut bacteria to trimethylamine, which imparts a fishy body odor; choline is a substrate for trimethylamine formation (35). There are differences in the bioavailability of choline and phosphatidylcholine eaten in the diet (5); however, most of the choline in the diet is in the form of phosphatidylcholine (36).
All mothers had expressed the intention to breastfeed for ≥90 d; all infants were breastfed for a minimum of 45 d. Infant assessments were scheduled at 10 and 12 mo, with age based on the child's estimated due date to correct for preterm or late delivery.
Adverse events in the phosphatidylcholine-supplemented and placebo groups were reviewed by a Data Safety Monitoring Board. All mothers of infants included in this report complied with the protocol, as indicated by capsule counts and elevations in blood choline concentrations in samples collected at intervals during pregnancy and lactation (37).
Dietary analysis of choline intake
All participants were asked to keep a complete 3-d food record, reflective of their usual intake, immediately before their 30-wk gestation and 45-d postpartum visits. Participants were asked to record everything that they ate and drank on 2 typical weekdays and 1 weekend day. Daily food intake records were analyzed by using the Esha Food Processor SQL program (version 10.3; ESHA Research). This nutrient analysis software references the USDA National Nutrient Database and includes food values for choline from the USDA Database for the Choline Content of Common Foods–2008 (http://www.nal.usda.gov/fnic/foodcomp/Data/Choline/Choln02.pdf). This software does not include values for betaine—a metabolite of choline. Hence, all known food betaine values (36, 38) were manually entered into the database before conducting the analyses and referenced according to the 5-digit Nutrient Databank Number. The total choline content of a particular food was calculated as the sum of the amounts of choline, phosphocholine, glycerophosphocholine, phosphatidylcholine, and sphingomyelin. Betaine was calculated independently. If the total choline or betaine content of a specific food item was unavailable, a nutritionally equivalent food was substituted in the analysis (eg, peach for apricot). Each day of the 3-d food record was analyzed individually and then averaged for each subject. The total choline intake was estimated by adding the average amount of choline ingested per day with the amount provided in the supplement (750 or 0 mg) multiplied by the individuals’ compliance rate, to increase the precision of intake variable.
Assessment of infant cognitive abilities
Infants were tested with a battery of cognitive assessments at 2 visits separated by 7–10 d at both 10 and 12 mo, for a total of 4 assessment visits. The 10- and 12-mo sessions were identical. Caregivers were present throughout the visit, holding their infants in their laps.
Short-term visuospatial memory
At each session, infants were tested twice on a short-term Visuospatial Memory Delayed Response Task (33, 39–41). The infant was seated on the caregiver's lap facing a testing table 80 cm high, 91 cm long, 30.5 cm wide, 8 cm tall with 3 embedded wells, 11 cm in diameter, and 5 cm deep arranged in a triangular pattern. For the 2-well protocol, an auxiliary top that covered the middle well and left only the outer 2 visible was put in place. There were 6 trials with 2 wells and then 6 trials with 3 wells, for a total of 12 trials per administration and 24 trials within a session. Within each 6-trial set, the order of delays administered was 3-9-15-3-9-15 s with location counterbalanced such that the stimulus was hidden 3 times on the left and 3 times on the right in the 2-well trials and 2 times on the right, 2 times on the left, and 2 times in the middle in the 3-well trials. The same order of location and delay was used for all tests and for all infants. The varied pattern of delays (3-9-15-3-9-15 s) controlled for fatigue: if an infant did not get a 15-s trial correct, but was able to choose correctly on the subsequent 3-s trial, we inferred that the issue with the 15-s trial was that the memory was not retained across the longer delay rather than that the infant was too tired to continue.
Before starting the task, infants were presented with an array of small toys (eg, plastic keys, rattles) from which to choose; the chosen toy was used in the trials. Up to 5 training trials with a delay of 1 s between hiding and encouragement to find it were presented on the first administration of the day and then the scored trials were presented. For each trial, the infant watched as the experimenter hid the object in a well. The wells were then covered simultaneously, eye contact was established with the infant, and the delay was imposed, whereas the researcher counted and clapped to draw the infant's gaze away from the wells during the delay. After the delay, the infant was encouraged to search for the object. Testing was discontinued when the infant lost interest and the experimenter was unable to reinstate interest through the use of new toys or when the researcher or parent determined that the infant was too fussy to continue. Trained observers coded the first reach on each trial and only reaches that resulted in the cover being removed were coded. If the infant reached with both hands at once, coding was completed based on visual regard. That is, the well at which the infant was looking at when the 2-handed reach occurred was coded as the choice. Memory was inferred when the infant found the hidden toy.
Long-term episodic memory
To test for long-term memory abilities, we used an imitation paradigm in which infants were given the opportunity to imitate an action modeled by the researcher after a delay (deferred imitation). Imitation paradigms are well accepted and widely used as a memory assessment for preverbal infants (42–47). Sixteen different objects and actions were tested: 4 different object-action sets in each of 4 sessions. A unique primary target action was defined for each object; alternative actions were available, if needed (see below). For each object in turn, there was a 30-s baseline period and a 30-s modeling period. The infant was allowed to explore an object for 30 s (baseline). After 30 s, the researcher retrieved the object and demonstrated the target action 3 times within 30 s to ensure that the infant had seen and understood it. If the infant spontaneously produced the target action during baseline, the alternative action was modeled. For example, if the infant rolled the paint roller on the table (primary target action) on her or his own, the action demonstrated with the paint roller was to roll it on the face (alternative action). This pattern of exploration (30 s) and demonstration of target action (30 s) occurred for each of the 4 objects in the set for a total of about 4 min. After a 15-min delay, which was filled with other tasks, the 4 objects were presented, in turn, and the infant was allowed to freely explore the object for 30 s. Long-term episodic memory (as measured by deferred imitation) was inferred when the infant repeated the target action demonstrated by the researcher. Items were scored during the task by the researcher.
Language development
Early language development was assessed with the MacArthur-Bates Short Form Vocabulary Checklist: Level I (48), a parent report of the words the infant understands and the words the infant says, using a list of 89 words that were systematically selected from the larger 396-item vocabulary checklist of the MacArthur-Bates Communicative Development Inventory (CDI): Words and Gestures (49). Caregivers completed the Short Form Vocabulary Checklist at the 10- and 12-mo visits; it was scored according to the guidelines provided in the peer-reviewed scientific article that described its derivation and presented the list (48). The words spoken score was used in analyses because it is less subjective than the words understood score, even though spoken vocabulary is limited at this age.
Global development
The Mullen Scales of Early Learning, AGS edition (50), was used to assess motor, language, and cognitive development. Mullen test items are standardized infant assessment procedures, such as tracking a moving object, moving blocks in or out of a container, solving puzzles, and responding to verbal requests or questions. The Mullen is divided into 5 separate scales: motor ability (fine and gross), visual reception, and language ability (receptive and expressive). There are age-standardized T scores and age-equivalency scores for each scale as well as a comprehensive index comprising the standardized scores of 4 of the subscales (visual reception, fine motor, and receptive and expressive language). Item administration and scoring were completed according to the standardized methods at 10 and 12 mo, with the administration of the test being split between the 2 visits at each age (fine motor and visual reception at the first session; gross motor and expressive and receptive language at the second session).
Data reduction and analyses
Short-Term Visuospatial Memory Task
Each infant participated in a maximum of 24 trials (2 sets of 12) at each session. Percentage correct scores were calculated for each set by dividing the number of correct responses by the number of trials (maximum of 12) on which the infant made a response. If the denominator of this ratio is too small, the estimate of performance is not reliable. By comparing cross-assessment correlations at various cutoffs, we determined that ≥4 trials were needed for a reliable data point. Thus, if an infant had attempted ≤3 trials, the percentage correct score was considered missing data. Across sessions and ages, this rule resulted in the loss of 6 to 13 participants’ scores in the first administration and 39–46 participants’ scores in the second administration of this task. The consistently high number of infants who did not complete 4 or more trials in the second administration of each session suggests that the infants were tired, bored, or frustrated with the task the second time. Thus, because of the large amount of missing data from the second administrations, only the percentage correct scores from the first administration at each session, averaged together, were used in the analyses.
Long-Term Episodic Memory Task
With the exception of one participant, all infants completed all 4 trials within each session. Paired-sample t tests showed no differences between the scores from the 2 sessions that were completed within age. Thus, the scores were averaged together, and a percentage correct within age was computed. All analyses were completed with this variable.
Analyses
The treatment and control group data were subjected to multivariate ANOVA by using the SPSS Inc data analyses package (version 19). Ten-month data and 12-mo data were analyzed separately. Short-term visuospatial memory percentage-correct scores, long-term episodic memory percentage-correct scores, and words spoken scores were entered as dependent variables for each age, in turn. The number of missing data points in the short-term visuospatial memory scores reduced the number of valid cases to 76. Thus, if it was determined that the short-term visuospatial memory scores were not integral to the model (ie, accounted for no variance), the analyses were repeated without those variables to restore the number of valid cases to 99.
RESULTS
Choline and betaine intake
Mothers in the choline group consumed a supplement of 750 mg choline/d in the form of phosphatidylcholine. This is ∼140% of the recommended adequate intake for lactating women and 170% of the recommended adequate intake for pregnant women. Three-day food records collected at 30 wk of gestation and 45 d postpartum were analyzed for dietary choline and betaine intakes. There was no difference between the groups in dietary choline intake at 30 wk gestation (363 ± 90.3 and 370 ± 113.9 mg/d in the placebo and choline groups, respectively) or at 45 d postpartum (369 ± 123.5 and 343 ± 92.8 mg/d in the placebo and choline groups, respectively). Similarly, there was no difference between the groups in dietary betaine intake at 45 d postpartum (296 ± 214.9 and 291 ± 245.1 mg/d in the placebo and choline groups, respectively). However, there was a difference between groups in dietary betaine intake at 30 wk gestation (225 ± 96.2 and 327 ± 267.3 mg/d in the placebo and choline groups, respectively; P < 0.05).
Safety of supplementation
The percentages of adverse events in the choline and placebo groups were not significantly different. Moreover, most of the adverse events were of minor severity, none were definitively related to the study, and no severe adverse events were reported. In addition, according to the study physician and external monitor, the adverse events were very typical of a normal obstetric population and did not occur with greater frequency or severity among the study participants.
Analyses of cognitive measures by treatment group
Descriptive statistics for the cognitive variables are provided in Table 2. In general, the data were normally distributed, and scores were within the range of typical development (33, 34, 48, 50). Age effects emerged as would be expected in cognitive development, with evidence of increased performance from 10 to 12 mo in the Short-Term Visuospatial Memory Task (P < 0.0001), in the Long-Term Episodic Memory Task (P < 0.0001), and in words spoken (P < 0.0001). Scores on the Mullen Early Learning composite scores at 10 mo or at 12 mo were above average (average = 100). The scores indicate age-appropriate overall development for the sample as a whole.
TABLE 2.
Descriptive statistics for cognitive tasks by treatment group
| Placebo |
Supplement |
||||||
| n | Mean ± SD | Range | n | Mean ± SD | Range | 1 | |
| 10 Mo | |||||||
| Visuospatial Memory Task | 50 | 0.64 ± 0.14 | 0.42–0.94 | 48 | 0.56 ± 0.19 | 0.25–1.0 | 0.243 |
| Long-Term Memory Task | 50 | 0.34 ± 0.02 | 0–0.88 | 49 | 0.33 ± 0.20 | 0–0.75 | 0.705 |
| Expressive Vocabulary | 50 | 1.28 ± 1.67 | 0–7 | 48 | 1.40 ± 1.60 | 0–6 | 0.697 |
| Global Development Index | 50 | 119.6 ± 10.0 | 98–139 | 48 | 119.3 ± 9.13 | 101–139 | 0.707 |
| 12 Mo | |||||||
| Visuospatial Memory Task | 50 | 0.73 ± 0.16 | 0.44–1.0 | 49 | 0.69 ± 0.16 | 0.29–1.0 | 0.327 |
| Long-Term Memory Task | 50 | 0.53 ± 0.20 | 0.13–1.0 | 49 | 0.45 ± 0.22 | 0–0.88 | 0.056 |
| Expressive Vocabulary | 50 | 3.96 ± 4.13 | 0–20 | 49 | 3.31 ± 3.00 | 0–11 | 0.368 |
| Global Development Index | 50 | 117.0 ± 11.6 | 85–139 | 49 | 115.2 ± 10.6 | 93–136 | 0.412 |
Multivariate ANOVA F test (Bonferroni adjustment, P = 0.0125).
In the 10-mo data, there were no findings of statistical significance. In the 12-mo data, a trend toward a group effect was found for the percentage correct on the Long-Term Episodic Memory Task (P = 0.056; 95% CI: 0.447, 0.531; Table 2). However, no 12-mo variables were significantly different. In sum, no differences were found at either age between the placebo and choline-supplemented groups on a composite index of global development, number of words spoken, short-term visuospatial memory abilities, or long-term episodic memory.
Exploration of potential confounders
Dietary data
Complete analyses of the diet data are reported elsewhere (37). However, because there was a significant difference between the groups in betaine intake at 30 wk gestation and dietary choline was not controlled, we report here the results of exploratory analyses for hypothesis generation. To explore the difference in betaine consumption as a potential covariate, multivariate analysis of 10- and 12-mo data were repeated with 30-wk betaine intake as a covariate. The trend toward a group effect of the intervention on the long-term episodic memory scores of 12-mo-olds was no longer present when betaine intake at 30 wk gestation was controlled for.
Because dietary choline was not controlled (ie, participants were free to eat at will), information from dietary recalls was compiled and an intake variable was computed that took into account diet intake plus capsule intake weighted by compliance (to improve accuracy of intake data). This variable was computed for 30 wk gestation and for 45 d postpartum time points. To assess the contribution of total choline intake (diet plus supplement) to cognitive variables (short-term visuospatial memory percentage correct scores, long-term episodic memory percentage correct scores, words spoken scores, and global development composite scores), stepwise regression analyses were conducted by using a backward elimination method to determine the best predictive model. Variables were first checked for multicollinearity. Variables entered into the analyses were average daily calories at both time points and betaine consumed at both time points. Total choline intake variables from the 2 time points were highly correlated such that multicollinearity issues precluded their simultaneous inclusion. Thus, analyses were run with each total choline intake variable in turn. For the 10-mo cognitive variables, only 30-wk betaine consumption and 45-d postpartum caloric intake entered into the model. Betaine consumption at 30 wk gestation entered as a negative predictor of the short-term visuospatial memory scores when the task was novel (P = 0.061); calories consumed at 45 d postpartum entered as a positive predictor of global development cognitive scores (P = 0.043). For the 12-mo cognitive variables, betaine consumption at 45 d postpartum entered as a significant positive predictor of short-term visuospatial memory when the task was novel (P = 0.058), words spoken (P = 0.017), and global development (P = 0.026). When analyses were repeated with the choline intake at 45 d postpartum variable, the patterns of prediction remained the same. Thus, betaine consumption should be assessed in human choline supplementation studies. Caution should be exercised if computing power analyses based on these exploratory results: no correction was made for multiplicity because we chose to better enable hypothesis generation by avoiding the inflation of a Type II error as is commonly recommended (51–53).
Genetic modulation of choline metabolism
Since the inception of this study, we have identified single nucleotide polymorphisms on genes related to choline metabolism that may predispose a pregnant woman to a higher need for exogenous choline (54, 55). The current study was not powered for genetic analyses. However, exploratory analyses of genetic groupings may suggest designs for follow-up studies (see Supplemental Materials under “Supplemental data” in the online issue).
DISCUSSION
The purpose of this study was to assess whether supplemental choline (as phosphatidylcholine) could be administered safely to pregnant and lactating women and to test the hypothesis that maternal supplementation with phosphatidylcholine during pregnancy and early lactation in humans would enhance the cognitive abilities of infants at 10 and 12 mo of age. Adverse events were not found to occur at a greater frequency among subjects taking supplemental choline relative to those taking placebo. Moreover, no differences were found at either age between the groups in the percentage correct scores on short-term visuospatial memory, on words spoken as measured by parent report, on long-term episodic memory, or on global development as measured by the Mullen Scales of Early Learning. We conclude that, when mothers are eating diets containing ∼65–80% of the recommended intake for choline, there is no advantage to supplementing these mothers with phosphatidylcholine (that delivers 750 mg/d) in terms of enhanced brain development in their infants. These results should be interpreted with caution. Our study was not adequately powered to determine whether some women, with genetic variations that might increase their requirements for choline, derive benefit from a choline supplement. In addition, our study was conducted in the United States, where diets contain good sources of choline. It is possible that choline supplementation might benefit fetal and infant brain development and thus, subsequent cognitive development in areas of the world where the average daily choline intake of women is estimated to be half that of the intake in the United States (13).
The rodent studies that reported enhanced hippocampal function after choline supplementation used comparatively higher doses of choline than we did; they added choline to drinking water or pelleted food to approximately quadruple normal dietary intake (17, 18, 21, 22). We used a supplement designed to increase the recommended choline intake (450 mg/d) by 2.6-fold; in the absence of safety data during pregnancy, we were hesitant to use the higher dose needed to quadruple intake (1350 mg choline/d). It is possible that the amount of supplement we administered was too small. Now that we have demonstrated that a supplement of phosphatidylcholine delivering 750 mg choline/d has no adverse effects, in future studies researchers may want to examine the effects of a larger dose of choline.
We used phosphatidylcholine, rather than choline, for our supplement. As discussed earlier, this was to avoid the fishy body odor that has been reported with choline supplementation, but which does not occur when phosphatidylcholine is used. The bioavailability of phosphatidylcholine is different from that of choline. Phosphatidylcholine is a lipid and can be absorbed intact or after being hydrolyzed by pancreatic lipases to form glycerophosphocholine (56). Approximately 50% is absorbed as the lipid and enters the lymphatic circulation, bypassing the liver, whereas the remainder, being aqueous soluble, enters the portal vein and is first presented to the liver. Choline, which is water soluble, is absorbed and enters the portal vein and, on first pass, is taken up by the liver (57). The liver converts some of this choline to betaine and exports the rest as phosphatidylcholine (58). Thus, it is possible that administering choline in the form of phosphatidylcholine rather than choline has different biological effects (57).
In our human study, we administered supplemental choline only during the second half of gestation and 90 d of lactation—a period that does not encompass the whole of human hippocampal neurogenesis (25 wk gestation through ≥4 y postnatally). The human hippocampus is functional in a rudimentary form at birth, but the structures of the medial temporal lobe, including the hippocampus, undergo significant morphologic development over an extended period of time (months to years). For example, 30% of granule cells in the human dentate gyrus do not start to proliferate and establish connections until after birth, with major connectivity to the frontal lobes not being established until 9–10 mo of age (31). Moreover, neurogenesis in the adult dentate gyrus has been reported (59). In the rodent studies on which we based our hypotheses, the treatment period encompassed most of the period of hippocampal neurogenesis in the rodent brain (18, 19, 21). Thus, a discrepancy exists in the translation.
Our time points for cognitive assessment were logistically confined. We examined effects across a 2-mo developmental period that is thought to be an important period for the development of cognitive functions subserved by the hippocampal structure. However, it is possible that the effects of choline supplementation will be more evident at an older age, after the hippocampal connections to the frontal lobes have been established and, thus, development of higher-order cognitive functions have progressed.
Future studies should take into account dietary intakes of both choline and betaine, because our results suggest an influence of betaine on cognition. The supplemented group had a higher mean betaine intake than did the placebo group. Data were not available to explain this difference. Dietary betaine is found mostly in grains, but also in foods such as shrimp, beets, and sweet potatoes. It is possible that the supplemented mothers were eating a large amount of grains. More detailed dietary data would be needed to determine why this difference existed.
The results of this trial suggest that, during pregnancy and lactation, middle-class American mothers consuming a Western diet do not benefit from a 750-mg/d phosphatidylcholine supplement with regard to fetal and infant brain development. The study established that this dose of phosphatidylcholine supplement is safe when used by pregnant and lactating women. Further research is needed to determine the utility of such supplementation in populations in which dietary choline is scarce, to determine whether there are subgroups of mothers with genetic variants in genes of choline metabolism who respond to supplemental choline, and to determine whether doses of supplemental choline higher than those that we used in this study influence brain development.
Acknowledgments
We thank the Solae Company for providing the phosphatidylcholine and corn oil supplements for this study, Brigitte Stephenson for assistance with participant management, and Julie Vick for compiling the dietary data.
The authors’ responsibilities were as follows—CLC: analyzed the data and led the writing of the manuscript; BDG: supervised the cognitive testing; LMF: participated in the supervision of the human study; K-AdC: supervised the analyses of choline and metabolites, DNA extraction, and single nucleotide polymorphism assays; and JSR and SHZ: were responsible for the conceptualization, implementation, and design of the human study and data interpretation. All authors participated in writing the manuscript. None of the authors had a personal or financial conflict of interest. The funding agencies had no role in the design, implementation, analysis, or interpretation of the data.
REFERENCES
- 1.Sweiry JH, Page KR, Dacke CG, Abramovich DR, Yudilevich DL. Evidence of saturable uptake mechanisms at maternal and fetal sides of the perfused human placenta by rapid paired-tracer dilution: studies with calcium and choline. J Dev Physiol 1986;8:435–45 [PubMed] [Google Scholar]
- 2.Sweiry JH, Yudilevich DL. Characterization of choline transport at maternal and fetal interfaces of the perfused guinea-pig placenta. J Physiol 1985;366:251–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeisel SH, Wurtman RJ. Developmental changes in rat blood choline concentration. Biochem J 1981;198:565–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozarda Ilcol Y, Uncu G, Ulus IH. Free and phospholipid-bound choline concentrations in serum during pregnancy, after delivery and in newborns. Arch Physiol Biochem 2002;110:393–9 [DOI] [PubMed] [Google Scholar]
- 5.Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr 2006;26:229–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmes-McNary MQ, Cheng WL, Mar MH, Fussell S, Zeisel SH. Choline and choline esters in human and rat milk and infant formulas. Am J Clin Nutr 1996;64:572–6 [DOI] [PubMed] [Google Scholar]
- 7.Zeisel SH, Char D, Sheard NF. Choline, phosphatidylcholine and sphingomyelin in human and bovine milk and infant formulas. J Nutr 1986;116:50–8 [DOI] [PubMed] [Google Scholar]
- 8.Chao CK, Pomfret EA, Zeisel SH. Uptake of choline by rat mammary-gland epithelial cells. Biochem J 1988;254:33–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho E, Zeisel SH, Jacques P, Selhub J, Dougherty L, Colditz GA, Willett WC. Dietary choline and betaine assessed by food-frequency questionnaire in relation to plasma total homocysteine concentration in the Framingham Offspring Study. Am J Clin Nutr 2006;83(4):905–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bidulescu A, Chambless LE, Siega-Riz AM, Zeisel SH, Heiss G. Usual choline and betaine dietary intake and incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. BMC Cardiovasc Disord 2007;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bidulescu A, Chambless LE, Siega-Riz AM, Zeisel SH, Heiss G. Repeatability and measurement error in the assessment of choline and betaine dietary intake: the Atherosclerosis Risk in Communities (ARIC) study. Nutr J 2009;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho E, Willett WC, Colditz GA, Fuchs CS, Wu K, Chan AT, Zeisel SH, Giovannucci EL. Dietary choline and betaine and the risk of distal colorectal adenoma in women. J Natl Cancer Inst 2007;99(16):1224–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gossell-Williams M, Fletcher H, McFarlane-Anderson N, Jacob A, Patel J, Zeisel S. Dietary intake of choline and plasma choline concentrations in pregnant women in Jamaica. West Indian Med J 2005;54:355–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Institute of Medicine, National Academy of Sciences Choline. Dietary reference intakes for folate, thiamin, riboflavin, niacin, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academy Press, 1998:390–422 [PubMed] [Google Scholar]
- 15.Pyapali GK, Turner D, Williams C, Meck W, Swartzwelder HS. Prenatal choline supplementation decreases the threshold for induction of long-term potentiation in young adult rats. J Neurophysiol 1998;79:1790–6 [DOI] [PubMed] [Google Scholar]
- 16.Montoya DA, White AM, Williams CL, Blusztajn JK, Meck WH, Swartzwelder HS. Prenatal choline exposure alters hippocampal responsiveness to cholinergic stimulation in adulthood. Brain Res Dev Brain Res 2000;123:25–32 [DOI] [PubMed] [Google Scholar]
- 17.Jones JP, Meck WH, Williams CL, Wilson WA, Swartzwelder HS. Choline availability to the developing rat fetus alters adult hippocampal long-term potentiation. Brain Res Dev Brain Res 1999;118(1-2):159–67 [DOI] [PubMed] [Google Scholar]
- 18.Meck WH, Williams C. Perinatal choline supplementation increases the threshold for chunking in spatial memory. Neuroreport 1997;8:3053–9 [DOI] [PubMed] [Google Scholar]
- 19.Meck WH, Williams C. Characterization of the facilitative effects of perinatal choline supplementation on timing and temporal memory. Neuroreport 1997;8:2831–5 [DOI] [PubMed] [Google Scholar]
- 20.Meck WH, Williams C. Simultaneous temporal processing is sensitive to prenatal choline availability in mature and aged rats. Neuroreport 1997;8:3045–51 [DOI] [PubMed] [Google Scholar]
- 21.Meck WH, Smith RA, Williams CL. Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Dev Psychobiol 1988;21:339–53 [DOI] [PubMed] [Google Scholar]
- 22.Meck WH, Smith RA, Williams CL. Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behav Neurosci 1989;103:1234–41 [DOI] [PubMed] [Google Scholar]
- 23.Meck WH, Williams CL. Choline supplementation during prenatal development reduces proactive interference in spatial memory. Brain Res Dev Brain Res 1999;118:51–9 [DOI] [PubMed] [Google Scholar]
- 24.Albright CD, Mar MH, Friedrich CB, Brown EC, Zeisel SH. Maternal choline availability alters the localization of p15Ink4B and p27Kip1 cyclin-dependent kinase inhibitors in the developing fetal rat brain hippocampus. Dev Neurosci 2001;23(2):100–6 [DOI] [PubMed] [Google Scholar]
- 25.Albright CD, Siwek DF, Craciunescu CN, Mar MH, Kowall NW, Williams CL, Zeisel SH. Choline availability during embryonic development alters the localization of calretinin in developing and aging mouse hippocampus. Nutr Neurosci 2003;6:129–34 [DOI] [PubMed] [Google Scholar]
- 26.Jones JP, Meck W, Williams CL, Wilson WA, Swartzwelder HS. Choline availability to the developing rat fetus alters adult hippocampal long-term potentiation. Brain Res Dev Brain Res 1999;118:159–67 [DOI] [PubMed] [Google Scholar]
- 27.Fisher MC, Zeisel SH, Mar MH, Sadler TW. Inhibitors of choline uptake and metabolism cause developmental abnormalities in neurulating mouse embryos. Teratology 2001;64:114–22 [DOI] [PubMed] [Google Scholar]
- 28.Fisher MC, Zeisel SH, Mar MH, Sadler TW. Perturbations in choline metabolism cause neural tube defects in mouse embryos in vitro. FASEB J 2002;16:619–21 [DOI] [PubMed] [Google Scholar]
- 29.Shaw GM, Carmichael SL, Laurent C, Rasmussen SA. Maternal nutrient intakes and risk of orofacial clefts. Epidemiology 2006;17:285–91 [DOI] [PubMed] [Google Scholar]
- 30.Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol 2004;160:102–9 [DOI] [PubMed] [Google Scholar]
- 31.Seress L. Morphological changes of the human hippocampal formation from midgestation to early childhood. In: Nelson CA, Luciana M, eds. Handbook of developmental cognitive neuroscience. Cambridge, MA: The MIT Press, 2001:45–58.
- 32.Carver LJ, Bauer PJ. The dawning of a past: the emergence of long-term explicit memory in infancy. J Exp Psychol Gen 2001;130:726–45 [PubMed] [Google Scholar]
- 33.Pelphrey KA, Reznick JS, Davis Goldman B, Sasson N, Morrow J, Donahoe A, Hodgson K. Development of visuospatial short-term memory in the second half of the 1st year. Dev Psychol 2004;40:836–51 [DOI] [PubMed] [Google Scholar]
- 34.Reznick JS, Morrow JD, Goldman BD, Snyder J. The onset of working memory in infants. Infancy 2004;6:145–54 [Google Scholar]
- 35.Zeisel SH, Wishnok JS, Blusztajn JK. Formation of methylamines from ingested choline and lecithin. J Pharmacol Exp Ther 1983;225:320–4 [PubMed] [Google Scholar]
- 36.Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr 2003;133:1302–7 [DOI] [PubMed] [Google Scholar]
- 37.Fischer LM, da Costa KA, Galanko J, Sha W, Stephenson B, Vick J, Zeisel SH. Choline intake and genetic polymorphisms influence choline metabolite concentrations in human breast milk and plasma. Am J Clin Nutr 2010;92:336–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeisel SH, Mar M-H, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr 2003;133:1302–7 (Published erratum appears in J Nutr 2003;133:2918–9.) [DOI] [PubMed] [Google Scholar]
- 39.Diamond A, Doar B. The performance of human infants on a measure of frontal cortex function, the delayed response task. Dev Psychobiol 1989;22:271–94 [DOI] [PubMed] [Google Scholar]
- 40.Schwartz BB, Reznick JS. Measuring infant spatial working memory using a modified delayed-response procedure. Memory 1999;7:1–17 [DOI] [PubMed] [Google Scholar]
- 41.Brody LR. Visual short-term cued recall memory in infancy. Child Dev 1981;52:242–50 [PubMed] [Google Scholar]
- 42.Meltzoff AN. Infant imitation after a 1-week delay: long-term memory for novel acts and multiple stimuli. Dev Psychol 1988;24:470–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meltzoff AN. Infant imitation and memory: nine-month-olds in immediate and deferred tests. Child Dev 1988;59:217–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barr R, Dowden A, Hayne H. Developmental changes in deferred imitation by 6- to 24-month-old infants. Infant Behav Dev 1996;19:159–70 [Google Scholar]
- 45.Barr R, Hayne H. Age-related changes in imitation: implications for memory development. In: Rovee-Collier C, Lipsitt LP, eds. Progress in infancy research. Vol 1. Mahwah, NJ: Lawrence Erlbaum Associates, Inc, Publishers, 2000:21–67.
- 46.Cheatham CL, Bauer PJ, Georgieff MK. Predicting individual differences in recall by infants born preterm and fullterm. Infancy 2006;10:17–42 [DOI] [PubMed] [Google Scholar]
- 47.Bauer PJ, Wenner JA, Dropik PL, Wewerka SS. Parameters of remembering and forgetting in the transition from infancy to early childhood. Monogr Soc Res Child Dev 2000;65:1–204 [PubMed] [Google Scholar]
- 48.Fenson L, Pethick SJ, Renda C, Cox JL, Dale PS, Reznick JS. Short-form versions of the MacArthur Communications Development Inventories. Appl Psycholinguist 2000;21:95–116 [Google Scholar]
- 49.Fenson L, Dale PS, Reznick JS, Bates E, Thal DJ, Pethick SJ. Variability in early communicative development. Monogr Soc Res Child Dev 1994;59:v-173 [PubMed] [Google Scholar]
- 50.Mullen EM. Mullen Scales of Early Learning, AGS edition: manual and item administration book. Circle Pines, MN: American Guidance Service, Inc, 1995 [Google Scholar]
- 51.Streiner DL, Norman GR. Correction for multiple testing: is there a resolution? Chest 2011;140:18–8 [DOI] [PubMed] [Google Scholar]
- 52.Schulz KF, Grimes DA. Multiplicity in randomised trials I: endpoints and treatments. Lancet 2005;365:1591–5 [DOI] [PubMed] [Google Scholar]
- 53.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–6 [PubMed] [Google Scholar]
- 54.da Costa KA, Kozyreva OG, Song J, Galanko JA, Fischer LM, Zeisel SH. Common genetic polymorphisms affect the human requirement for the nutrient choline. FASEB J 2006;20(9):1336–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeisel SH. Gene response elements, genetic polymorphisms and epigenetics influence the human dietary requirement for choline. IUBMB Life 2007;59(6):380–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fox JM, Betzing H, Lekim D. Pharmacokinetics of orally ingested phosphatidylcholine. In: Barbeau A, Growdon JH, Wurtman RJ, eds. Nutrition and the brain. New York, NY: Raven Press, 1979:95–108.
- 57.Cheng W-L, Holmes-McNary MQ, Mar M-H, Lien EL, Zeisel SH. Bioavailability of choline and choline esters from milk in rat pups. J Nutr Biochem 1996;7:457–64 [Google Scholar]
- 58.Zeisel SH, Story DL, Wurtman RJ, Brunengraber H. Uptake of free choline by isolated perfused rat liver. Proc Natl Acad Sci USA 1980;77:4417–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med 1998;4:1313–7 [DOI] [PubMed] [Google Scholar]