Abstract
This study was conducted to determine whether dietary Se deficiency precluded overproduction of glutathione peroxidase-1 (GPX1) activity in mice overexpressing (OE) this gene and thus rescued their type 2 diabetes–like phenotypes. A total of 20 male OE and wild-type (WT) mice were fed an Se-deficient (<0.02 mg/kg) diet or an Se-supplemented (0.3 mg/kg as sodium selenite) diet from 1 to 5 mo of age. Dietary Se deficiency eliminated or attenuated (P < 0.05) genotype differences in concentrations of blood glucose, plasma insulin, and/or hepatic lipids, insulin sensitivity, and glucose-stimulated insulin secretion at the end of the study. Dietary Se deficiency decreased (P < 0.05) OE islet mRNA levels of 2 key transcriptional activators (Beta2 and Foxa2) and removed genotype differences in islet mRNA levels of 7 genes (Beta2, Cfos, Foxa2, Pregluc, Ins1, p53, and Sur1) related to insulin synthesis and secretion. Compared with those of the Se-adequate OE mice, the Se-deficient OE mice had lower (P < 0.05) hepatic mRNA levels of 2 key rate-limiting enzymes for lipogenesis (Acc1) and glycolysis (Gk1), along with lower (P < 0.05) activities of hepatic glucokinase and muscle phosphoenolpyruvate carboxykinase. Dietary Se deficiency also decreased (P < 0.05) blood glucose and hepatic lipid concentrations in the WT mice. In conclusion, dietary Se deficiency precluded the overproduction of GPX1 in full-fed OE mice and partially rescued their metabolic syndromes. This alleviation resulted from modulating the expression and/or function of proinsulin genes, lipogenesis rate-limiting enzyme genes, and key glycolysis and gluconeogenesis enzymes in islets, liver, and muscle.
Introduction
Selenium has been considered to have antidiabetic (1, 2) properties due to its insulin-mimetic effect (3). Indeed, Se deficiency is associated with diabetes (4–6), and administration of Se to streptozotocin-induced diabetic rats restored their glycemic control (7, 8). However, a number of recent human studies have shown a hyperglycemic, hyperlipidemic, and prodiabetic effect of Se supplements (9–11). Our laboratory showed that a prolonged feeding of high dietary Se (3 mg/kg) induced mild gestational diabetes in first-parity pregnant rats and insulin resistance in their offspring (12). Although other groups have also shown similar (13) effects of Se on body glucose and lipid metabolism, mechanisms and health impacts of such effects of Se remain unclear or controversial.
Glutathione peroxidase-1 (GPX1)7 was the first-identified selenoprotein in mammals (14) and has been widely considered to be a major antioxidant intracellular enzyme (15). Whereas GPX1 activity is extremely low in islets (16, 17) and is altered in development of diabetes (18) and its complications (19–22), increased activity of this enzyme in erythrocytes was actually associated with hyperinsulinemia and insulin resistance in pregnant women (23). Strikingly, our laboratory found that overexpression of GPX1 in mice induced hyperglycemia, hyperinsulinemia, insulin resistance, and obesity at 6 mo of age (24). To rescue these phenotypes and reveal the underlying mechanisms, we first restricted the diet of the GPX1 overexpressing (OE) mice from 2 to 6 mo of age (25). Because this extended diet restriction ameliorated all of these type 2 diabetes-like phenotypes except for fasting hyperinsulinemia and hypersecretion of insulin by glucose stimulation, these 2 abnormalities seemed to be primary effects of GPX1 overproduction. To further eliminate these 2 phenotypes for a full rescue of these mice, we conducted a second experiment and did not supplement Se in the diets of the feed-restricted OE mice to preclude their GPX1 overproduction (26). However, the dietary Se depletion showed no additional benefit, above that of the diet restriction alone, to the OE phenotypes. It is likely that the latter treatment exerted metabolic impacts on the OE mice that were too drastic, which disallowed demonstration of further effects of the former treatment. Metabolic benefit of excluding GPX1 activity overproduction might be detected only in the full-fed OE mice.
Synthesis and secretion of insulin in pancreatic islets are regulated by transcriptional factors, signal molecules, and functional proteins encoded by genes such as pancreatic and duodenal homeobox 1 (Pdx1), neurogenic differentiation 1(Beta2), hnf1 homeobox a (Hnf1a), forkhead box a2 (Foxa2), v-maf musculoaponeurotic fibrosarcoma oncogene family protein a (Mafa), glucose transporter 2 (Glut2), and glucokinase Gk1 (27–33). Hepatic lipogenesis is controlled by regulatory proteins and functional enzymes encoded by genes such as sterol regulatory element binding transcription factor 1a (Srebp1a), sterol regulatory element binding factor 2 (Srebp2), acetyl-coenzyme A carboxylase 1 (Acc1), fatty acid synthase (Fasn), and peroxisome proliferator-activated receptor γ (Pparγ) (34–37). Glucokinase plays a key role in glucose oxidation (glycolysis), and phosphoenolpyruvate carboxykinase (PEPCK) is the rate-limiting enzyme in gluconeogenesis (38). As a major signal protein of cell death/survival, p53 is also involved in carbohydrate and lipid metabolism (39, 40). We have previously shown the distinct impacts of GPX1 knockout or overexpression on expression and function of many of these factors (25, 41). Plausibly, disorders of glucose and lipid metabolism in the OE mice might result from dysregulation of those factors by the overproduction of GPX1. Therefore, our objective was to determine the following: 1) if prolonged dietary Se depletion in OE mice rescued their type 2 diabetes–like phenotypes and 2) if the presumed rescue was mediated by modulating expression and function of key factors related to insulin synthesis, secretion, and function in islets and lipogenesis, glycolysis, and gluconeogenesis in liver and muscle.
Materials and Methods
Mice, diets, physiologic tests, and tissue sample collections.
Our mouse experiments were approved by the Institutional Animal Care and Use Committee at Cornell University. Mice were reared in plastic cages in an animal room with a constant temperature (22°C) and a 12-h light:dark cycle and were given free access to feed and distilled water. The OE mice were derived from a B6C3 (C57B1×C3H) hybrid line (Taconic) (42). A total of 20 weanling male (1 mo old) wild-type (WT) and OE mice (n = 5 for each genotype by diet) were fed a Torula yeast- and sucrose-based diet (42) that was deficient in Se (<0.02 mg Se/kg) or that was adequate in Se (0.3 mg Se/kg as sodium selenite) for 4 mo. Mice were feed-deprived for 8 h overnight before measurements, tests, and tissue sample collection. Individual body weights, blood glucose concentrations, plasma insulin concentrations, insulin and glucose tolerance tests (0.5 U insulin/kg body weight and 1 g dextrose/kg, respectively), and glucose-stimulated insulin secretion (GSIS; 1 g dextrose/kg) were recorded or determined at initial and then monthly until the end of study. Blood samples were collected via tail bleeding, and blood glucose was determined by using a glucometer (Bayer). Plasma insulin was determined by using a rat/mouse insulin ELISA kit (Crystal Chem). At the end of study, all experimental mice were killed by exsanguination via heart puncture with the use of a heparinized syringe after CO2 anesthesia to collect blood (heart), pancreatic islets, liver, and muscle that were stored at −80°C before analysis. The pancreatic islets were isolated from mice using a standard procedure with minor modifications (25).
Molecular and biochemical assays.
Total RNA was isolated from liver and pancreatic islets using a Trizol kit (Invitrogen), and the subsequent analyses by quantitative real-time PCR of 21 insulin synthesis- and secretion-related genes in islets and 10 lipogenesis-related genes in liver were carried out as previously described (25, 26). Each reaction was performed in triplicate with validated primers listed in Supplemental Table 1. Liver lipid was extracted by using a chloroform:methanol method, and commercial kits (Wako Chemicals) were used to measure concentrations of TG (L-type triglyceride M kit), total cholesterol (Cholesterol E kit), and nonesterified fatty acid (NEFA-HR kit) in the extractions. Hepatic GPX1 activity was measured at 25°C by using the coupled assay of NADPH oxidation as previously described (43). Hepatic GK activity was measured in a reaction coupled with glucose-6-phosphate dehydrogenase through monitoring production of NADPH (44). Liver and muscle PEPCK activities were determined by using a spectrophotometric assay (45). Western blot analysis of hepatic p53 protein was performed as described (46) by using a primary rabbit antibody against mouse p53 protein (Cell Signaling), and the relative density of the protein bands was quantified by using Canvas 7 software (Deneba Systems). Protein concentration was determined by using a bicinchoninic acid protein assay kit (Thermo Scientific).
Statistical analysis.
Data were analyzed by using SAS (release 6.11; SAS Institute). Dietary Se and genotype effects were tested by 2-way ANOVA with (body weight and physiologic data) or without (gene, protein, lipid, and enzyme data) time-repeated measurements. If there was a main effect, Tukey t test was used for post hoc comparisons of means. Data are presented as means ± SE, and the significance level of treatment effects or mean differences was set at P ≤ 0.05.
Results
Metabolic phenotypes.
The OE mice had higher (P < 0.05) hepatic GPX1 activity than the WT mice, whereas dietary Se deficiency decreased (P < 0.05) the activity in both genotypes (Fig. 1A). The final body weight of OE mice was 27% heavier (P < 0.05) than that of the WT mice (Fig. 1B), and this genotype difference (P < 0.05) appeared as early as the first month (data not shown). However, dietary Se deficiency did not produce any significant effect on body weight of either genotype. In contrast, dietary Se deficiency decreased (P < 0.05) blood glucose concentrations in both OE and WT mice compared with their respective Se-adequate controls at the end of study (Fig. 1C). Consequently, dietary Se deficiency minimized blood glucose differences between the Se-adequate WT and OE mice. Dietary Se deficiency also decreased (P < 0.05) postmortem plasma insulin concentrations (Fig. 1D) and improved (P < 0.05) blood glucose tolerance in the OE mice (Fig. 2A). However, dietary Se deficiency impaired the early phase (15 and 30 min) of blood glucose clearance in the WT mice (Fig. 2A).
FIGURE 1.
Effects of dietary Se concentration on hepatic GPX1 activity (A), body weight (B), blood glucose concentration (C), and postmortem plasma insulin concentration (D) in WT and OE mice at 5 mo of age. Values are means ± SE, n = 3–4 (A and D) or n = 5–10 (B and C). Means for a given measure without a common letter differ, P < 0.05. GPX1, glutathione peroxidase-1; OE, GPX1 overexpressing; OE(+), Se-adequate GPX1 overexpressing mice; OE(−), Se-deficient GPX1 overexpressing mice; WT, wild-type; WT(+), Se-adequate wild-type mice; WT(−), Se-deficient wild-type mice.
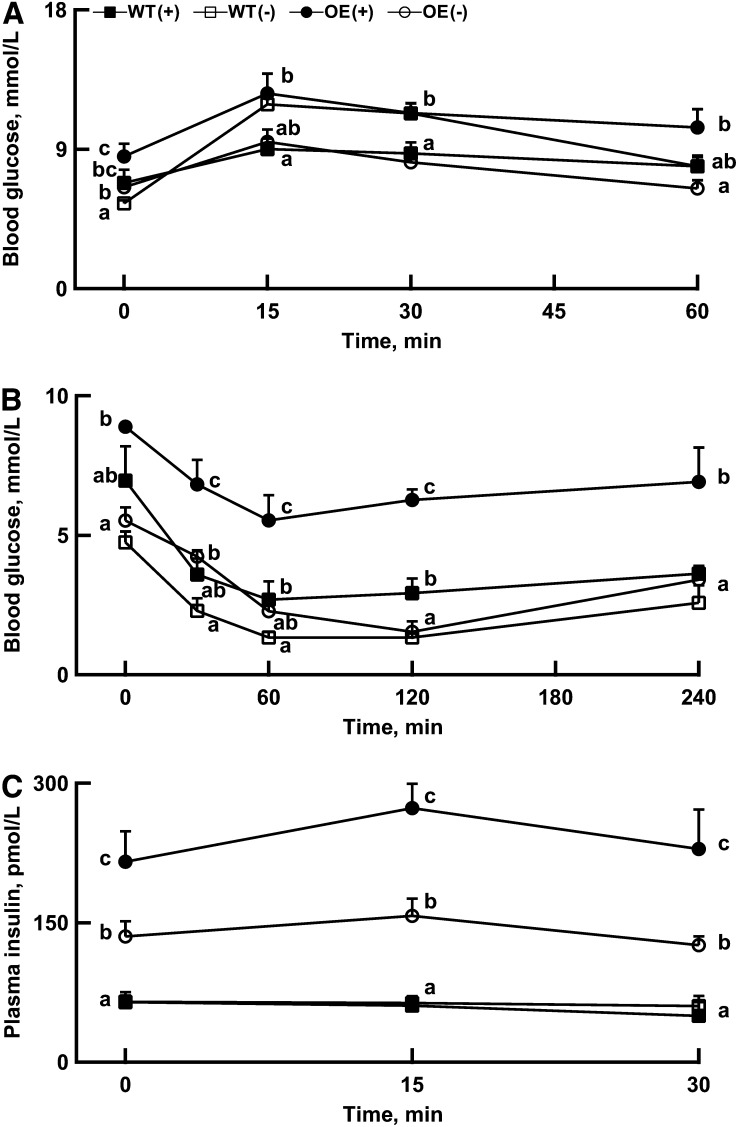
FIGURE 2.
Effects of dietary Se concentration on body glucose tolerance test (1 g dextrose/kg) (A), body insulin tolerance test (0.5 U insulin/kg) (B), and glucose-stimulated insulin secretion (1 g dextrose/kg) (C) in WT and OE mice at 1 wk prior to the end of the experiment (5 mo of age). Values are means ± SE, n = 5. Means for a given measure at a given time point without a common letter differ, P < 0.05. OE, glutathione peroxidase-1 (GPX1) overexpressing; OE(+), Se-adequate GPX1 overexpressing mice; OE(−), Se-deficient GPX1 overexpressing mice; WT, wild-type; WT(+), Se-adequate wild-type mice; WT(−), Se-deficient wild-type mice.
Dietary Se deficiency improved (P < 0.05) insulin sensitivity at various time points in both genotypes, and the magnitude of improvement was greater in the OE than in the WT mice (Fig. 2B). The effects of dietary Se and genotype on the relative changes of body insulin tolerances were similar to those of absolute changes (Supplemental Fig. 1A). However, the relative impairment of body glucose tolerance by dietary Se deficiency in the WT mice seemed to be more apparent than that of the absolute responses (Supplemental Fig. 1B). Dietary Se deficiency attenuated (P < 0.05) plasma insulin concentrations at 0, 15, and 30 min after GSIS by 37%, 42%, and 45%, respectively, in the OE mice but showed little effect in the WT mice (Fig. 2C). In Se adequacy, hepatic concentrations of TG, total cholesterol, and nonesterified fatty acid (Fig. 3A–C) were 31%, 154%, and 37% greater (P < 0.05), respectively, in the OE mice than in the WT mice. However, these genotype differences were either minimized or eliminated by dietary Se deficiency.
FIGURE 3.
Effects of dietary Se concentration on hepatic concentrations of TG (A), TC (B), and NEFA (C) in WT and OE mice at 5 mo of age. Values are means ± SE, n = 5. Means for a given measure without a common letter differ, P < 0.05. NEFA, nonesterified fatty acid; OE, glutathione peroxidase-1 (GPX1) overexpressing; OE(+), Se-adequate GPX1 overexpressing mice; OE(−), Se-deficient GPX1 overexpressing mice; TC, total cholesterol; WT, wild-type; WT(+), Se-adequate wild-type mice; WT(−), Se-deficient wild-type mice.
Islet gene expression.
Among the assayed genes in islets, 16 were affected (P < 0.05) by dietary Se and/or genotype. Compared with islets from the Se-adequate OE mice, those from the Se-deficient OE mice had lower (P < 0.05) mRNA levels of Beta2 and Foxa2 and tended to have lower levels (P < 0.09) of Cfos and Pregluc. But they had greater (P < 0.05) mRNA levels of Cat, Hnf1a, Hnf4α, and Kir6.2 (Fig. 4A). In the WT islets, dietary Se deficiency enhanced (P < 0.05) mRNA levels of Cat, Cfos, Hnf4α, Foxo1, Gk1, Ins1, and p53 (Fig. 4A, B). Genotype differences between the Se-adequate WT and OE mice in islet mRNA levels of 7 genes (Beta2, Cfos, Foxa2, Pregluc, Insl, p53, and Sur1) were removed by dietary Se deficiency. Neither genotype nor dietary Se affected islet mRNA levels of Irs2, Jund, Mafa, Sirt1, or Ucp2 (Supplemental Fig. 2).
FIGURE 4.
Effects of dietary Se concentration on pancreatic islet mRNA abundances of insulin-related genes in WT and OE mice at 5 mo of age. The figure shows genes in the OE islets that were responsive to dietary Se deficiency (A) and those genes that were less responsive to the same treatment (B). Values are means ± SE, n = 5. Means for a given gene without a common letter differ, P < 0.05. OE, glutathione peroxidase-1 (GPX1) overexpressing; OE(+), Se-adequate GPX1 overexpressing mice; OE(−), Se-deficient GPX1 overexpressing mice; WT, wild-type; WT(+), Se-adequate wild-type mice; WT(−), Se-deficient wild-type mice.
Hepatic lipogenesis gene expression.
Hepatic Gk1 mRNA abundance was 62-fold greater (P < 0.01) in the Se-adequate OE mice than in the Se-adequate WT mice (Fig. 5), whereas dietary Se deficiency produced (P < 0.05) an opposite effect on Gk1 expression between the 2 genotypes (increased in the WT mice and decreased in the OE mice). Furthermore, dietary Se deficiency increased (P < 0.05) Pparγ in the OE mice but decreased (P < 0.05) Cyp7a1, Srebp1a, and Srebp2 in the WT mice and Acc1 in the OE mice. In addition, the OE mice had greater (P < 0.05) mRNA levels of Acc1, Cyp7a1, Fasn, Mccc1, and Pparγ but lower (P < 0.05) mRNA levels of F1,6bp and Hmgcs2 than did the WT mice.
FIGURE 5.
Effects of dietary Se concentration on hepatic mRNA abundances of lipogenesis-related genes in WT and OE mice at 5 mo of age. Values are means ± SE, n = 5. Means for a given gene without a common letter differ, P < 0.05. OE, glutathione peroxidase-1 (GPX1) overexpressing; OE(+), Se-adequate GPX1 overexpressing mice; OE(−), Se-deficient GPX1 overexpressing mice; WT, wild-type; WT(+), Se-adequate wild-type mice; WT(−), Se-deficient wild-type mice.
Tissue GK and PEPCK activities and p53 protein.
Hepatic GK activity in the Se-adequate OE mice was 2.5-fold greater (P < 0.05) than that of the Se-adequate WT mice (Fig. 6A). Dietary Se deficiency suppressed (P < 0.05) the enzyme activity in the OE mice to a level similar to that of the Se-adequate WT mice. Likewise, muscle PEPCK activity was 6-fold (P < 0.05) greater in the Se-adequate OE mice than in the Se-adequate WT mice (Fig. 6B). There was a 38% decrease (P < 0.05) in the enzyme activity in muscle of the Se-deficient OE mice compared with their Se-adequate counterparts. Hepatic PEPCK activity was also lower (P < 0.05) with dietary Se deficiency in the WT mice. Liver p53 protein in the Se-deficient WT mice was 67% higher (P < 0.05) than that in the Se-adequate WT mice (Fig. 6C).
FIGURE 6.
Effects of dietary Se concentration on hepatic GK activity (A), hepatic and muscular PEPCK activities (B), and hepatic p53 protein (C) in WT and OE mice at 5 mo of age. Values are means ± SE, n = 5. The band image of Western blot (C) was a representative of 5 independent experiments. Means for a given measure without a common letter differ, P < 0.05. GK, glucokinase; OE, glutathione peroxidase-1 (GPX1) overexpressing; OE(+), Se-adequate GPX1 overexpressing mice; OE(−), Se-deficient GPX1 overexpressing mice; PEPCK, phosphoenolpyruvate carboxykinase; WT, wild-type; WT(+), Se-adequate wild-type mice; WT(−), Se-deficient wild-type mice.
Discussion
The present study represents the third attempt in a series of experiments to rescue type 2 diabetes–like phenotypes in OE mice. After we obtained a partial reversal of these phenotypes by diet restriction (25) and observed no additional benefit of dietary Se deficiency in the diet-restricted OE mice (26), this study was conducted to explore whether dietary Se deficiency in the OE mice given free access to feed (full-fed) could completely rescue their phenotypes. Although dietary Se deficiency indeed precluded the GPX1 overproduction in the OE mice and thus minimized the difference in hepatic GPX1 activity from the WT mice, 3 of their phenotypes, including hyperglycemia, insulin resistance, and elevated hepatic lipid profiles (24), were nearly rescued. Meanwhile, their hyperinsulinemia and aggravated GSIS were also improved by dietary Se deficiency. Interestingly, the overall impact of dietary Se deficiency in the full-fed OE mice resembled that of diet restriction in Se-adequate OE mice (25). Because dietary Se deficiency and diet restriction were supposed to produce distinctly different primary effects, it was interesting to see indistinct impacts of these 2 treatments on the OE phenotypes. More specifically, diet restriction in the Se-adequate OE mice eliminated obesity and brought their body weight down to a level lower than in the WT mice but had little effect on activities of GPX1 and the other 2 selenoproteins in liver, pancreas, muscle, and plasma (25). In contrast, dietary Se deficiency in the full-fed OE mice decreased their liver GPX1 activity but not their body weight. Apparently, diet restriction alleviated the OE phenotypes by mechanisms related to obesity control, whereas the actions of dietary Se deficiency were mediated by precluding the overproduction of GPX1 activity and perhaps the downregulation of other selenoproteins as well (25, 26). Intriguingly, these 2 treatments showed no additive benefit on the OE phenotype in our previous experiment (26). In that case, diet restriction seemed to be too drastic to allow identification of metabolic effects of dietary Se deficiency. Furthermore, neither dietary Se deficiency from age 1 to 5 mo nor diet restriction from age 2 to 6 mo could completely rescue hyperinsulinemia or aggravated GSIS. These 2 disorders are likely predisposed by GPX1 overproduction in prenatal or neonatal stages.
Two “proinsulin” genes, Beta2 (28, 47) and Foxa2 (30), were decreased by dietary Se deficiency in the OE mice. Because these 2 genes encode key transcriptional factors for β cell differentiation and insulin synthesis (28, 48), downregulation of their mRNA and presumably their functional protein levels in the OE mice was consistent with their hypoinsulinemic response to dietary Se depletion. Furthermore, dietary Se deficiency also removed the genotype differences between the Se-adequate WT and OE islets in another 5 genes (Cfos, Pregluc, Ins1, p53, and Sur1) related to insulin synthesis and secretion. These changes suggest potential mechanisms to explain the attenuated hyperinsulinemia and improved GSIS in the Se-deficient OE mice. With regard to the elevated islet Cat, Hnf1a, Hnf4α, and Kir6.2 mRNA levels by dietary Se deficiency in the OE mice, physiologic implications remain unclear to us. Upregulation of these proinsulin genes may be perceived as a feedback response to the improved metabolic condition and/or downregulation of other proinsulin genes. Among the 7 genes upregulated by dietary Se deficiency in islets of the full-fed WT mice, mRNA levels of 2 (Ins1and Gk1) were also enhanced by dietary Se deficiency in islets of the restricted-fed WT mice (26). Thus, Ins1 and Gk1 are highly responsive to dietary Se supply or body Se status per se.
The improved hepatic lipid profile in the Se-deficient OE mice, compared with their Se-adequate controls, was concurrent with a downregulation of the GK mRNA level and activity. Being an insulin-dependent key factor involved in lipogenesis (49), GK activity is closely related to energy production through glycolysis and reactions in tricarboxylic acid cycle (50), and consequently provides substrate precursors such as acetyl-CoA and NADPH for biosynthesis of cholesterol and other lipids. In Se adequacy, hepatic GK mRNA level and activity were >62- and 2.5-fold greater, respectively, in the OE mice than in the WT mice. Clearly, the decrease in hepatic GK activity by dietary Se deficiency in the OE mice to the level in WT mice helped suppress that pathway of lipogenesis. Furthermore, downregulation of hepatic Acc1 in the Se-deficient OE mice was also attributed to their decreased hepatic lipid profile because ACC1 is a rate-limiting enzyme of lipogenesis that is activated by insulin (51). Although the implications of the elevated hepatic Pparγ expression in the Se-deficient OE mice on their hepatic lipid status remain unclear, the decreased muscle PEPCK activity was consistent with their hypoglycemic response to dietary Se deficiency, presumably by attenuating gluconeogenesis (52). The hypoglycemic and hepatic lipid-lowering effects of dietary Se deficiency in the WT mice were associated with a downregulation of GK and PEPCK activities and an upregulation of p53 protein in liver. Whereas key roles of GK in glycolysis and PEPCK in gluconeogenesis are well documented (38), the tumor suppressor p53 protein recently has been shown to be a novel regulator of lipid metabolism involved in the development of obesity and fatty liver (39, 40). Long-term dietary selenomethionine consumption has been shown to increase exon-specific DNA methylation of the p53 gene in a Se dose–dependent manner in rat liver (53). Thus, the elevated hepatic p53 protein in the Se-deficient WT mice not only helps explain the attenuated lipogenesis but also unveils a novel link between Se/GPX1 and p53 on glucose and lipid metabolism. In addition, increased hepatic Gk1 mRNA in the Se-deficient WT mice might represent a feedback mechanism in response to the decreased GK activity (54). Furthermore, downregulation of hepatic Srebp1a and Srebp2 mRNA in the Se-deficient WT mice should have inhibited lipogenesis because these 2 genes encode key transcriptional factors for the pathway (55).
In summary, dietary Se deficiency disallowed the GPX1 overproduction in the full-fed OE mice and nearly rescued all of their type 2 diabetes–like phenotypes. As proposed in Figure 7, the observed improvement was associated with downregulation of proinsulin genes in islets, lipogenesis rate-limiting enzyme genes in liver, and key glycolysis and gluconeogenesis enzymes in liver and/or muscle. Although molecular or physiologic implications for several gene expression changes remain elusive, our study has shown a strong regulation of hepatic GK mRNA and activity by GPX1 overproduction and dietary Se deficiency, and a novel link of Se/GPX1 to p53 on body energy metabolism. Despite our focus on the role of GPX1 in OE phenotypes in the present study, other Se-dependent proteins, including selenoprotein P (56, 57), methionine-R-sulfoxide reductase B1 (13), thioredoxin reductase 3 (13), and selenoprotein S (58), might also be involved in glucose metabolism or diabetes. Because expressions of these proteins are affected by dietary Se (12, 13), and perhaps by the overproduction of GPX1 to a certain extent (13), our ongoing research aims to elucidate their relative roles in dietary Se deficiency–mediated changes in OE phenotypes.
FIGURE 7.
Scheme of potential regulatory pathways and mechanisms for the impacts of dietary Se deficiency on the type 2 diabetes–like phenotypes of OE mice. GK, glucokinase; GPX1, glutathione peroxidase-1; GSIS, glucose-stimulated insulin secretion; NEFA, nonesterified fatty acid; OE, GPX1 overexpressing; OE(+), Se-adequate GPX1 overexpressing mice; OE(−), Se-deficient GPX1 overexpressing mice; PEPCK, phosphoenolpyruvate carboxykinase; TC, total cholesterol; WT, wild-type; WT(+), Se-adequate wild-type mice; WT(−), Se-deficient wild-type mice.
Supplementary Material
Acknowledgments
X.G.L. designed the research; X.Y., M.P.P., C.A.R., M.Z.V., L.L., and X.G.L. conducted the experiments and analyzed the data; X.G.L., M.Z.V., and X.Y. wrote the manuscript; and X.G.L. had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: GK, glucokinase; GPX1, glutathione peroxidase-1; GSIS, glucose-stimulated insulin secretion; OE, glutathione peroxidase-1–overexpressing; PEPCK, phosphoenolpyruvate carboxykinase; WT, wild-type.
Literature Cited
- 1.Mueller AS, Pallauf J. Compendium of the antidiabetic effects of supranutritional selenate doses. In vivo and in vitro investigations with type II diabetic db/db mice. J Nutr Biochem. 2006;17:548–60 [DOI] [PubMed] [Google Scholar]
- 2.Faure P. Protective effects of antioxidant micronutrients (vitamin E, zinc and selenium) in type 2 diabetes mellitus. Clin Chem Lab Med. 2003;41:995–8 [DOI] [PubMed] [Google Scholar]
- 3.Ezaki O. The insulin-like effects of selenate in rat adipocytes. J Biol Chem. 1990;265:1124–8 [PubMed] [Google Scholar]
- 4.Akbaraly TN, Arnaud J, Rayman MP, Hininger-Favier I, Roussel AM, Berr C, Fontbonne A. Plasma selenium and risk of dysglycemia in an elderly French population: results from the prospective Epidemiology of Vascular Ageing Study. Nutr Metab (Lond). 2010;7:21–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navarro-Alarcón M, Lopez-G de la SH, Perez-Valero V, Lopez-Martinez C. Serum and urine selenium concentrations as indicators of body status in patients with diabetes mellitus. Sci Total Environ. 1999;228:79–85 [DOI] [PubMed] [Google Scholar]
- 6.Kljai K, Runje R. Selenium and glycogen levels in diabetic patients. Biol Trace Elem Res. 2001;83:223–9 [DOI] [PubMed] [Google Scholar]
- 7.McNeill JH, Delgatty HL, Battell ML. Insulinlike effects of sodium selenate in streptozocin-induced diabetic rats. Diabetes. 1991;40:1675–8 [DOI] [PubMed] [Google Scholar]
- 8.Battell ML, Delgatty HL, McNeill JH. Sodium selenate corrects glucose tolerance and heart function in STZ diabetic rats. Mol Cell Biochem. 1998;179:27–34 [DOI] [PubMed] [Google Scholar]
- 9.Bleys J, Navas-Acien A, Guallar E. Serum selenium and diabetes in U.S. adults. Diabetes Care. 2007;30:829–34 [DOI] [PubMed] [Google Scholar]
- 10.Bleys J, Navas-Acien A, Stranges S, Menke A, Miller ER, III, Guallar E. Serum selenium and serum lipids in US adults. Am J Clin Nutr. 2008;88:416–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stranges S, Sieri S, Vinceti M, Grioni S, Guallar E, Laclaustra M, Muti P, Berrino F, Krogh V. A prospective study of dietary selenium intake and risk of type 2 diabetes. BMC Public Health. 2010;10:564–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng MS, Li X, Liu Y, Zhao H, Zhou JC, Li K, Huang JQ, Sun LH, Tang JY, Xia XJ, et al. A high-selenium diet induces insulin resistance in gestating rats and their offspring. Free Radic Biol Med. 2012;52:1335–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labunskyy VM, Lee BC, Handy DE, Loscalzo J, Hatfield DL, Gladyshev VN. Both maximal expression of selenoproteins and selenoprotein deficiency can promote development of type 2 diabetes-like phenotype in mice. Antioxid Redox Signal. 2011;14:2327–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–90 [DOI] [PubMed] [Google Scholar]
- 15.Lei XG, Cheng WH, McClung JP. Metabolic regulation and function of glutathione peroxidase-1. Annu Rev Nutr. 2007;27:41–61 [DOI] [PubMed] [Google Scholar]
- 16.Grankvist K, Marklund SL, Täljedal IB. CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem J. 1981;199:393–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20:463–6 [DOI] [PubMed] [Google Scholar]
- 18.Peuchant E, Brun JL, Rigalleau V, Dubourg L, Thomas MJ, Daniel JY, Leng JJ, Gin H. Oxidative and antioxidative status in pregnant women with either gestational or type 1 diabetes. Clin Biochem. 2004;37:293–8 [DOI] [PubMed] [Google Scholar]
- 19.Hamanishi T, Furuta H, Kato H, Doi A, Tamai M, Shimomura H, Sakagashira S, Nishi M, Sasaki H, Sanke T, et al. Functional variants in the glutathione peroxidase-1 (GPx-1) gene are associated with increased intima-media thickness of carotid arteries and risk of macrovascular diseases in Japanese type 2 diabetic patients. Diabetes. 2004;53:2455–60 [DOI] [PubMed] [Google Scholar]
- 20.Lewis P, Stefanovic N, Pete J, Calkin AC, Giunti S, Thallas-Bonke V, Jandeleit-Dahm KA, Allen TJ, Kola I, Cooper ME, et al. Lack of the antioxidant enzyme glutathione peroxidase-1 accelerates atherosclerosis in diabetic apolipoprotein E-deficient mice. Circulation. 2007;115:2178–87 [DOI] [PubMed] [Google Scholar]
- 21.Nemoto M, Nishimura R, Sasaki T, Hiki Y, Miyashita Y, Nishioka M, Fujimoto K, Sakuma T, Ohashi T, Fukuda K, et al. Genetic association of glutathione peroxidase-1 with coronary artery calcification in type 2 diabetes: a case control study with multi-slice computed tomography. Cardiovasc Diabetol. 2007;6:23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuzuya M, Ando F, Iguchi A, Shimokata H. Glutathione peroxidase 1 Pro198Leu variant contributes to the metabolic syndrome in men in a large Japanese cohort. Am J Clin Nutr. 2008;87:1939–44 [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Scholl TO, Leskiw MJ, Donaldson MR, Stein TP. Association of glutathione peroxidase activity with insulin resistance and dietary fat intake during normal pregnancy. J Clin Endocrinol Metab. 2003;88:5963–8 [DOI] [PubMed] [Google Scholar]
- 24.McClung JP, Roneker CA, Mu W, Lisk DJ, Langlais P, Liu F, Lei XG. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci USA. 2004;101:8852–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang XD, Vatamaniuk MZ, Wang SK, Roneker CA, Simmons RA, Lei XG. Molecular mechanisms for hyperinsulinaemia induced by overproduction of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia. 2008;51:1515–24 [DOI] [PubMed] [Google Scholar]
- 26.Pepper MP, Vatamaniuk MZ, Yan X, Roneker CA, Lei XG. Impacts of dietary selenium deficiency on metabolic phenotypes of diet-restricted GPX1-overexpressing mice. Antioxid Redox Signal. 2011;14:383–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. Beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12:1763–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu C, Stein GH, Pan N, Goebbels S, Hörnberg H, Nave KA, Herrera P, White P, Kaestner KH, Sussel L, et al. Pancreatic beta cells require NeuroD to achieve and maintain functional maturity. Cell Metab. 2010;11:298–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shih DQ, Screenan S, Munoz KN, Philipson L, Pontoglio M, Yaniv M, Polonsky KS, Stoffel M. Loss of HNF-1alpha function in mice leads to abnormal expression of genes involved in pancreatic islet development and metabolism. Diabetes. 2001;50:2472–80 [DOI] [PubMed] [Google Scholar]
- 30.Lantz KA, Vatamaniuk MZ, Brestelli JE, Friedman JR, Matschinsky FM, Kaestner KH. Foxa2 regulates multiple pathways of insulin secretion. J Clin Invest. 2004;114:512–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao L, Guo M, Matsuoka TA, Hagman DK, Parazzoli SD, Poitout V, Stein R. The islet beta cell-enriched MafA activator is a key regulator of insulin gene transcription. J Biol Chem. 2005;280:11887–94 [DOI] [PubMed] [Google Scholar]
- 32.Thorens B, Weir GC, Leahy JL, Lodish HF, Bonner-Weir S. Reduced expression of the liver/beta-cell glucose transporter isoform inglucose-insensitive pancreatic beta cells of diabetic rats. Proc Natl Acad Sci USA. 1990;87:6492–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winzell MS, Coghlan M, Leighton B, Frangioudakis G, Smith DM, Storlien LH, Ahrén B. Chronic glucokinase activation reduces glycaemia and improves glucose tolerance in high-fat diet fed mice. Eur J Pharmacol. 2011;663:80–6 [DOI] [PubMed] [Google Scholar]
- 34.Shimano H. Sterol regulatory element-binding protein family as global regulators of lipid synthetic genes in energy metabolism. Vitam Horm. 2002;65:167–94 [DOI] [PubMed] [Google Scholar]
- 35.Mao J, DeMayo FJ, Li H, Abu-Elheiga L, Gu Z, Shaikenov TE, Kordari P, Chirala SS, Heird WC, Wakil SJ. Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc Natl Acad Sci USA. 2006;103:8552–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paulauskis JD, Sul HS. Cloning and expression of mouse fatty acid synthase and other specific mRNAs. Developmental and hormonal regulation in 3T3–L1 cells. J Biol Chem. 1988;263:7049–54 [PubMed] [Google Scholar]
- 37.Jones JR, Barrick C, Kim KA, Lindner J, Blondeau B, Fujimoto Y, Shiota M, Kesterson RA, Kahn BB, Magnuson MA. Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc Natl Acad Sci USA. 2005;102:6207–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Granner D, Pilkis S. The genes of hepatic glucose metabolism. J Biol Chem. 1990;265:10173–6 [PubMed] [Google Scholar]
- 39.Yahagi N, Shimano H, Matsuzaka T, Sekiya M, Najima Y, Okazaki S, Okazaki H, Tamura Y, Iizuka Y, Inoue N, et al. p53 involvement in the pathogenesis of fatty liver disease. J Biol Chem. 2004;279:20571–5 [DOI] [PubMed] [Google Scholar]
- 40.Ashur-Fabian O, Har-Zahav A, Shaish A, Wiener Amram H, Margalit O, Weizer-Stern O, Dominissini D, Harats D, Amariglio N, Rechavi G. apoB and apobec1, two genes key to lipid metabolism, are transcriptionally regulated by p53. Cell Cycle. 2010;9:3761–70 [PubMed] [Google Scholar]
- 41.Wang X, Vatamaniuk MZ, Roneker CA, Pepper MP, Hu LG, Simmons RA, Lei XG. Knockouts of SOD1 and GPX1 exert different impacts on murine islet function and pancreatic integrity. Antioxid Redox Signal. 2011;14:391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng WH, Ho YS, Ross DA, Han Y, Combs GF, Jr, Lei XG. Overexpression of cellular glutathione peroxidase does not affect expression of plasma glutathione peroxidase or phospholipid hydroperoxide glutathione peroxidase in mice offered diets adequate or deficient in selenium. J Nutr. 1997;127:675–80 [DOI] [PubMed] [Google Scholar]
- 43.Lei XG, Evenson JK, Thompson KM, Sunde RA. Glutathione peroxidase and phospholipid hydroperoxide glutathione peroxidase are differentially regulated in rats by dietary selenium. J Nutr. 1995;125:1438–46 [DOI] [PubMed] [Google Scholar]
- 44.Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, Datta SR, Greenberg ME, Licklider LJ, Lowell BB, et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424:952–6 [DOI] [PubMed] [Google Scholar]
- 45.Duff DA, Snell K. Limitations of commonly used spectrophotometric assay methods for phosphoenolypyruvate carboxykinase activity in crude extracts of muscle. Biochem J. 1982;206:147–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng W, Fu YX, Porres JM, Ross DA, Lei XG. Selenium dependent cellular glutathione peroxidase protects mice against a pro-oxidant-induced oxidation of NADPH, NADH, lipids, and protein. FASEB J. 1999;13:1467–75 [DOI] [PubMed] [Google Scholar]
- 47.Malecki MT, Jhala US, Antonellis A, Fields L, Doria A, Orban T, Saad M, Warram JH, Montminy M, Krolewski AS. Mutations in NEUROD1 are associated with the development of type 2 diabetes mellitus. Nat Genet. 1999;23:323–8 [DOI] [PubMed] [Google Scholar]
- 48.Lee CS, Sund NJ, Vatamaniuk MZ, Matschinsky FM, Stoffers DA, Kaestner KH. Foxa2 controls Pdx1 gene expression in pancreatic beta-cells in vivo. Diabetes. 2002;51:2546–51 [DOI] [PubMed] [Google Scholar]
- 49.Foretz M, Guichard C, Ferré P, Foufelle F. Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc Natl Acad Sci USA. 1999;96:12737–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matschinsky FM. Banting Lecture 1995: a lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes. 1996;45:223–41 [DOI] [PubMed] [Google Scholar]
- 51.Scott KE, Wheeler FB, Davis AL, Thomas MJ, Ntambi JM, Seals DF, Kridel SJ. Metabolic regulation of invadopodia and invasion by acetyl-CoA carboxylase 1 and de novo lipogenesis. PLoS ONE. 2012;7:e29761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang J, Kalhan SC, Hanson RW. What is the metabolic role of phosphoenolpyruvate carboxykinase? J Biol Chem. 2009;284:27025–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeng H, Yan L, Cheng WH, Uthus EO. Dietary selenomethionine increases exon-specific DNA methylation of the p53 gene in rat liver and colon mucosa. J Nutr. 2011;141:1464–8 [DOI] [PubMed] [Google Scholar]
- 54.Jackson DA, Pombo A, Iborra F. The balance sheet for transcription: an analysis of nuclear RNA metabolism in mammalian cells. FASEB J. 2000;14:242–54 [PubMed] [Google Scholar]
- 55.Edwards PA, Tabor D, Kast HR, Venkateswaran A. Regulation of gene expression by SREBP and SCAP. Biochim Biophys Acta. 2000;1529:103–13 [DOI] [PubMed] [Google Scholar]
- 56.Misu H, Takamura T, Takayama H, Hayashi H, Matsuzawa-Nagata N, Kurita S, Ishikura K, Ando H, Takeshita Y, Ota T, et al. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. 2010;12:483–95 [DOI] [PubMed] [Google Scholar]
- 57.Speckmann B, Walter PL, Alili L, Reinehr R, Sies H, Klotz LO, Steinbrenner H. Selenoprotein P expression is controlled through interaction of the coactivator PGC-1alpha with FoxO1a and hepatocyte nuclear factor 4alpha transcription factors. Hepatology. 2008;48:1998–2006 [DOI] [PubMed] [Google Scholar]
- 58.Gao Y, Feng HC, Walder K, Bolton K, Sunderland T, Bishara N, Quick M, Kantham L, Collier GR. Regulation of the selenoprotein SelS by glucose deprivation and endoplasmic reticulum stress: SelS is a novel glucose-regulated protein. FEBS Lett. 2004;563:185–90 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.