Abstract
Background:
Anterior cruciate ligament injury increases risk for accelerated development of osteoarthritis. The effect of exercise on articular cartilage following joint injury is not well understood. Biochemical biomarkers of collagen degradation and proteoglycan turnover are potential indicators for early articular cartilage degeneration.
Hypothesis:
This study tests the hypothesis that serum concentrations of CS846 and CTXii correlate with structural changes to articular cartilage following joint injury in exercised animals.
Study Design:
Controlled laboratory study.
Methods:
Twenty-four Sprague-Dawley rats underwent either arthrotomy alone (sham surgery) or anterior cruciate ligament transection (ACLT). Animals were recovered for 3 weeks and then exercised on a treadmill at 18 m per minute, 1 hour per day, 5 days per week, until sacrifice either 6 or 12 weeks later. Articular cartilage was assessed grossly, and histology was graded using modified Mankin, toluidine blue, and modified David-Vaudey scales. Serum collected preoperatively and at sacrifice was assayed by ELISA for CTXii and CS846.
Results:
At 6 weeks, gross grades (P < 0.01), modified Mankin scores (P < 0.03), and toluidine blue scores (P < 0.04) were higher, reflecting increased degeneration in ACLT animals compared with sham surgery animals. Serum CS846 increased after 6 weeks in ACLT animals (P < 0.05). Serum CTXii levels strongly correlated with Mankin degenerative scores (coefficient = 0.81, P < 0.01) and David-Vaudey histology grades (coefficient = 0.73, P < 0.01) at 6 weeks. While gross grades remained higher at 12 weeks in ACLT animals (P < 0.04), no differences were seen in serum CS846 and CTXii. Histology scores also showed no differences between ACLT and sham due to increasing degeneration in the sham surgery group.
Conclusion:
The strong correlation between serum CTXii and microstructural changes to articular cartilage following joint injury demonstrates potential use of serum biomarkers for early detection of cartilage degeneration. Increasing cartilage degeneration in exercised sham-surgery animals suggests that early loading may have negative effects on articular cartilage due to either mechanical injury or hemarthrosis after arthrotomy.
Clinical Relevance:
Patients with anterior cruciate ligament injury are at increased risk for development of posttraumatic osteoarthritis. CTXii may be useful for early detection of joint degeneration. Further study on the effects of exercise after injury is important to postinjury and postoperative rehabilitation.
Keywords: serum CTXii, serum CS846, biochemical biomarkers, anterior cruciate ligament transection, articular cartilage, hemarthrosis, rat
Osteoarthritis (OA) is characterized by progressive degeneration of articular cartilage and is a leading cause of disability. The disease involves the entire joint and is strongly associated with chondrocyte loss and degradation of articular cartilage.11 Osteoarthritis consists of multiple etiologies, of which joint injuries result in accelerated degeneration that contributes to significant long-term disability and health care expenditures.2
Anterior cruciate ligament (ACL) injury is associated with accelerated development of OA19,25 and commonly occurs in young active patients through sports or traumatic injuries. Clinical studies have shown that approximately 50% of ACL-injured patients develop radiographic signs of OA within 10 to 20 years of injury.21,22,25 Early detection and treatment of articular cartilage and joint metabolic dysfunction following injury may delay or reduce the incidence of posttraumatic OA after ACL injury. Biochemical biomarkers of collagen degradation and proteoglycan turnover have potential to show articular cartilage breakdown and metabolic changes before the onset of radiographically evident OA. Biochemical markers of articular cartilage structural change prior to detection by radiography and magnetic resonance imaging would be useful in the development of early intervention strategies.
Articular cartilage is primarily composed of type II collagen and proteoglycans such as aggrecan. Fragments of C-terminal crosslinked telopeptide type II collagen (CTXii), a breakdown product of collagen type II, are detectable in urine, serum, and synovial fluid.5 CTXii has been shown to be predictive of joint degeneration in small and large animal injury models1,9,23 and in human patients suffering from degenerative OA.7,18,20
Aggrecan chondroitin sulfate 846 epitope (CS846) is another potential marker of early degeneration. It is a by-product of proteoglycan metabolism and is detectable in serum and synovial fluid.27,28 CS846 has been shown to differentiate between radiographic and nonradiographic OA in human subjects7 but has not been studied in animal models of ACL injury.
The effects of exercise on joint degeneration have varied in the literature, with no clear consensus on the implications for unstable and injured joints. Light to moderate exercise on a treadmill reduced articular cartilage degeneration following ACL transection (ACLT) in a short-term rat study.13 However, in the same study, intense exercise accelerated articular cartilage degeneration following ACLT, illustrating a differential response based on exercise intensity.13 Other studies show that intense exercise alone can induce articular cartilage degeneration in animals,26,29 that exercise potentiates the destructive effects of hemarthrosis,16 and that exercise is known to modulate biochemical biomarker levels.12
Because biomarkers of collagen and proteoglycan turnover may be indicative of early structural changes to articular cartilage, the purpose of this study was to test the hypothesis that serum concentrations of CS846 and CTXii correlate with articular cartilage degeneration following ACLT in animals undergoing a standardized exercise regimen.
Materials and Methods
Experimental Design
Following approval by the Institutional Animal Care and Use Committee, 24 three-month-old Sprague-Dawley rats (~325 g) were assigned to either sham surgery (n = 12) or ACLT (n = 12). Sham-surgery animals underwent arthrotomy only, while ACLT animals underwent ACLT to the right stifle joint. All animals were recovered for 3 weeks before undergoing a standardized exercise program on a treadmill until sacrifice after 6 or 12 weeks of exercise (9 or 15 weeks after surgery). Following sacrifice, blood was obtained, and the knee (stifle) joints were harvested, assessed grossly with India ink, and then processed for histology.
Surgical Procedure
Following induction of anesthesia, a longitudinal skin incision was performed to the right knee joint. A medial parapatellar arthrotomy was used to enter the joint.32 The arthrotomy was then repaired in the sham-surgery animals. For the ACLT animals, the patella was dislocated laterally, the knee flexed, and the ACL and posterior cruciate ligament visualized under 4× loupe manipulation. The ACL was then transected with jeweler’s scissors. Complete transection of the ACL was confirmed via a positive anterior drawer test. The joint was then repaired using 5-0 Vicryl and 4-0 nylon (Ethicon, Somerville, New Jersey).
Exercise Program
All animals underwent a standardized treadmill exercise program at moderate intensity to mimic an average ACL-injured population. Animals were allowed to rest 10 to 14 days following surgery and then began a week of exercise training to adapt to the treadmill (Columbus Instruments, Columbus, Ohio). During this week, the rate of exercise was incrementally increased from 10 m per minute to 18 m per minute, while the duration of exercise was increased from 10 to 50 minutes, through increasing exercise time duration by 10 minutes per day. Following the training period, animals were exercised on the treadmill for 1 hour a day, 5 times a week, for 6 or 12 weeks at a rate of 18 m per minute. The exercise rate was based on past reports in the literature where moderate- to high-intensity exercise was defined as running approximately 1100 meters a day at speeds ranging from 15 to 20 m per minute.1,13,26
Serum Collection
One milliliter of venous blood was withdrawn from the jugular vein at the time of surgery. At the time of sacrifice, 4 mL of venous blood was withdrawn via cardiac puncture immediately following euthanasia. All blood samples were collected in serum separator tubes, allowed to clot for 2 hours at room temperature, and centrifuged at 2000 g for 15 minutes at 4°C, after which the collected serum was aliquoted and stored at −80°C prior to analysis.
Gross Imaging and Grading
After euthanasia, the rat knee joint was exposed and extraneous soft tissue removed. The distal femur was stained with India ink (BD, Franklin Lakes, New York; dilution 1:10) and imaged with an Olympus MVX stereomicroscope and DP71 camera (Olympus, Center Valley, Pennsylvania). The distal femur was graded by 2 independent, blinded reviewers using a previously published scale for gross assessment of articular cartilage.32 Grade 1 indicated an intact articular cartilage surface; grade 2, minimal fibrillation; grade 3, overt fibrillation; and grade 4, erosion with exposed bone.
Histological Staining and Grading
Following gross assessment, the intact joint was fixed in neutral buffered formalin for 72 hours, decalcified in 10% EDTA, bisected sagittally into the medial and lateral compartments, embedded in paraffin, and cut in 5-µm sections in the sagittal plane. Slides from the central region of the medial and lateral compartments were stained with hematoxylin/eosin, safranin O/fast green, picrosirius red, and toluidine blue, using established protocols.6,31 Histological scoring was performed by 2 independent, blinded observers using the modified Mankin score,30 a previously published toluidine blue score for proteoglycan assessment,10 and a modified David-Vaudey score for polarized light microscopy assessment of matrix structure.8 David-Vaudey matrix scores of 0 (normal) or 1 (superficial loss of birefringence) were condensed to grade 0 (normal/superficial loss) because of thin superficial cartilage in rats. A total of 4 surfaces were graded: the medial femoral condyle, medial tibial plateau, lateral femoral condyle, and tibial plateau. Scores were then totaled for each joint, and the mean total scores were calculated and reported.
Biomarker Assessment and Analysis
Serum was analyzed for CS846 and CTXii using commercially available ELISA assays from IBEX Technologies (Montreal, Canada) and Immuno Diagnostic Systems (IDS, Scottsdale, Arizona), respectively, according to the manufacturers’ instructions. Standards and samples were assayed in duplicate on 96-well plates (50 µL/sample). CS846 and CTXii concentrations were calculated from a standard curve and normalized to preoperative values. Because of inadequate preoperative sampling from some rats, the sham group had 4 rats for CTXii analysis and 5 rats for CS846 analysis for the 12-week time point.
Statistical Analysis
Statistical analysis of results was conducted with SPSS 17.0 using nonparametric tests. The Mann-Whitney test was used for statistical assessment between sham and ACLT animals for biomarkers, gross assessment, and histology findings. The Kriskal-Wallis test, followed by the Wilcoxon signed-rank test for intergroup comparisons, was used to determine statistical differences within the ACLT or sham population over time. The Spearman rho test was used to assess the correlation among biomarker levels, histology grades, and gross grades. Data are reported as mean ± standard error of the mean unless otherwise noted. Significance was defined as P < 0.05.
Results
Gross Assessment
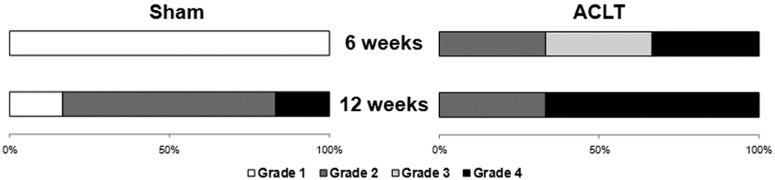
The articular cartilage in ACLT animals exhibited gross degeneration after 6 and 12 weeks of standardized exercise (Figure 1). At 6 weeks, India ink and stereomicroscopy revealed signs of minimal and overt fibrillation in 4 of 6 ACLT animals (grades 2 and 3) and signs of erosion with exposed bone in 2 of 6 ACLT animals (grade 4; Figure 2). In contrast, India ink and stereomicroscopy revealed no signs of fibrillation or erosion (grade 1) in sham animals following 6 weeks of exercise; the articular surface appeared smooth, glistening, and intact. At 6 weeks, the mean gross score was threefold higher in ACLT animals (3.2 ± 0.5) compared with sham animals (1.0 ± 0.1, P < 0.01).
Figure 1.
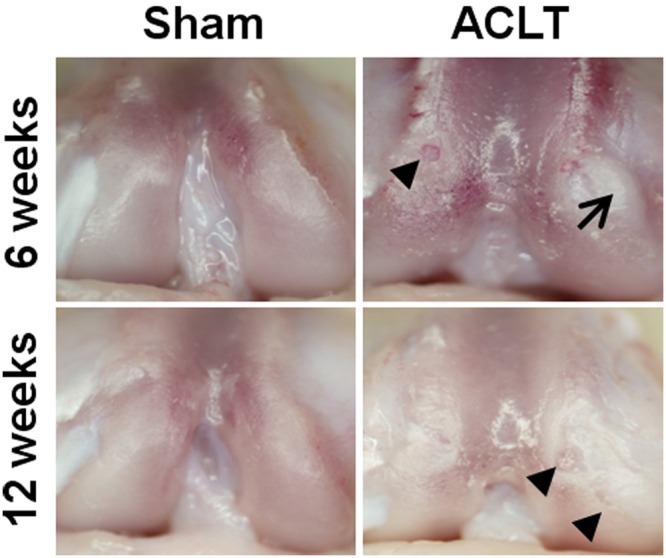
Articular cartilage erosion and fibrillation and fibrous tissue development were grossly evident after anterior cruciate ligament transection (ACLT). Selected gross images of rat knee femurs after 6 and 12 weeks of standardized exercise. Erosions (arrowheads) and fibrous tissue development (arrow) were observed in the ACLT group at both time points. Pannus formation was observed in 1 of 6 sham animals at 12 weeks.
Figure 2.
Gross grades were higher in anterior cruciate ligament transection (ACLT) animals compared with sham. After 6 weeks of exercise, gross scores were all grade 1 for the sham and were equally distributed among grades 2, 3, and 4 for ACLT animals (2 rats per grade). After 12 weeks of exercise, most sham animals had minimal fibrillations (grade 2), and the majority of ACLT animals had cartilage erosion with exposed bone (grade 4). Grade 1 indicates an intact articular cartilage surface; grade 2, minimal fibrillation; grade 3, overt fibrillation; and grade 4, erosion with exposed bone.
Articular cartilage erosion was more prominent following 12 weeks of exercise (Figure 1). In ACLT animals, 4 of 6 showed gross signs of erosion with exposed bone (grade 4), while the remaining 2 exhibited minor cartilage fibrillation following India ink application (grade 2; Figure 2). At 12 weeks, 5 of 6 sham animals showed signs of early fibrillation during India ink and stereomicroscopy gross assessment (grade 2/3; P = 0.01 vs 6-week sham). The final sham animal had extensive infiltration of soft tissue pannus covering the joint surfaces, which was classified as grade 4 even though subchondral bone was not exposed. The mean gross score was elevated with ACLT animals (3.3 ± 0.7) compared with sham animals (2.0 ± 0.4, P < 0.04).
Qualitative Histological Assessments
Articular cartilage structure, cellularity, glycosaminoglycan staining, and matrix structure were assessed on histological sections using 3 established histology grading systems.
Modified Mankin degeneration score
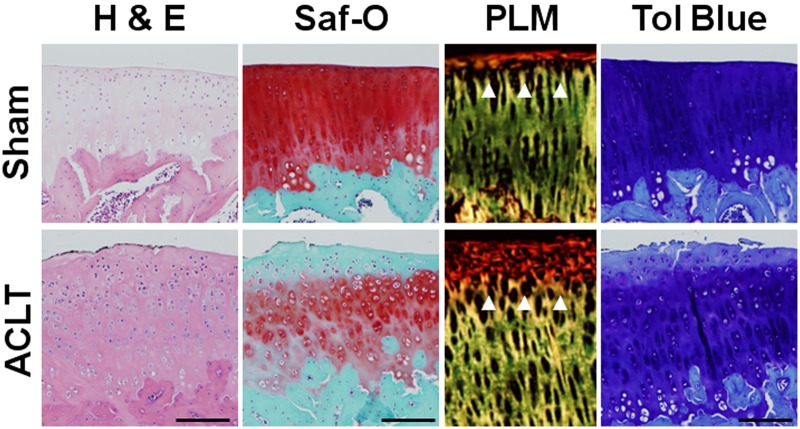
Articular cartilage structural degeneration was evident in histological sections from ACLT animals stained with hematoxylin/eosin and safranin O. Signs of degeneration included surface disruption and irregularity, fibrillation, and focal loss of chondrocytes (Figure 3). With higher scores reflective of increasing cartilage degeneration, the total modified Mankin score was twofold higher in ACLT animals (15.5 ± 3.7) compared to sham animals (8.0 ± 4.4; P < 0.03) after 6 weeks of standardized exercise (Figure 4A). In addition, a trend toward increasing Mankin scores was observed between 6-week (8.0 ± 4.4) and 12-week (12.7 ± 5.0) sham-surgery animals (P = 0.20). No statistical differences were found between groups at 12 weeks (P > 0.05). At 6 weeks, the total modified Mankin score strongly correlated with gross grades (correlation coefficient = 0.716, P < 0.01).
Figure 3.
Histological staining showed increased articular cartilage degeneration after 6 weeks of exercise in anterior cruciate ligament transection (ACLT) animals. Representative histology images from a centrally located articular cartilage section of the medial tibial plateau showing pathology consistent with average reported histology grades following 6 weeks of standardized exercise. Included are representative images of hematoxylin/eosin (H&E), safranin O (Saf-O), toluidine blue (Tol Blue), and polarized light microscopy (PLM) of stained sections. The observed increase in cell cloning, mild fibrillations, and loss of Saf-O staining were consistent with elevated Mankin scores after ACLT. In addition, the disruption of superficial collagen organization (white arrowheads) seen under PLM assessment was similar to observed surface fibrillations. The reduction in proteoglycan staining seen with Saf-O was confirmed by Tol Blue, further confirming loss of proteoglycan staining after ACLT. In contrast to ACLT groups, the sham exercise group had normal-appearing articular cartilage structure and strong proteoglycan staining after 6 weeks of exercise. Scale bar = 200 µm.
Figure 4.
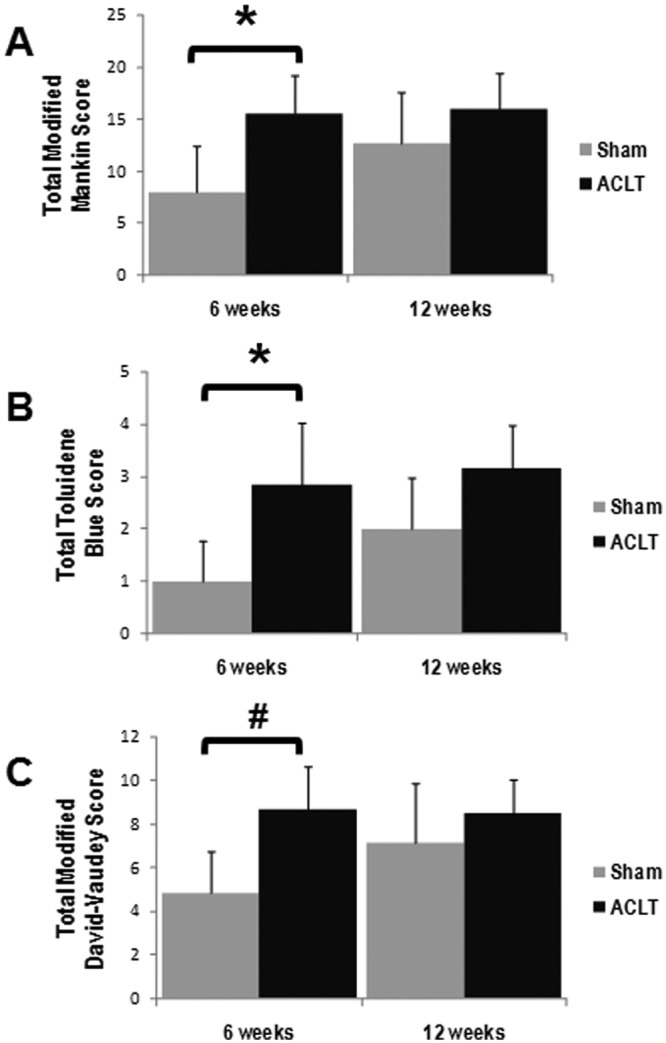
Increasing histological evidence for articular degeneration after both anterior cruciate ligament transection (ACLT) and arthrotomy was evident at 12 weeks. At 6 weeks, more extensive articular cartilage degeneration was seen in ACLT animals compared to sham (arthrotomy alone), indicated by higher total modified Mankin scores (A), greater loss of glycosaminoglycans by toluidine blue scores (B), and more matrix disruption by David-Vaudy scoring (C). However, at 12 weeks, all histological scores trended higher for the sham-surgery group, and no statistical differences between the scores for the ACLT and sham surgery groups were observed (*P < 0.05, #P ~ 0.05).
Toluidine blue score: Proteoglycan assessment
Proteoglycan staining was reduced in superficial and intermediate articular cartilage layers in histological sections from ACLT animals following 6 weeks of exercise (Figure 3). Higher toluidine blue scores indicate increased loss of proteoglycan staining in articular cartilage. The total toluidine blue score was nearly threefold higher in ACLT (2.8 ± 1.2) compared with sham animals (1.0 ± 0.8, P < 0.04) at 6 weeks (Figure 4B). No statistical differences were found between groups after 12 weeks of exercise (P > 0.05).
David-Vaudey matrix score
Collagen matrix organization was assessed by the modified David-Vaudey matrix score with polarized light microscopy. Increased superficial matrix disorganization was apparent in histology from ACLT animals (Figure 3). While the matrix scores trended higher after 6 weeks of exercise (Figure 4C; P = 0.09), they were not statistically different between groups at the 12-week timepoint (P > 0.05). At 6 weeks, the total modified David-Vaudey matrix score showed modest correlations with gross grading (correlation coefficient = 0.689, P < 0.02).
Serum CS846
Serum CS846, a marker of proteoglycan metabolism, was assessed in sham and ACLT animals undergoing standardized exercise. Serum CS846 increased in ACLT animals after 6 weeks. The mean serum CS846 increased nearly twofold following 6 weeks of exercise in ACLT animals (P = 0.05) before returning to preoperative levels at 12 weeks (P < 0.03; Figure 5). In sham animals, serum CS846 also increased roughly twofold at 6 weeks; however, this increase was not statistically significant due to greater variability between animals (P > 0.05). CS846 levels decreased nearly 20% from preoperative levels in sham animals following 12 weeks of standardized exercise (P < 0.05, n = 4). No statistical differences were found between sham-surgery and ACL-injured groups at either timepoint (P > 0.05).
Figure 5.
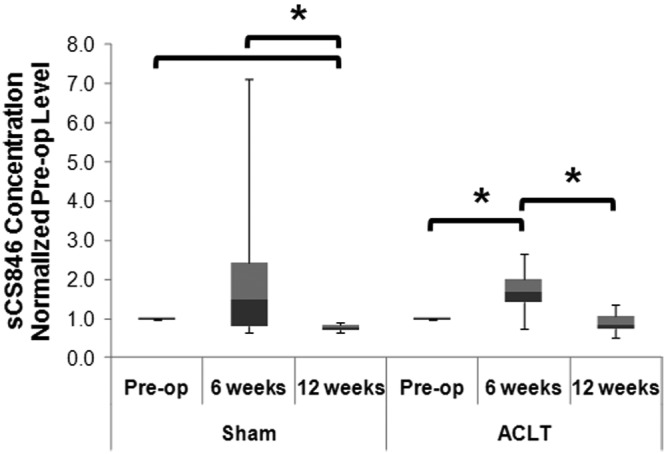
Serum CS846 is elevated with mild cartilage degeneration. Serum CS846 levels increased following anterior cruciate ligament transection (ACLT) or sham surgery after 6 weeks of exercise in all groups (by Kruskal-Wallis reported on the graph) with intergroup comparisons assessed by the Wilcoxon rank test. After 12 weeks of exercise, the concentration of CS846 returned to preoperative levels (*P < 0.05).
Serum CTXii
Serum CTXii, a biomarker for collagen type II degradation, trended higher following 6 weeks of exercise in ACLT animals (P = 0.116, n = 6). No change in biomarker levels was found in sham animals at 6 weeks (P > 0.05). After 12 weeks, the level of CTXii decreased in ACLT animals (P < 0.03) and trended lower in sham animals (P = 0.07, n = 5). No statistical differences were found between groups (P > 0.05).
Serum CTXii Correlates with Structural and Gross Findings
When serum CTXii levels for the entire 6-week cohort (both ACLT and sham surgery) were separated by gross articular cartilage score into mild degeneration (scores 1 and 2) and severe degeneration (scores 3 and 4), the CTXii was higher in animals with severe degeneration (1.66 ± 0.26) compared to animals with mild degeneration (0.93 ± 0.10; P < 0.03).
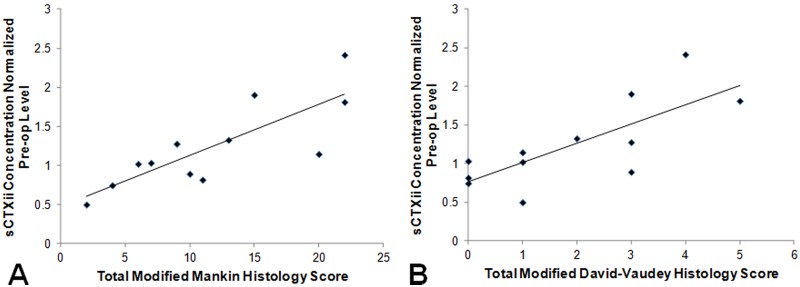
Furthermore, strong relationships were found between CTXii level and histological grading after 6 weeks of standardized exercise (Figure 6). Higher levels of CTXii correlated with greater articular cartilage degeneration (higher histology grades) assessed by the total modified Mankin score (correlation coefficient = 0.813, P < 0.01, n = 12) and by the total modified David-Vaudey matrix grade (correlation coefficient = 0.729, P < 0.01, n = 12). In addition, a moderate relationship was found between CTXii levels and gross grades at 6 weeks (correlation coefficient = 0.618, P < 0.04, n = 12).
Figure 6.
CTXii correlates with histology. At 6 weeks, higher levels of CTXii correlated with greater articular cartilage degeneration (higher histology grades) assessed by (A) the total modified Mankin score (correlation coefficient = 0.813, P < 0.01, n = 12) and (B) the total modified David-Vaudey matrix grade (correlation coefficient = 0.729, P < 0.01, n = 12). A linear fit was applied (black line) to better demonstrate the correlation between CTXii level and histology score.
Discussion
In this study, the relationship between serum biochemical biomarkers CS846 and CTXii and structural changes to articular cartilage was assessed in animals undergoing standardized exercise following sham surgery or ACLT. We found more extensive articular cartilage degeneration by gross assessment and histological assessment in ACLT animals. In addition, a transient increase in serum CS846 levels was found for ACLT animals after 6 weeks prior to returning to preoperative levels after 12 weeks. Finally, strong correlations were found between histological scores and CTXii levels and between histological scores and gross grades. A moderate correlation was observed between CTXii and gross grades. Anterior cruciate ligament transection animals undergoing standardized exercise had more extensive structural joint degeneration then sham animals, which correlated with levels of biochemical biomarkers of collagen type II breakdown.
Cartilage degeneration was more severe in ACLT animals compared to sham-surgery animals. Gross pathology included articular cartilage erosion, cartilage fibrillation, and fibrous tissue formation with ACLT. Gross inspection documented extensive articular cartilage fibrillation after 6 weeks and tissues with erosion of cartilage to subchondral bone after 12 weeks of exercise in ACLT animals. Findings were consistent with previous published studies showing increased articular cartilage degeneration following ACLT with and without moderate to intense exercise.1,13,14,17,29 In a study by Appleton et al, also in Sprague-Dawley rats, articular cartilage fissuring was found as early as 2 weeks after ACLT and medial meniscectomy in rats undergoing exercise on a rotary wheel.1 Joint damage occurred more rapidly in the Appleton study, likely due to the use of ACLT combined with meniscectomy, which has been linked with accelerated OA progression and joint degeneration compared to ACLT alone.15
Similar to gross findings, more extensive articular cartilage degeneration was found histologically in both the condyles and tibial plateau following ACLT. The total modified Mankin score and total toluidine blue score increased with ACLT compared to sham, consistent with more extensive gross and histologically evident articular cartilage damage. However, at 12 weeks, the difference between ACLT and sham was no longer statistically different. Sham animals undergoing exercise developed articular cartilage surface fissuring at 12 weeks, resulting in higher total Mankin scores compared with 6 weeks. The observed degeneration after arthrotomy alone could be due to injury or bleeding with resulting inflammation associated with the sham surgery followed by exercise. The health of articular cartilage following sham surgery has not been thoroughly discussed in the literature. However, Hooiveld et al showed that even a single exposure of healthy joints to blood in a canine model resulted in joint degeneration if the joint was loaded.16 While the arthrotomy may have resulted in subtle mechanical injury, it would have induced joint bleeding and hemarthrosis. The evidence for mild degeneration in this study is consistent with findings from other groups where exercise intensified the negative effects of other factors, such as hemarthrosis, on articular cartilage health.
In a study by Galois and colleagues,13,14 low and moderate levels of exercise protected the joints of Wistar rats with ACLT over 28 days compared with ACLT. In contrast, intense exercise in the same study was not chondroprotective. Because of the shorter study time frame, it is unclear if the protective effect seen over 28 days would continue with longer periods of exercise. Continued exercise in their study might result in early signs of cartilage degeneration during a longer time course, as seen in our study. Furthermore, Appleton and colleagues showed more extensive degeneration in forced mobilization of ACLT animals compared with standard ACLT animals,1 while Tang et al showed that intense exercise by itself leads to increased articular cartilage fissuring and degeneration even without joint injury after 6 weeks.29 The exercise requirement used in our study—18 m per minute, 1 hour a day, for 5 days—did not result in observable cartilage breakdown in sham animals at 6 weeks but likely contributed to articular cartilage fissuring observed at 12 weeks. However, we cannot exclude the possibility that the sham surgery itself or associated hemarthrosis contributed to the eventual development of fissuring and early degeneration.
Serum concentrations of CS846, a biochemical biomarker of proteoglycan turnover, increased following 6 weeks of exercise in ACLT populations, demonstrating increased proteoglycan synthesis and metabolism. Frisbie and colleagues also showed increased synovial fluid and serum levels of CS846 following treadmill exercise and during OA development in an equine model.12 The elevated serum levels of CS846 in our study did not persist through 12 weeks. CS846 has been shown to vary with disease severity. Consistent with our findings, Mazzuca et al showed in a human clinical study that serum concentrations of CS846 increased with early disease/injury but decreased with more advanced OA and cartilage erosion.24
A strong relationship was found between serum CTXii levels and histological scoring assessments, indicative of structural and cellular changes to the articular cartilage. This is one of the first studies to correlate serum CTXii directly with assessment of articular cartilage structural change. Increased CTXii levels correlated with higher histology scores, indicative of more extensive cartilage degeneration. Serum CTXii may therefore be a useful outcome measure for evaluation of novel structurally modifying therapies.
Serum CTXii levels trended higher in ACLT animals compared with sham animals after 6 weeks of exercise. Appleton et al demonstrated increased concentrations of urine CTXii in a Sprague-Dawley ACLT and meniscectomy model at 4 weeks after joint injury, but the increase was transient and returned to near presurgical concentrations by 8 weeks.1 This transient increase is likely related to breakdown of collagen type II within the joint and the resulting breakdown products passing to the serum and then the urine for excretion during the most extensive period of degeneration. The selection of the 9-week (6 weeks of exercise) and 15-week (12 weeks of exercise) time points was based on the exercise protocol. These time points may not be optimal to capture the earliest changes in biochemical biomarkers following ACLT injury in this model. Further studies are needed to more completely characterize early changes in serum biochemical biomarkers over time in the rat ACL transaction model.
Several studies1,3,4 have shown normalization of biochemical biomarkers and inflammatory markers following the acute or early phase of joint injury, with normalization typically occurring over 6 months in human studies.3 The mechanism of biomarker normalization is unclear but may be due to resolution of inflammation in the joint, resulting in loss of the catabolic environment and allowing for a return to joint homeostasis and normal joint metabolism. An alternative viewpoint could be that homeostasis is not restored after joint injury but that chondrocyte loss through death or metabolic dysregulation occurs to reduce biomarkers to basal levels. Biochemical biomarkers are likely most useful soon after injury and in conjunction with imaging studies. Further analysis is needed into the pathogenesis of joint injury to assess the mechanism(s) affecting return of biochemical biomarkers to basal levels over time.
An exercise regimen was incorporated because running is a more natural condition than cage habitation for rats and because ACL injury most often occurs in active individuals. Selection of this regimen led to limitations, including a smaller sample size and variability in the motivation of different rats to consistently exercise on the treadmill throughout the 60-minute exercise period. These factors may contribute to the variability seen within experimental groups.
To conclude, this study found a strong relationship between serum CTXii, a biochemical biomarker of collagen type II degradation, and histological findings of articular cartilage degeneration following ACL transaction in a rat model. Serum CTXii may have potential as a biochemical biomarker to assess early cartilage damage and efficacy of novel chondroprotective interventions prior to development of cartilage loss and advanced joint degeneration.
Acknowledgments
The authors thank Kimberlee K. Suter and Michele L. Mulkeen for technical assistance. This study was funded by the National Institutes of Health R01 AR052784 (CR Chu), the Albert Ferguson Endowed Chair (CR Chu), the Pittsburgh Foundation (CH Coyle/CR Chu), and the Orthopedic Research Education Foundation (S Henry/CR Chu).
Contributions: CHC and SHE contributed equally to the study. CHC, SEH and CRC conceived and designed the study. SEH, AMH, CHC and MJO contributed to data acquisition and analysis as well as critical review of the manuscript. Data interpretation and manuscript preparation were performed by CHC and CRC. CRC is responsible for the integrity of the work as a whole.
References
- 1. Appleton CT, McErlain DD, Pitelka V, et al. Forced mobilization accelerates pathogenesis: characterization of a preclinical surgical model of osteoarthritis. Arthritis Res Ther. 2007;9(1):R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20(10):739-744 [DOI] [PubMed] [Google Scholar]
- 3. Cameron ML, Fu FH, Paessler HH, Schneider M, Evans CH. Synovial fluid cytokine concentrations as possible prognostic indicators in the ACL-deficient knee. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):38-44 [DOI] [PubMed] [Google Scholar]
- 4. Catterall JB, Stabler TV, Flannery CR, Kraus VB. Changes in serum and synovial fluid biomarkers after acute injury (NCT00332254). Arthritis Res Ther. 2010;12(6):R229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Charni N, Juillet F, Garnero P. Urinary type II collagen helical peptide (HELIX-II) as a new biochemical marker of cartilage degradation in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2005;52(4):1081-1090 [DOI] [PubMed] [Google Scholar]
- 6. Chu CR, Coyle CH, Chu CT, et al. In vivo effects of single intra-articular injection of 0.5% bupivacaine on articular cartilage. J Bone Joint Surg Am. 2010;92(3):599-608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cibere J, Zhang H, Garnero P, et al. Association of biomarkers with pre-radiographically defined and radiographically defined knee osteoarthritis in a population-based study. Arthritis Rheum. 2009;60(5):1372-1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. David-Vaudey E, Ghosh S, Ries M, Majumdar S. T2 relaxation time measurements in osteoarthritis. Magn Reson Imaging. 2004;22(5):673-682 [DOI] [PubMed] [Google Scholar]
- 9. Duclos ME, Roualdes O, Cararo R, Rousseau JC, Roger T, Hartmann DJ. Significance of the serum CTX-II level in an osteoarthritis animal model: a 5-month longitudinal study. Osteoarthritis Cartilage. 2010;18(11):1467-1476 [DOI] [PubMed] [Google Scholar]
- 10. Ekenstedt KJ, Sonntag WE, Loeser RF, Lindgren BR, Carlson CS. Effects of chronic growth hormone and insulin-like growth factor 1 deficiency on osteoarthritis severity in rat knee joints. Arthritis Rheum. 2006;54(12):3850-3858 [DOI] [PubMed] [Google Scholar]
- 11. Felson DT, Neogi T. Osteoarthritis: is it a disease of cartilage or of bone? Arthritis Rheum. 2004;50(2):341-344 [DOI] [PubMed] [Google Scholar]
- 12. Frisbie DD, Al-Sobayil F, Billinghurst RC, Kawcak CE, McIlwraith CW. Changes in synovial fluid and serum biomarkers with exercise and early osteoarthritis in horses. Osteoarthritis Cartilage. 2008;16(10):1196-1204 [DOI] [PubMed] [Google Scholar]
- 13. Galois L, Etienne S, Grossin L, et al. Dose-response relationship for exercise on severity of experimental osteoarthritis in rats: a pilot study. Osteoarthritis Cartilage. 2004;12(10):779-786 [DOI] [PubMed] [Google Scholar]
- 14. Galois L, Etienne S, Grossin L, et al. Moderate-impact exercise is associated with decreased severity of experimental osteoarthritis in rats. Rheumatology (Oxford). 2003;42(5):692-693 [DOI] [PubMed] [Google Scholar]
- 15. Hayami T, Pickarski M, Zhuo Y, Wesolowski GA, Rodan GA, Duong le T. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone. 2006;38(2):234-243 [DOI] [PubMed] [Google Scholar]
- 16. Hooiveld MJ, Roosendaal G, Jacobs KM, et al. Initiation of degenerative joint damage by experimental bleeding combined with loading of the joint: a possible mechanism of hemophilic arthropathy. Arthritis Rheum. 2004;50(6):2024-2031 [DOI] [PubMed] [Google Scholar]
- 17. Jay GD, Fleming BC, Watkins BA, et al. Prevention of cartilage degeneration and restoration of chondroprotection by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 2010;62(8):2382-2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jordan KM, Syddall HE, Garnero P, et al. Urinary CTX-II and glucosyl-galactosyl-pyridinoline are associated with the presence and severity of radiographic knee osteoarthritis in men. Ann Rheum Dis. 2006;65(7):871-877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kannus P, Jarvinen M. Posttraumatic anterior cruciate ligament insufficiency as a cause of osteoarthritis in a knee joint. Clin Rheumatol. 1989;8(2):251-260 [DOI] [PubMed] [Google Scholar]
- 20. Lohmander LS, Atley LM, Pietka TA, Eyre DR. The release of crosslinked peptides from type II collagen into human synovial fluid is increased soon after joint injury and in osteoarthritis. Arthritis Rheum. 2003;48(11):3130-3139 [DOI] [PubMed] [Google Scholar]
- 21. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769 [DOI] [PubMed] [Google Scholar]
- 22. Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145-3152 [DOI] [PubMed] [Google Scholar]
- 23. Matyas JR, Atley L, Ionescu M, Eyre DR, Poole AR. Analysis of cartilage biomarkers in the early phases of canine experimental osteoarthritis. Arthritis Rheum. 2004;50(2):543-552 [DOI] [PubMed] [Google Scholar]
- 24. Mazzuca SA, Poole AR, Brandt KD, Katz BP, Lane KA, Lobanok T. Associations between joint space narrowing and molecular markers of collagen and proteoglycan turnover in patients with knee osteoarthritis. J Rheumatol. 2006;33(6):1147-1151 [PubMed] [Google Scholar]
- 25. Neuman P, Englund M, Kostogiannis I, Friden T, Roos H, Dahlberg LE. Prevalence of tibiofemoral osteoarthritis 15 years after nonoperative treatment of anterior cruciate ligament injury: a prospective cohort study. Am J Sports Med. 2008;36(9):1717-1725 [DOI] [PubMed] [Google Scholar]
- 26. Pap G, Eberhardt R, Sturmer I, et al. Development of osteoarthritis in the knee joints of Wistar rats after strenuous running exercise in a running wheel by intracranial self-stimulation. Pathol Res Pract. 1998;194(1):41-47 [DOI] [PubMed] [Google Scholar]
- 27. Poole AR, Ionescu M, Swan A, Dieppe PA. Changes in cartilage metabolism in arthritis are reflected by altered serum and synovial fluid levels of the cartilage proteoglycan aggrecan: implications for pathogenesis. J Clin Invest. 1994;94(1):25-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rizkalla G, Reiner A, Bogoch E, Poole AR. Studies of the articular cartilage proteoglycan aggrecan in health and osteoarthritis: evidence for molecular heterogeneity and extensive molecular changes in disease. J Clin Invest. 1992;90(6):2268-2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tang T, Muneta T, Ju YJ, et al. Serum keratan sulfate transiently increases in the early stage of osteoarthritis during strenuous running of rats: protective effect of intraarticular hyaluronan injection. Arthritis Res Ther. 2008;10(1):R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van der Sluijs JA, Geesink RG, van der Linden AJ, Bulstra SK, Kuyer R, Drukker J. The reliability of the Mankin score for osteoarthritis. J Orthop Res. 1992;10(1):58-61 [DOI] [PubMed] [Google Scholar]
- 31. Williams A, Qian Y, Bear D, Chu CR. Assessing degeneration of human articular cartilage with ultra-short echo time (UTE) T2* mapping. Osteoarthritis Cartilage. 2010;18(4):539-546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yoshioka M, Coutts RD, Amiel D, Hacker SA. Characterization of a model of osteoarthritis in the rabbit knee. Osteoarthritis Cartilage. 1996;4(2):87-98 [DOI] [PubMed] [Google Scholar]