Abstract
Kinetic models enable nutrient needs and kinetic behaviors to be quantified and provide mechanistic insights into metabolism. Therefore, we modeled and quantified the kinetics, bioavailability, and metabolism of RRR-α-tocopherol in 12 healthy adults. Six men and 6 women, aged 27 ± 6 y, each ingested 1.81 nmol of [5−14CH3]-(2R, 4′R, 8′R)-α-tocopherol; each dose had 3.70 kBq of 14C. Complete collections of urine and feces were made over the first 21 d from dosing. Serial blood samples were drawn over the first 70 d from dosing. All specimens were analyzed for RRR-α-tocopherol. Specimens were also analyzed for 14C using accelerator MS. From these data, we modeled and quantified the kinetics of RRR-α-tocopherol in vivo in humans. The model had 11 compartments, 3 delay compartments, and reservoirs for urine and feces. Bioavailability of RRR-α-tocopherol was 81 ± 1%. The model estimated residence time and half-life of the slowest turning-over compartment of α-tocopherol (adipose tissue) at 499 ± 702 d and 184 ± 48 d, respectively. The total body store of RRR-α-tocopherol was 25,900 ± 6=220 μmol (11 ± 3 g) and we calculated the adipose tissue level to be 1.53 μmol/g (657 μg/g). We found that a daily intake of 9.2 μmol (4 mg) of RRR-α-tocopherol maintained plasma RRR-α-tocopherol concentrations at 23 μmol/L. These findings suggest that the dietary requirement for vitamin E may be less than that currently recommended and these results will be important for future updates of intake recommendations.
Introduction
Vitamin E’s sole function appears to be as a nonspecific, chain-breaking antioxidant that prevents propagation of free-radical reactions (1, 2). The dietary requirement for vitamin E has been estimated from intakes providing plasma α-tocopherol concentrations that limit hydrogen peroxide-induced hemolysis. Based on conservative analyses of the sparse data, a plasma α-tocopherol concentration of 12 μmol/L was chosen as the target value, and 12 mg/d was identified as the intake level that would provide that target plasma concentration (3) and was established as the estimated average requirement (EAR).
Although the DRI level for vitamin E is based on the best available information, there is still a need for more convincing data on which to base these intake levels. Information about α-tocopherol bioavailability, body storage, metabolic exchange, and elimination rates would be very valuable for determining intake levels for delivering specific amounts to the systemic circulation and for replacing amounts lost to irreversible elimination. Several kinetic studies have been conducted in the past, but many of them have been fairly short in duration (4–12) and thus may have missed information about slower turning-over compartments or utilized fairly large doses of tocopherol (5, 10–15) and thus may have assessed kinetics more representative of supplement intake than dietary intake if the dose influenced the kinetic behavior. Therefore, we conducted a study in which 12 volunteers consumed a very small dose of 14C-RRR-α-tocopherol and provided complete collection of feces and urine for 21 d and serial blood samples for 70 d. Enrichment of biological samples was used to develop a compartmental model of α-tocopherol metabolism in humans to determine kinetic parameters useful for guiding development of improved reference intake levels.
Participants and Methods
Participants and study design.
Twelve healthy volunteers (6 men, 6 women) participated in an intervention study in which each participant consumed [5-14CH3]-(2R, 4′R, 8′R)-α-tocopherol then provided serial blood, urine, and fecal samples so that the kinetics of the α-tocopherol could be determined. To ensure ethical treatment of the participants, the study was approved by the Institutional Review Board at the University of California at Davis Medical Center and informed consent was obtained from the study participants. Each participant was dosed (per-os) with 1.81 nmol (0.78 mg, 3.7 kBq) of [5-14CH3]-(2R, 4′R, 8′R)-α-tocopherol [synthesis previously described (16, 17)] that was mixed with 60 g of 2% fat milk in a container and swallowed. The container was then rinsed with another 60 g of 2% fat milk that was also swallowed. Further details of the study can be found in Chuang et al. (16).
Specimen preparation and analysis.
Aliquots of RBC, urine, and feces specimens were vacuum-dried in smooth-wall, tin capsules (Elemental Microanalysis) and analyzed for total carbon at the University of California Davis Analytical Laboratory (18). Every 10th sample was measured in duplicate. The CV (%) of total carbon was 3.5% for RBC, 1.1% for feces, and 1.6% for urine. Aliquots of the plasma, RBC, urine, and fecal specimens were measured for their concentrations of 14C using accelerator MS (19). Finally, the plasma and RBC concentrations of α-tocopherol were also measured by HPLC (20).
Compartmental modeling.
Compartmental modeling was used to analyze plasma, erythrocyte, urine, and feces concentrations of [5-14CH3]-(2R, 4′R, 8′R)-α-tocopherol (the tracer) in plasma and erythrocytes that were collected over a 70-d period from dosing and in urine and feces that was collected over a 21-d period from dosing (16). Tracer enrichment in plasma and RBC was measured as a concentration (percent 14C enrichment/L) and was converted to fraction of dose based on calculated blood volume (21, 22) and measured RBC volume. For each participant, the total number of data points collected ranged between 113 and 145, with specific collections for each participant as follows: 54–60 data points each for plasma, 23–30 data points each for RBC, 25–26 data points each for urine, and 10–29 data points each for feces, for a total of 1554 data points used in modeling. For most participants, the urine and fecal collections provided at least 15 points each on the terminal slope, allowing calculation of longer half-life values than previously possible. Transfer of the tracer and its metabolites between compartments was expressed as first-order linear differential equations. The WinSAAM software package (v.3.0.7) was used for the kinetic analysis. The mathematical predictions of 14C-labeled compounds in plasma, erythrocytes, urine, and feces were compared with measured data points and model parameters were adjusted until model prediction was in good accord with observed data. Multiple compartments contributed to the 14C enrichment of plasma and RBC. Thus, for the 14C-labeled analytes in plasma, the contents of compartment 15 (rapidly absorbed plasma α-tocopherol), 4 (chylomicron α-tocopherol), 10 (lipoprotein α-tocopherol), and 9 carboxyethyl hydroxychroman (CEHC) were summed and compared with the measured data. Similarly for the 14C-labeled analytes in erythrocytes, the contents of compartments 16 (RBC extrinsically bound to membrane α-tocopherol) and 18 (RBC intrinsically incorporated α-tocopherol) were summed and compared with measured data. A least squares procedure was then used to minimize the difference between model prediction and observed data. Model structures and sets of parameter values were compared using sums of squared residuals, such that the model structure and the parameter set providing the lowest sums of squared residuals were chosen. Model uniqueness was ensured by requiring that all parameter values were characterized by fractional SD (CV) < 0.6. Data from each participant were modeled individually.
Results
The study participants were 27 ± 7 y old, weighed 67 ± 11 kg, and had a BMI of 22 ± 2 kg/m2 and a packed RBC volume of 41 ± 3% (means ± SD). Baseline plasma α-tocopherol concentrations were 23 ± 3 μmol/L and baseline erythrocyte α-tocopherol concentrations were 2.8 ± 0.4 μmol/L. Further details can be found in Chuang et al. (16).
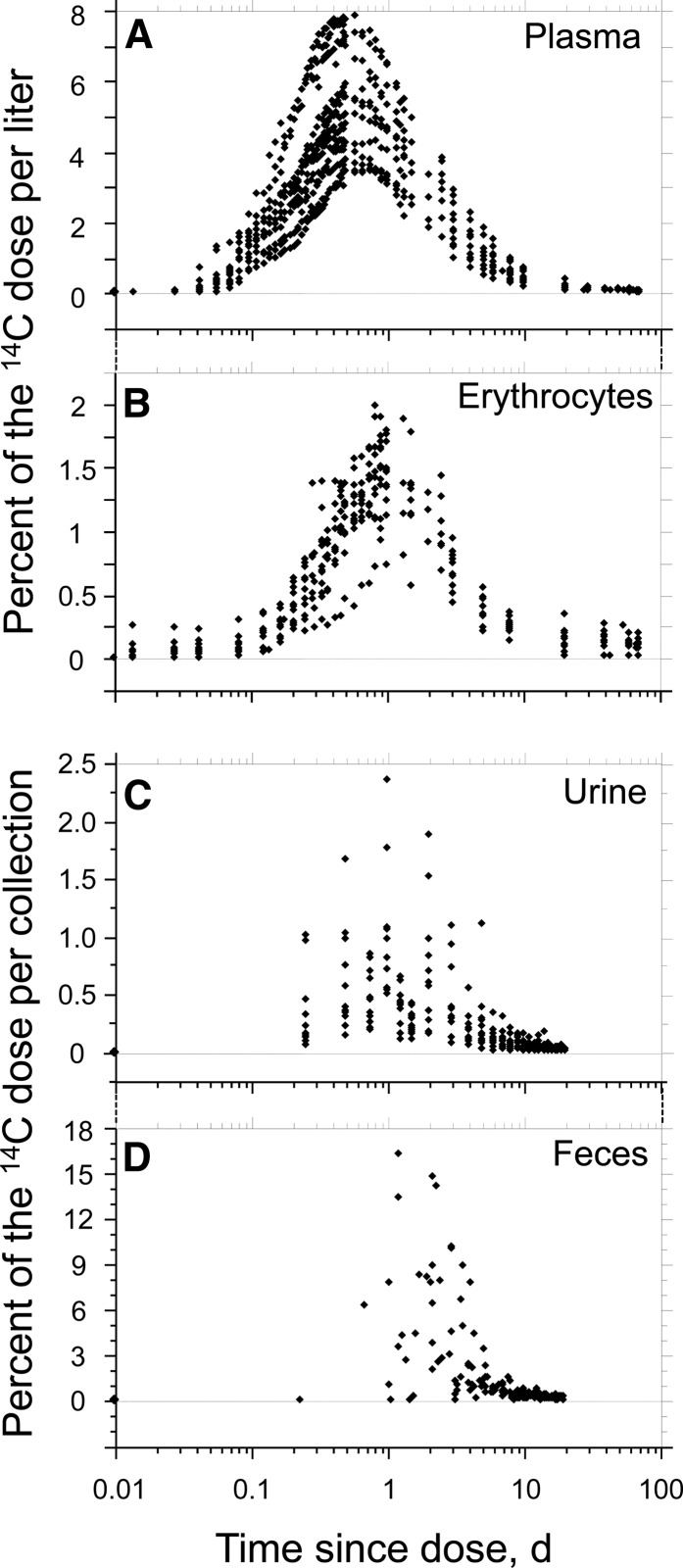
Plasma, erythrocyte, urine, and fecal concentrations of 14C by time since dose are shown in Figure 1. The peak concentrations of 14C in plasma ranged from ∼3.5 to ∼8% of dose/L. The peak concentrations of 14C in erythrocytes ranged from ∼0.75 to ∼2% of dose/L. In addition, the plasma concentration of 14C peaked at 12 h after dosing, whereas the erythrocyte concentrations of 14C peaked at ∼24 h after dosing. Plasma and erythrocyte concentrations of 14C each returned to baseline at ∼10 d after dosing. Finally, the peak concentrations of 14C in urine ranged from ∼0.075 to ∼2.375% of dose. The peak concentrations of 14C in feces ranged from ∼1 to 16.5% of dose.
FIGURE 1.
Distribution of 14C in plasma (A), erythrocytes (B), urine (C), and feces (D) of healthy adults after ingestion of [5-14CH3]-(2R, 4′R, 8′R)-α-tocopherol.
The compartment numbers in brackets, described below and in Figure 2, represent arbitrary numbers used in model development and designation of equations in the modeling software. The model consisted of 11 compartments, 3 delay compartments [compartments 2, 17, 18], plus reservoirs for urine and feces. The model compartments and reservoirs included a mouth/stomach [compartment 1] for entry of the dose; stomach-emptying delay with a mean ± SD delay time of 0.0343 ± 0.00675 d [compartment 2]; upper gastrointestine [compartment 3]; colon [compartment 25]; a plasma compartment for rapid absorption of α-tocopherol [compartment 15]; plasma chylomicrons [compartment 4]; plasma lipoproteins [compartment 10]; plasma [compartment 9]; hepatocytes to accept chylomicrons [compartment 7]; a multi-organ compartment [which would include hepatic stellate cells (HSC), brain, spleen, etc.] [compartment 6]; adipose [compartment 26]; RBC membranes (extrinsic binding) [compartment 16]; marrow [compartment 17] represented as a fixed 6-d delay; RBC (intrinsic incorporation) represented as a fixed delay for an average lifespan of 120 d [compartment 18]; feces [compartment 5]; and urine [compartment 8]. The fluxes in Figure 2 are described in detail in Table 1.
FIGURE 2.
The final kinetic model of RRR-α-tocopherol used to fit the observed data from all participants. The circles represent compartments. The numbers represent the steady-state flows (μmol/d) in the direction of the arrows. The dashed arrows represent where a delay was used. HSC, hepatic stellate cell.
TABLE 1.
Steady-state flows and fractional transfer rates of RRR-α-tocopherol of all participants from donor to recipient compartments1
| From donor compartment | To recipient compartment | Flow rate | Fractional transfer coefficient |
| Name (ID no. on Fig. 2) | Name (ID no. on Fig. 2) | μmol/d | d−1 |
| Dose (#1)2 | Rapidly absorbed plasma (#15) | 0.051 ± 0.134 | 0.91 ± 2.14 |
| Rapidly absorbed plasma (#15) | Hepatocytes (#7) | 0.042 ± 0.132 | 217 ± 187 |
| Rapidly absorbed plasma (#15) | RBC extrinsic (#16) | 0.010 ± 0.011 | 284 ± 187 |
| Dose (#1) | Upper gastrointestine (#3) | 9.20 ± 2.37 | 30.4 ± 6.74 |
| Upper gastrointestine (#3) | Colon (#25) | 1.80 ± 0.82 | 0.28 ± 0.16 |
| Colon (#25) | Feces (#5) | 7.22 ± 2.49 | 1.17 ± 0.73 |
| Upper gastrointestine (#3) | Plasma chylomicrons (#4) | 7.40 ± 1.85 | 1.15 ± 0.40 |
| Plasma chylomicrons (#4) | Hepatocytes (#7) | 7.16 ± 1.86 | 64.4 ± 8.06 |
| Plasma chylomicrons (#4) | RBC extrinsic (#16) | 0.24 ± 0.13 | 2.35 ± 1.54 |
| Hepatocytes (#7) | Plasma lipoproteins (#10) | 6.87 ± 1.83 | 102 ± 253 |
| Hepatocytes (#7) | Plasma CEHC (#9) | 0.33 ± 0.16 | 3.83 ± 8.62 |
| Plasma lipoproteins (#10) | Bone marrow (#17) | 0.21 ± 0.15 | 0.0033 ± 0.0022 |
| Bone marrow (#17) | RBC intrinsic (#18) | 0.21 ± 0.15 | 0.33 ± 5.8·10−17 |
| RBC intrinsic (#18) | HSC, brain, spleen, etc. (#6) | 0.21 ± 0.15 | 0.0083 (fixed)3 |
| Plasma lipoproteins (#10) | RBC extrinsic (#16) | 44.8 ± 53.6 | 0.74 ± 1.03 |
| RBC extrinsic (#16) | Plasma lipoproteins (#10) | 45.0 ± 53.6 | 3.48 ± 5.05 |
| Plasma lipoproteins (#10) | Adipose tissue (#26) | 103 ± 40.3 | 1.61 ± 0.63 |
| Adipose tissue (#26) | Plasma lipoproteins (#10) | 103 ± 40.3 | 0.004 ± 0.001 |
| Plasma lipoproteins (#10) | HSC, brain, spleen, etc. (#6) | 195 ± 105 | 3.12 ± 1.83 |
| HSC, brain, spleen, etc. (#6) | Plasma lipoproteins (#10) | 188 ± 105 | 0.51 ± 0.21 |
| HSC, brain, spleen, etc. (#6) | Colon (#25) | 5.42 ± 2.10 | 0.015 ± 0.008 |
| HSC, brain, spleen, etc. (#6) | Plasma CEHC (#9) | 1.70 ± 1.26 | 0.0059 ± 0.0087 |
| Plasma CEHC (#9) | Urine (#8) | 2.03 ± 1.36 | 17.8 ± 21.7 |
Values are mean ± SD, = 12. CEHC, carboxyethyl hydroxychroman; HSC, hepatic stellate cell.
Numbers in parentheses refer to the compartments indicated in Figure 2.
Fixed in accord with the average RBC lifespan of 120 d.
Graphs of the goodness of fit of our observed data and current knowledge of physiology for a sample participant are shown in Figure 3. Visual inspection of the fraction of 14C-doses in plasma, erythrocytes, urine, and feces showed good fits between model prediction and observed data for all participants. The CV for fractional transfer coefficients among compartments (not shown) were generally <60% (and in most cases <20%) for adjustable parameters, indicating good mathematical confidence in parameter values.
FIGURE 3.
The model predicted (lines) and the observed data (squares) of 14C in plasma (A), erythrocytes (B), urine (C), and feces (D) of participant number 10 after ingestion of [5-14CH3]-(2R, 4′R, 8′R)-α-tocopherol.
Model results showed that the dose was efficiently absorbed and transferred throughout the system. Mean absorption of the dose was 81 ± 1%. Mean transfer rates among compartments ranged from 0.01 to 195 μmol/d (Table 1). Mean daily fecal [compartment 5] and urine [compartment 8] losses were found to be 7.2 ± 2.49 μmol/d and 2.0 ± 1.36 μmol/d, respectively. Total body RRR-α-tocopherol stores were found to be ∼25,900 ± 6220 μmol, which would be equivalent to ∼11 g (Table 2), and ranged from ∼17,000 to 36,000 μmol. Approximately 99% of the total body store was associated with a slowly turning-over compartment, which was assumed to be primarily adipose tissue [compartment 26]. Residence times and half-lives for model compartments are shown in Table 3.
TABLE 2.
Model-predicted concentrations of all participants of RRR-α-tocopherol in selected compartments1
| Tissue | RRR-α-tocopherol concentrations |
| Name (ID no. on Fig. 2) | μmol |
| Plasma chylomicrons2 (#4) | 0.12 ± 0.04 |
| Plasma lipoproteins (#10) | 64.8 ± 14.3 |
| Plasma CEHC (#9) | 0.46 ± 0.47 |
| Colon (#25) | 8.53 ± 5.87 |
| Hepatocytes (#7) | 0.82 ± 1.28 |
| HSC, brain, spleen, etc. (#6) | 392 ± 134 |
| Adipose tissue (#26) | 25,400 ± 6100 |
| RBC extrinsic (#16) | 13.6 ± 6.32 |
| RBC intrinsic (#18) | 25.1 ± 18.5 |
| Other | 7.70 ± 3.10 |
| Total | 25,900 ± 6220 |
Values are mean ± SD, = 12. CEHC, carboxyethyl hydroxychroman; HSC, hepatic stellate cell.
Numbers in parentheses refer to the compartments indicated in Figure 2.
TABLE 3.
Residence times and half lives of RRR-α-tocopherol of all participants in selected compartments 1
| Tissue | Residence time | Half-life |
| Name (ID no. on Fig. 2) | d | |
| Plasma chylomicrons (#4)2 | 0.015 ± 0.002 | 0.011 ± 0.002 |
| Plasma lipoproteins (#10) | 0.214 ± 0.073 | 0.148 ± 0.050 |
| Rapidly absorbed plasma (#15) | 0.0020 ± 7 · 10−9 | 0.0014 ± 5 · 10−9 |
| HSC, brain, spleen, etc. (#6) | 2.42 ± 1.43 | 1.53 ± 0.61 |
| Hepatocytes (#7) | 0.094 ± 0.125 | 0.066 ± 0.086 |
| Adipose tissue (#26) | 499 ± 702 | 184 ± 48.0 |
| RBC extrinsic (#16) | 0.565 ± 0.337 | 0.394 ± 0.232 |
Values are mean ± SD, = 12. HSC, hepatic stellate cell.
Numbers in parentheses refer to the compartments indicated in Figure 2.
Discussion
The model presented in this paper extends our prior model of tocopherol kinetics (23). The present model was expanded to include compartments for RBC enrichment with 14C. In addition, a few other minor modifications were made to the model to include information uncovered by the new data, as discussed below. The present model, using a compartmental modeling approach, is a more complete analysis than our prior noncompartmental approach (16).
For this study, 14C enrichment of RBC was measured compared with our prior study (23); therefore, the model was expanded so that 14C enrichment of RBC could be included in the compartmental analysis. Initially, a path was added to incorporate α-tocopherol into RBC during their differentiation. Compartment 17 was first in this path and was a 6-d delay representing differentiation of RBC in marrow (24, 25). Compartment 17 received α-tocopherol from compartment 10. The second compartment in this path was an intrinsic RBC compartment [compartment 18], which delivered α-tocopherol to the multi-organ compartment [compartment 6], in accord with RBC clearance by spleen and liver (26).
Another addition to our prior model (23) was a rapidly absorbed plasma compartment [compartment 15], representing a very rapid absorption of a small amount of the ingested α-tocopherol. The flow through this pathway accounted for only a small fraction (0.007) of the absorbed dose; thus, this flux was very small. Compartment 15 was required to account for the very early appearance of the 14C tracer in the plasma, because the tracer was observed by the first time point of blood collection at 0.0139 d (Fig. 1A). Mathematically moving α-tocopherol through the upper gastrointestine for absorption via chylomicrons was not sufficiently quick to generate the 14C tracer in plasma at the early time points. A previously published compartmental model of vitamin A kinetics (27) required a similar pathway for tracer to be transferred quickly from the upper gastrointestinal tract to plasma retinol bound to retinol binding protein, preceding (and thus by-passing) the traditionally understood pathway of vitamin A absorption as retinyl ester into chylomicrons. Compartment 15, representing quick transfer of α-tocopherol into the blood, may be characterized by transfer across the stomach wall or sublingual absorption as observed with vitamin B-12 (28).
In addition, our data showed that α-tocopherol was associated with RBC more quickly than can be accounted for by incorporation into RBC through marrow. The 14C tracer was found to be associated with RBC within 0.0139 d since dosing (Fig. 1B), presumably by extrinsic incorporation into the RBC membrane due to its hydrophobicity. We therefore added paths from the rapidly absorbed plasma compartment [compartment 15] and the plasma chylomicrons [compartment 4] to a newly added extrinsic RBC compartment [compartment 16]. Previous findings support the existence of this exchange. A previous in vitro study demonstrated that a large proportion of α-tocopherol can be transferred between lipoprotein particles. In culture, incubation of LDL particles containing labeled tocopherol with unlabeled HDL particles resulted in 20% of the labeled tocopherol being transferred from LDL to HDL in 24 h (29). Alternatively, incubation of HDL particles containing labeled tocopherol with unlabeled LDL particles resulted in 75% of labeled tocopherol being transferred from HDL to LDL in 24 h (29).
The fractional transfer rate observed in the present study was similar to that observed in an ex vivo study of rat lipoproteins and erythrocytes. Bjornson et al. (30) estimated a fractional transfer rate of tocopherol from rat lipoproteins to erythrocytes to be 0.188/h (4.5/d), whereas we found fractional transfer from chylomicrons to RBC extrinsic to be 2.35/d and fractional transfer of α-tocopherol from plasma lipoproteins to RBC extrinsic to be 0.74/d. These values represent a fairly small portion of total movement out of chylomicrons and lipoproteins overall (4% of chylomicron α-tocopherol and 12% of general plasma lipoprotein α-tocopherol transferring to RBC membranes). Moreover, in the cell culture study, most of the transfer of labeled tocopherol between HDL and LDL occurred very quickly, within the first 30 min of incubation (29), which is also in accord with the present model findings. Massey (31) also found rapid transfer of α-tocopherol between lipoprotein particles, and Kitabchi and Wimalasena (32, 33) demonstrated α-tocopherol rapidly binds to specific binding sites on human erythrocytes.
The mean value for total body α-tocopherol was calculated to be ∼26 mmol (11 g) (Table 2). Most (99%) of this α-tocopherol was associated with compartment 26, which turned over very slowly and was assumed to be adipose tissue. The average adipose mass of the study participants was estimated from the average body weight (67 kg) and the estimated percent body fat of 25%, giving an α-tocopherol concentration in adipose of 1.53 μmol/g (657 μg/g). These values are in good accord with the measured values reported by Parker (34), who determined α-tocopherol in adipose biopsies of adults to find that the α-tocopherol concentration in adipose tissue ranged from 0.142 to 1.89 μmol/g (61–811 μg/g). Another study compared tocopherol concentrations for individuals favoring different types of oil for cooking. Mean α-tocopherol values in adipose were 0.219, 0.198, and 0.260 μmol/g (94, 85, and 112 μg/g) for those that used soybean oil, palm shortening, and corn oil, respectively (35). The total tocopherol concentration of adipose tissue in young adult accident victims was measured to be 0.581 μmol/g (250 μg/g) tissue (36). McMasters et al. (37) reported somewhat higher values in individuals where total tocopherol concentrations in adipose ranged from 32.6 to 467 μmol/g before supplementation and 44.2 to 782 μmol/g adipose tissue after supplementation with 1 g/d tocopherol for 2 wk. Kayden et al. (38) reported the tocopherol content of adipose tissue in apparently healthy participants to range from 0.371 to 2.18 μmol/ μmol (190–1117 ng/mg) TG. Because adipose tissue is comprised of 40% fat (39), this is equivalent to 0.176–1.04 μmol tocopherol/g adipose tissue.
The highest rates of transfer of α-tocopherol between compartments occurred between compartment 10 (plasma lipoproteins) and compartment 6, with an exchange flow of almost 200 μmol/d. This would ensure that the availability of α-tocopherol would mitigate oxidative stress throughout tissues. Another high flow was observed from compartment 10 to 26 (adipose tissue); it was just above 100 μmol/d. To remain in steady state, the same mass per day flowed out of adipose tissue, but due to the very large compartment size, this flow was achieved with a very small fractional transfer rate of 0.004 ± 0.001/d, meaning that only 0.4% of the α-tocopherol was transferred out of the adipose tissue per day. The third largest exchange rate was between compartments 10 and 16 (RBC extrinsic), again to mitigate oxidative stress to RBC.
The longest half-life (184 ± 48 d) of the major α-tocopherol storage compartment is longer than that reported for other studies (4–7). For many previous kinetic studies, sampling times extended only days after dosing; thus, longer term kinetic behavior could not be assessed. The longer sampling regime over months in the current study allowed the determination of the longer half-life. The decline in plasma labeled tocopherol during the first few days after dosing represents removal of tocopherol from plasma into stores and as utilization. Assuming irreversible loss, this decline will result in an underestimation of tocopherol half-life (and overestimation of rate of tocopherol depletion). The importance of longer sampling times for accurate assessment of half-lives was previously demonstrated (16). The Elgin tocopherol depletion study (40) also demonstrated that tocopherol depletion requires ∼6 y. Estimating tocopherol half-life from the Elgin study data suggests a half-life of ∼25 mo. During the Elgin depletion, erythrocyte susceptibility to peroxide hemolysis increased steadily over the first 30 mo as plasma tocopherol concentrations decreased, then hemolysis leveled off, suggesting a tocopherol threshold had been met below which plasma tocopherol was no longer able to protect against oxidative stress, the purported primary role of tocopherol in the body (2).
The model developed imposed steady-state conditions where input equals output and the law of mass conservation is assumed. This implies that any α-tocopherol consumed is eliminated in the feces and urine by an equivalent amount of α-tocopherol (or metabolite) already in the body so that no net retention occurs. From our present model, we determined the mean absorption (± SD) of the α-tocopherol dose was 80.8 ± 5.98%. By comparison, absorption estimated by the balance method (dose minus 3-d fecal loss) was 81.9 ± 5.89%. Some reports have used peak plasma concentration to estimate absorption, but this method substantially underestimates absorption. For example, the peak plasma method, applied to our data, estimates absorption of 5.39 ± 1.58%. A compartment model method and the balance method are far superior to the peak plasma method for estimating absorption. Use of an isotope adds to the reliability of the balance method, because an isotopic label is unaffected by gut microbiota, in contrast to measurement of an intact compound, which might be either degraded or synthesized by gut microbiota, resulting in overestimation or underestimation of absorption. Using Figure 2 illustrates how the true absorption is calculated as 81% by [dose − (feces − fecal metabolic loss)] · 100/dose or [9.25 − (7.22–5.42)] · 100/9.25. Whereas, if only feces were collected, then one can only calculate apparent absorption as 22% by (dose − feces) · 100/dose or (9.25–7.22) · 100/9.25. Therefore, without accounting for fecal metabolic losses by using a thorough compartmental modeling approach, one can only accurately estimate the apparent absorption that may partly explain the wide range of α-tocopherol absorption values reported in the literature.
Based on α-tocopherol kinetics after volunteers consumed apples fortified with deuterium-labeled α-tocopherol, adults should consume enough α-tocopherol to absorb 5 mg/d for repletion of tissues of daily losses (41). Using the peak plasma method to determine absorption (estimated to be 33%), they calculated a required intake of 15 mg/d to deliver 5 mg/d to tissues. However, the peak plasma method underestimates absorption. With an absorption value of 81%, as described in the above paragraph, adults would need to consume 6 mg α-tocopherol/d to provide 5 mg/d for repletion of losses previously estimated (41).
The current EAR for vitamin E for adults has been estimated from intakes providing plasma α-tocopherol concentrations capable of mitigating hydrogen peroxide-induced hemolysis. A plasma concentration of 12 μmol/L of α-tocopherol was chosen as the target value and 12 mg/d was identified as the intake concentration that would provide the target plasma concentration (3). Thus, 12 mg/d of α-tocopherol was established as the EAR and 15 mg/d was established as the RDA (3). However, the kinetic analysis presented here suggests that a much lower daily intake of RRR α-tocopherol would be sufficient to maintain plasma concentrations at 12 μmol/L. Participants in the present study had plasma α-tocopherol concentrations ranging from 19 to 27 μmol/L. The α-tocopherol intake sufficient to maintain these plasma concentrations was 9.2 + 0.2 μmol/d (4.0 + 0.1 mg/d). This further suggests that the actual requirement for vitamin E is lower than the current EAR and RDA. Furthermore, there has been support (42) for the 1989 RDA for vitamin E of 10 mg/d (43) over the higher value of 15 mg/d established in 2000 (3).
In summary, a compartmental model has been developed that describes RRR-α-tocopherol kinetics in healthy adults by focusing on the absorption, storage, and elimination of RRR-α-tocopherol. Our compartmental model will be useful for the development of improved RDA values for vitamin E. Moreover, these results provide multiple indications that the current EAR and RDA for vitamin E may be set higher than necessary.
Acknowledgments
The authors thank Dr. Michael Green for helpful discussions on model structure. A.J.C. designed and conducted the research; A.J.C. and D.M.H. performed and interpreted laboratory analysis; J.A.N. performed the mathematical modeling with input from A.J.C. and J.G.F.; A.J.C., J.A.N., J.G.F., and H.C.F. interpreted the model results and wrote the manuscript; and A.J.C., J.A.N., and J.G.F. had primary responsibility for final content. All authors read and approved the final manuscript.
Literature Cited
- 1.Wolf G. The discovery of the antioxidant function of vitamin E: the contribution of Henry A. Mattill. J Nutr. 2005;135:363–6 [DOI] [PubMed] [Google Scholar]
- 2.Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007;43:4–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Food and Nutrition Board Panel on Antioxidants and Related Compounds, Institute of Medicine and National Academy of Sciences, Editors. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC; National Academies Press; 2000. [PubMed]
- 4.Hall WL, Jeanes YM, Lodge JK. Hyperlipidemic subjects have reduced uptake of newly absorbed vitamin E into their plasma lipoproteins, erythrocytes, platelets, and lymphocytes, as studied by deuterium-labeled alpha-tocopherol biokinetics. J Nutr. 2005;135:58–63 [DOI] [PubMed] [Google Scholar]
- 5.Jeanes YM, Hall WL, Lodge JK. Comparative (2)H-labelled alpha-tocopherol biokinetics in plasma, lipoproteins, erythrocytes, platelets and lymphocytes in normolipidaemic males. Br J Nutr. 2005;94:92–9 [DOI] [PubMed] [Google Scholar]
- 6.Traber MG, Ramakrishnan R, Kayden HJ. Human plasma vitamin E kinetics demonstrate rapid recycling of plasma RRR-alpha-tocopherol. Proc Natl Acad Sci USA. 1994;91:10005–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferslew KE, Acuff RV, Daigneault EA, Woolley TW, Stanton PE., Jr Pharmacokinetics and bioavailability of the RRR and all racemic stereoisomers of alpha-tocopherol in humans after single oral administration. J Clin Pharmacol. 1993;33:84–8 [DOI] [PubMed] [Google Scholar]
- 8.Kelleher J, Losowsky MS. The absorption of alpha-tocopherol in man. Br J Nutr. 1970;24:1033–47 [DOI] [PubMed] [Google Scholar]
- 9.MacMahon MT, Neale G. The absorption of alpha-tocopherol in control subjects and in patients with intestinal malabsorption. Clin Sci. 1970;38:197–210 [DOI] [PubMed] [Google Scholar]
- 10.Traber MG, Burton GW, Hughes L, Ingold KU, Hidaka H, Malloy M, Kane J, Hyams J, Kayden HJ. Discrimination between forms of vitamin E by humans with and without genetic abnormalities of lipoprotein metabolism. J Lipid Res. 1992;33:1171–82 [PubMed] [Google Scholar]
- 11.Traber MG, Burton GW, Ingold KU, Kayden HJ. RRR- and SRR-alpha-tocopherols are secreted without discrimination in human chylomicrons, but RRR-alpha-tocopherol is preferentially secreted in very low density lipoproteins. J Lipid Res. 1990;31:675–85 [PubMed] [Google Scholar]
- 12.Parks EJ, Dare D, Frazier KB, Hellerstein MK, Neese RA, Hughes E, Traber MG. Dependence of plasma alpha-tocopherol flux on very low-density triglyceride clearance in humans. Free Radic Biol Med. 2000;29:1151–9 [DOI] [PubMed] [Google Scholar]
- 13.Bruno RS, Leonard SW, Li J, Bray TM, Traber MG. Lower plasma alpha-carboxyethyl-hydroxychroman after deuterium-labeled alpha-tocopherol supplementation suggests decreased vitamin E metabolism in smokers. Am J Clin Nutr. 2005;81:1052–9 [DOI] [PubMed] [Google Scholar]
- 14.Vaule H, Leonard SW, Traber MG. Vitamin E delivery to human skin: studies using deuterated alpha-tocopherol measured by APCI LC-MS. Free Radic Biol Med. 2004;36:456–63 [DOI] [PubMed] [Google Scholar]
- 15.Leonard SW, Paterson E, Atkinson JK, Ramakrishnan R, Cross CE, Traber MG. Studies in humans using deuterium-labeled alpha- and gamma-tocopherols demonstrate faster plasma gamma-tocopherol disappearance and greater gamma-metabolite production. Free Radic Biol Med. 2005;38:857–66 [DOI] [PubMed] [Google Scholar]
- 16.Chuang JC, Matel HD, Nambiar KP, Kim SH, Fadel JG, Holstege DM, Clifford AJ. Quantitation of [5–14CH3]-(2R, 4′R, 8′R)-alpha-tocopherol in humans. J Nutr. 2011;141:1482–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Netscher T, Mazzini F, Jestin R. Tocopherols by hudride reduction of dialkylamino derivatives. Eur J Org Chem. 2007;2007:1176–83 [Google Scholar]
- 18. Microchemical determination of carbon, hydrogen, and nitrogen, automated method. In: Official Methods of Analysis of AOAC International. Gaithersburg (MD): AOAC International; 2006. p. 5–6.
- 19.Kim SH, Kelly PB, Clifford AJ. Biological/biomedical accelerator mass spectrometry targets. 1. optimizing the CO2 reduction step using zinc dust. Anal Chem. 2008;80:7651–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siluk D, Oliveira RV, Esther-Rodriguez-Rosas M, Ling S, Bos A, Ferrucci L, Wainer IW. A validated liquid chromatography method for the simultaneous determination of vitamins A and E in human plasma. J Pharm Biomed Anal. 2007;44:1001–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yiengst MJ, Shock NW. Blood and plasma volume in adult males. J Appl Physiol. 1962;17:195–8 [DOI] [PubMed] [Google Scholar]
- 22.Wadsworth GR. The blood volume of normal women. Blood. 1954;9:1205–7 [PubMed] [Google Scholar]
- 23.Clifford AJ, de Moura FF, Ho CC, Chuang JC, Follett J, Fadel JG, Novotny JA. A feasibility study quantifying in vivo human alpha-tocopherol metabolism. Am J Clin Nutr. 2006;84:1430–41 [DOI] [PubMed] [Google Scholar]
- 24.Koury MJ. Self-renewal in late-stage erythropoiesis. Blood. 2011;117:2562–4 [DOI] [PubMed] [Google Scholar]
- 25.Lin Y, Dueker SR, Follett JR, Fadel JG, Arjomand A, Schneider PD, Miller JW, Green R, Buchholz BA, Vogel JS, et al. Quantitation of in vivo human folate metabolism. Am J Clin Nutr. 2004;80:680–91 [DOI] [PubMed] [Google Scholar]
- 26.Klausner MA, Hirsch LJ, Leblond PF, Chamberlain JK, Klemperer MR, Segel GB. Contrasting splenic mechanisms in the blood clearance of red blood cells and colloidal particles. Blood. 1975;46:965–76 [PubMed] [Google Scholar]
- 27.Cifelli CJ, Green JB, Wang Z, Yin S, Russell RM, Tang G, Green MH. Kinetic analysis shows that vitamin A disposal rate in humans is positively correlated with vitamin A stores. J Nutr. 2008;138:971–7 [DOI] [PubMed] [Google Scholar]
- 28.Sharabi A, Cohen E, Sulkes J, Garty M. Replacement therapy for vitamin B12 deficiency: comparison between the sublingual and oral route. Br J Clin Pharmacol. 2003;56:635–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Traber MG, Lane JC, Lagmay NR, Kayden HJ. Studies on the transfer of tocopherol between lipoproteins. Lipids. 1992;27:657–63 [DOI] [PubMed] [Google Scholar]
- 30.Bjornson LK, Gniewkowski C, Kayden HJ. Comparison of exchange of alpha-tocopherol and free cholesterol between rat plasma lipoproteins and erythrocytes. J Lipid Res. 1975;16:39–53 [PubMed] [Google Scholar]
- 31.Massey JB. Kinetics of transfer of alpha-tocopherol between model and native plasma lipoproteins. Biochim Biophys Acta. 1984;793:387–92 [DOI] [PubMed] [Google Scholar]
- 32.Kitabchi AE, Wimalasena J. Demonstration of specific binding sites for 3H-RRR-alpha-tocopherol on human erythrocytes. Ann N Y Acad Sci. 1982;393:300–14 [DOI] [PubMed] [Google Scholar]
- 33.Kitabchi AE, Wimalasena J. Specific binding sites for D-alpha-tocopherol on human erythrocytes. Biochim Biophys Acta. 1982;684:200–6 [DOI] [PubMed] [Google Scholar]
- 34.Parker RS. Carotenoid and tocopherol composition of human adipose tissue. Am J Clin Nutr. 1988;47:33–6 [DOI] [PubMed] [Google Scholar]
- 35.El-Sohemy A, Baylin A, Ascherio A, Kabagambe E, Spiegelman D, Campos H. Population-based study of alpha- and gamma-tocopherol in plasma and adipose tissue as biomarkers of intake in Costa Rican adults. Am J Clin Nutr. 2001;74:356–63 [DOI] [PubMed] [Google Scholar]
- 36.Dju MY, Mason KE, Filer LJ., Jr Vitamin E (tocopherol) in human tissues from birth to old age. Am J Clin Nutr. 1958;6:50–60 [DOI] [PubMed] [Google Scholar]
- 37.McMasters V, Lewis JK, Kinsell LW, Van der Veen J, Olcott HS. Effect of supplementing the diet of man with tocopherol on the tocopherol levels of adipose tissue and plasma. Am J Clin Nutr. 1965;17:357–9 [DOI] [PubMed] [Google Scholar]
- 38.Kayden HJ, Hatam LJ, Traber MG. The measurement of nanograms of tocopherol from needle aspiration biopsies of adipose tissue: normal and abetalipoproteinemic subjects. J Lipid Res. 1983;24:652–6 [PubMed] [Google Scholar]
- 39.Snyder WS, Cook MJ, Nasset ES, Karhausen LR, Howells GP, Tipton IH. Report of the task group on reference man ICRP Publication 23. New York: International Commission on Radiological Protection; 1974 [Google Scholar]
- 40.Horwitt MK, Century B, Zeman AA. Erythrocyte survival time and reticulocyte levels after tocopherol depletion in man. Am J Clin Nutr. 1963;12:99–106 [DOI] [PubMed] [Google Scholar]
- 41.Bruno RS, Leonard SW, Park SI, Zhao Y, Traber MG. Human vitamin E requirements assessed with the use of apples fortified with deuterium-labeled alpha-tocopheryl acetate. Am J Clin Nutr. 2006;83:299–304 [DOI] [PubMed] [Google Scholar]
- 42.Horwitt MK. Critique of the requirement for vitamin E. Am J Clin Nutr. 2001;73:1003–5 [DOI] [PubMed] [Google Scholar]
- 43. NRC, editor. Recommended Dietary Allowances. 10th ed. Washington, DC: National Academy of Sciences; 1989.