Abstract
Pancreatic zinc (Zn2+) concentrations are linked to diabetes and pancreatic dysfunction, but Zn2+ is also required for insulin processing and packaging. Zn2+ released with insulin increases β-cell pancreatic death after streptozotocin toxin exposure in vitro and in vivo. Triosephosphate accumulation, caused by NAD+ loss and glycolytic enzyme dysfunction, occur in type-1 diabetics (T1DM) and animal models. We previously showed these mechanisms are also involved in Zn2+ neurotoxicity and are attenuated by nicotinamide- or pyruvate-induced restoration of NAD+ concentrations, Zn2+ restriction, or inhibition of Sir2 proteins. We tested the hypothesis that similar Zn2+- and NAD+-mediated mechanisms are involved in β-cell toxicity in models of ongoing T1DM using mouse insulinoma cells, islets, and nonobese diabetic (NOD) mice. Zn2+, streptozotocin, and cytokines caused NAD+ loss and death in insulinoma cells and islets, which were attenuated by Zn2+ restriction, pyruvate, nicotinamide, NAD+, and inhibitors of Sir2 proteins. We measured diabetes incidence and mortality in NOD mice and demonstrated that pyruvate supplementation, or genetic or dietary Zn2+ reduction, attenuated these measures. T-lymphocyte infiltration, punctate Zn2+ staining, and β-cell loss increased with time in islets of NOD mice. Dietary Zn2+ restriction or Zn2+ transporter 5 knockout reduced pancreatic Zn2+ staining and increased β-cell mass, glucose homeostasis, and survival in NOD mice, whereas Zn2+ supplementation had the opposite effects. Pancreatic Zn2+ reduction or NAD+ restoration (pyruvate or nicotinamide supplementation) are suggested as novel targets for attenuating T1DM.
Introduction
Type 1 diabetes (T1DM)11 is an autoimmune disease resulting from specific T-lymphocyte– and reactive oxygen species (ROS)-mediated destruction of the insulin-producing β-cells of the islets of Langerhans in the pancreas (1). It affects 1 in 300 people in the U.S. and is a major cause of mortality due to cardiovascular disease before 30 y of age (2). As such, the mechanisms of β-cell death in T1DM need to be better understood. Previous studies have shown the involvement of zinc (Zn2+) toxicity in streptozotocin models of T1DM in vitro or in vivo (3–5). We have now examined more physiologic models of T1DM for the role of Zn2+ toxicity. We have tested the hypothesis that Zn2+- and NAD+-mediated mechanisms are involved in β-cell toxicity in the mixed cytokine model of ongoing T1DM using mouse insulinoma cells and islets and in the nonobese diabetic (NOD) mouse.
NOD mouse model.
The NOD mouse loses β-cells and glycemic control through autoimmune-mediated mechanisms accurately mimicking T1DM (1). Immune-mediated ROS generation results in oxidative injury, depolarization, and degranulation of β-cells, causing secretory granular Zn2+ release [reviewed in (6)]. Inhibition of the metabolic enzymes GAPDH and pyruvate dehydrogenase causes an increase in triosephosphates as occurs in T1DM patients, perhaps mediated by a reduced NAD+:NADH ratio (7). Nicotinamide attenuates diabetes incidence in NOD mice, presumably by restoring this ratio (8). These mechanisms are identical to those proposed for Zn2+ neurotoxicity (9–11), where pyruvate, nicotinamide, NAD+, and inhibition of Sir2 proteins attenuate neurotoxicity.
Streptozotocin.
Streptozotocin is selectively toxic to pancreatic islets and a mouse β-cell insulinoma cell line (MIN6) causing reductions in NAD+:NADH, glucose oxidation, and glucose-induced insulin secretion (3, 12). Streptozotocin induces Zn2+ release, alkylates DNA-activating poly-ADP ribose polymerase and NF-κB, and depletes NAD+ and ATP (3, 13, 14). Oxygen radical scavengers, nicotinamide, Zn2+ chelators, and pyruvate reduce streptozotocin-induced diabetes incidence [this study and (3–5,15)].
Pancreatic Zn2+.
The pancreatic intracellular Zn2+ concentration ([Zn2+]i) is very high and within the pancreas Zn2+ is concentrated in secretory granules, where it allows insulin processing and hexahedral Zn2+/insulin crystal formation (3). Upon glucose induction and β-cell depolarization, insulin-bound and noninsulin-bound Zn2+ are released, both of which are cytotoxic to adjoining β-cells. This helps to explain the specificity of β-cell death (4, 16). Because of this, dietary Zn2+ supplementation has become controversial. The reasoning for supplementation is that type-2 diabetics (T2DM) and the elderly tend to be Zn2+ deficient. Zn2+ supplementation has been proposed to attenuate diabetes incidence (17) and T2DM but not T1DM animal models were reported to be Zn2+ deficient [reviewed in (18)]. This bimodality of Zn2+ also occurs in neurons, where Zn2+ is required for cell survival and supplementation is beneficial if concentrations are reduced but is toxic under pathologic conditions where labile Zn2+ is in excess (19, 20). Dietary Zn2+ changes have been shown to induce changes in Zn2+ transporters, depending on cell type, cellular localization, and the Zn2+ transporter [reviewed in (21)]. Intestinal expression of Zn2+ importers, ZIP4 in particular, has been shown to be upregulated by dietary restriction. Pancreatic acinar cell expression of the Zn2+ exporters ZnT1 and ZnT2 were shown to be reduced by dietary Zn2+ restriction (22). Under pathophysiologic, high-glucose, depolarizing conditions, the influx of Zn2+ into islets and β-cells is predominantly mediated by voltage-gated calcium channels rather than by transporters (23).
Zn2+ homeostasis in β-cells is genetically linked to T1DM and T2DM. Two slc30a family Zn2+ transporters [Zinc transporter (ZNT) 5 and ZNT8] are preferentially or specifically expressed in β-cells and are involved in physiologic pancreatic Zn2+ uptake into the Golgi and packaging into secretory granules (24, 25). ZNT8 is a major epitope for autoantigen generation in T1DM, adversely affecting β-cell Zn2+ transport and increasing pancreatic autoimmune attack (26). A common single nucleotide polymorphism in ZNT8 is also linked to reduced susceptibility to T2DM (27). However, global and/or β-cell knockout of ZnT5 or ZnT8 are not clinically diabetic. This suggests redundancy in β-cell Zn2+ transport function (24, 28, 29).
In the studies described herein, we propose that diabetic immune-generated cytokines and ROS cause intracellular release of Zn2+ or reuptake of noninsulin-bound Zn2+ released exogenously by β-cell degranulation. This increased [Zn2+]i potentiates loss of NAD+ concentrations through Sir2 proteins, resulting in glycolytic inhibition, β-cell death, and diabetes incidence in ongoing T1DM (Supplemental Fig. 1).
Materials and Methods
Cell culture and toxicity studies.
Cultures of the mouse insulinoma cell line MIN6 (from Dr. John Corbett, while at Washington University) were maintained as described (3); where indicated, cultures were preloaded with 10 μmol/L ZnCl2 in the growth medium that did not affect cell number or viability. Then 35 μmol/L ZnCl2 in serum-free minimal essential medium or 300 μmol/L ZnCl2 in DMEM + 15% FBS was used for normal-density toxicities, and these conditions achieved similar levels of toxicity (Expt. 1). ZnCl2 (400 μmol/L) in HEPES buffered salt solution with or without 60 mmol/L KCl replacing NaCl and Ca2+ channel antagonists was present for 5 or 15 min in the [Zn2+]i measurements as described (30). For glucose deprivation (GD), normal-density cultures were washed 7 times in minimal essential medium lacking glucose and exposed in this medium plus normal growth additives with or without optimized concentrations of additional compounds for 24 h. The compounds used were: N,N,N‘N’-tetrakis(-)[2-pyridylmethyl]-ethylenediamine (TPEN), a Zn2+-specific chelator; sirtinol or 2-hydroxynaphthaldehyde (Naph), sirtuin (Sir2 protein) inhibitors; nimodipine and mibefradil, specific L- and T-type calcium channel antagonists (10 μmol/L), respectively; and compounds that restored NAD+ concentrations (pyruvate, nicotinamide, and NAD+) at optimized concentrations (data not shown). High-density cultures (HDs) were used for streptozotocin and cytokine toxicities. MIN6 cells were collected, counted, resuspended in DMEM + 15% FBS, and plated at HD/low extracellular volume (∼5–10 × 1010 cells/L, in 0.04 mL) in V-shaped, 96-well plates (9). These HDs were plated in 7.5 mmol/L streptozotocin or a mixture of cytokines (250 μg/L IL-1β, 8 μg/L, TNFα, and 200 μg/L IFNγ) with coexposure to the compounds tested. High cytokine concentrations were required due to the HDs and short exposure (6 h, Expt. 2). Cell viability was assayed at varying times later by 3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide (MTT) staining (0.1% final) of individual wells of a tissue culture plate, the absorbance at 595 nm was then measured (n = 8–20 wells of cells from at least 3 independent experiments), and significance was tested using 1-way ANOVA (Expts. 1 and 2). Staining with propidium iodide (2.5 mg/L) for normal-density Zn2+ and GD toxicity studies followed by measurement of fluorescence gave comparable results (data not shown). HDs were only assayed by MTT, because a monolayer culture is required for propidium iodide staining.
Isolated islet generation.
Islets were generated from C57/Bl6/J mice (Jackson Labs) (31), recovered overnight, and handpicked into 24-well plates. Equivalent numbers of islets (∼30) were put into each well in growth medium without phenol red. Alamar Blue (BioSource) was added (5%) to determine islet viability and basal fluorescence measured for islet content (excitation = 535 nm, emission = 595 nm). Islets were washed and exposed to 300 μmol/L Zn2+ for 20 h or a mixture of IL-1β, TNFα, and IFNγ (10, 4, 50 μg/L) for 50 h (with or without 6 mmol/L pyruvate, nicotinamide, NAD+) in DMEM + 15% FBS. Higher concentrations of exogenous Zn2+ were necessary to induce death of islets, because 15% FBS is necessary for islet survival but binds a substantial quantity of Zn2+. Alamar Blue was re-added and fluorescence directly measured after 4 h (32). Death was normalized and expressed as a percentage of the complete death induced by 20 μmol/L A23187 (Ca2+ ionophore), and significance was tested using 1-way ANOVA (Expt. 3). NAD+ increased the basal Alamar Blue signal and therefore sham wash + NAD+ Alamar Blue fluorescence was used as 100% viability for the toxicity in the presence of NAD+.
Determination of NAD+ concentrations.
For the NAD+ measurements, 1.67 × 106 MIN6 cells or 50 islets were lysed by the addition of hot 75% ethanol/0.05 mol/L K2HPO4 after a 3-h exposure to 300 μmol/L Zn2+ or 15 mmol/L streptozotocin in DMEM + 15% FBS (MIN6 only) as previously described (10). We used the same exposure to 300 μmol/L Zn2+ in DMEM + 15% FBS for MIN6 cultures for comparison of NAD+ concentrations in MIN6 cultures to NAD+ concentrations in islets and significance was tested using 1-way ANOVA (Expt. 4).
65Zn2+ accumulation.
These experiments were performed as described for neurons (30). MIN6 cultures were washed in HEPES solution containing the desired drugs and 65Zn2+ at 37°C for 5–15 min (2 mCi/L, 1–5 Ci/g; DuPont NEN; total Zn2+ = 20 μmol/L) in the presence or absence of 60 mmol/L K+ and voltage-gated Ca2+ channel antagonists as indicated. A 5-min depolarizing exposure and a 15-min nondepolarizing exposure caused equivalent accumulation of 65Zn2+ and were used as the time points for the addition of antagonists. The stimulus solution was washed out 3 times with ice-cold quench buffer, cells lysed in SDS and counted (Supplemental Table 1), and significance was tested using 1-way ANOVA (Expt. 5).
[Zn2+]i measurements.
To monitor [Zn2+]i, MIN6 cultures were washed and loaded with 5 μmol/L FluoZin-3-AM (excitation = 485 nm; emission = 530 nm) for 30 min. Cultures were then exposed as indicated and cellular fluorescence measured and compared with fluorescence in the presence of a Zn2+ ionophore (delta max) compared with fluorescence in the presence of the Zn2+ chelator TPEN (delta min). Calculations were performed exactly as described (30) and significance was tested using 1-way ANOVA (Expt. 6). FluoZin-3 is not influenced by the Ca2+ or magnesium concentrations but weakly responds to other transition metals like lanthanum, mercury, or cadmium (33).
Colony maintenance and trials.
The NOD inbred mouse strain (Taconic) was maintained at Louisiana State University Health Sciences Center’s animal facility. Housing, killing, and anesthesia for all animals concurred with the institutional Animal Studies Committee guidelines and the Public Health Service Guide for the Care and Use of Laboratory Animals. Mice were killed by CO2 asphyxiation followed by thoracotomy. The animal handler and tissue processor were unaware of the treatment conditions. Treatment began on 11 groups of age-matched, female, NOD mice (higher disease penetrance in females) at 6 wk of age. Plastic cages and water bottles with no metal were used for the duration to minimize Zn2+ contamination. The treatment groups tested were nonpurified diet (Harlan diet no. 2019) + saline injection, + 200 mg Zn2+/L in the drinking water, + pyruvate (0.5 g/kg i.p. tri-weekly), and + Zn2+ injections (100 μmol/kg/wk i.p. ZnSO4). We also tested feeding a Zn2+-deficient purified diet [TD.85419; 0.5–1.5 mg Zn2+/kg diet, Harlan Teklad (34)] with 0, 1, 2, or 60 mg Zn2+ (from ZnSO4)/kg diet supplied in purified (18 mOhm) water (Expt. 7). In addition, ZnT5 heterozygous knockout mice (24) were backcrossed to NOD mice for 10 generations followed by interbreeding to obtain ZnT5−/−, ZnT5+/−, and ZnT5+/+ female mice in an NOD background, which were fed nonpurified diet (Expt. 8). Water and food ingestion and body weight were monitored weekly and did not significantly vary between groups (∼5 mL of water and 5 g of food per mouse per day at 16 wk, decreasing to ∼4 mL and 4 g at 32 wk). Blood glucose from fed mice was monitored every Monday afternoon starting at 10 wk; blood glucose from mice deprived of food for 6 h was determined periodically (glucose oxidase). These blood glucose samples gave qualitatively similar results. Mice akinetic with prodding or unable to eat and drink were killed and mortality was recorded; significance was tested using Kaplan-Meier estimates of the survival functions, with subsequent logrank tests and Sidak adjustment of P values. Multiple low-dose streptozotocin was performed (55 mg/kg i.p. injection each day for 5 d in C57/Bl6) and blood glucose was measured on d 0, 1, 4, 7, 14, and 21 (Supplemental Table 2) and significance was tested using a Student’s t test (Expt. 9).
Zn2+-restricted and nonpurified diets.
The Zn2+-restricted diet and nonpurified diet were from Harlan Teklad, batch-labeled TD.85419 and no. 2019, respectively. The Zn2+-deficient diet was previously described (34) and the nonpurified diet is a proprietary standard diet (2019) made of corn and wheat with ∼19% protein, 14.7% total fiber, and 9% fat by weight, 0.060 g of Zn2+/kg diet, and 3.3 kcal/g of diet energy density. The datasheet for 2019 lists the ingredients and calculated nutrient value (Harlan Laboratories). The mineral and vitamin mixes used in 2019 are given in Supplemental Tables 3 and 4.
Zn2+ staining.
Ten-μm cryostat sections were dried and immersed in 5 μmol/L N-(6-methoxy-quinolyl)-para-toluenesulfonamide (TSQ) (Invitrogen/Life Technologies) for 2 min as described (3) (Expt. 10). Punctate Zn2+ staining was determined using SISAnalysis counting software (Soft Imaging System) in 20 equivalently spaced sections (from 100) after TSQ staining (Expt. 11). ZinPyr-1 (ZP1; 5 μmol/L, TefLabs) staining was performed using dye in PBS for 2 min (11). Islet images were captured at identical exposures using a fluorescence microscope (Nikon TS-100) with a 10–20× air objective (Expt. 12). The intensity of insulin and ZP1 staining was measured by Image J software from NIH. Four to 8 images were measured per animal and 6 animals/group. The mean intensities were calculated and compared between the treated and untreated groups by a Student’s t test. Immunohistochemistry and hematoxylin/eosin staining were performed on adjacent postfixed sections.
Immunofluorescent technique.
Sections were postfixed, permeabilized in 0.25% Triton X-100, labeled using anti-insulin (1:1000, Dako), developed, mounted with ProLong (Invitrogen/Life Technologies), and examined by epifluorescence microscopy (Nikon TS-100). The β-cell area was determined using SIS Analysis software in every tenth section (from 100) after insulin staining and significance was tested using 1-way ANOVA (Expt. 12).
Data analysis and statistics.
All data are presented as mean ± SEM. The n is given for each experiment in the legends. Each set of results was compared with the appropriate sham wash controls and toxin exposure alone by 1-way ANOVA using the Dunnett’s post hoc test and significance achieved by P < 0.05. The statistical analyses comparing the insulin or ZP1 staining intensities between 2 groups were compared by a Student’s t test. The statistical analyses for survival time or time until diabetes developed were performed where the curves for each genotype or treatment were compared with wild-type littermates or the normal Zn2+-containing diet. This was done using Kaplan-Meier estimates of the survival functions, with subsequent logrank tests and Sidak adjustment of P values from multiple comparisons among the genotype or treatment conditions (P < 0.05) (35).
Reagents.
Unless otherwise stated, all reagents were from Sigma Chemical.
Results
MIN6 [Zn2+]i concentrations increased after Zn2+ and ROS exposure and were sensitive to voltage-gated Ca2+ channel blockade.
That toxic Zn2+ enters β-cells first needed to be confirmed. Depolarization resulted in increased [Zn2+]i as measured by FluoZin3, which could be attenuated using voltage-gated calcium antagonists. The contribution of Zn2+ uptake transporters was less evident in these acute uptake experiments, though the basal concentrations of both [Zn2+]i and 65Zn2+ accumulation were partially dependent on temperature (data not shown). H2O2 also induced an increase in [Zn2+]i, presumably by intracellular release (Fig. 1). 65Zn2+ accumulation studies in MIN6 cultures confirmed these results using the additional Ca2+ channel antagonists Gd3+, Ni2+, or Ca2+ (Supplemental Table 1). These results agree with another report that studied [Zn2+]i in islets, dispersed islet cells, and insulinoma cell lines (23).
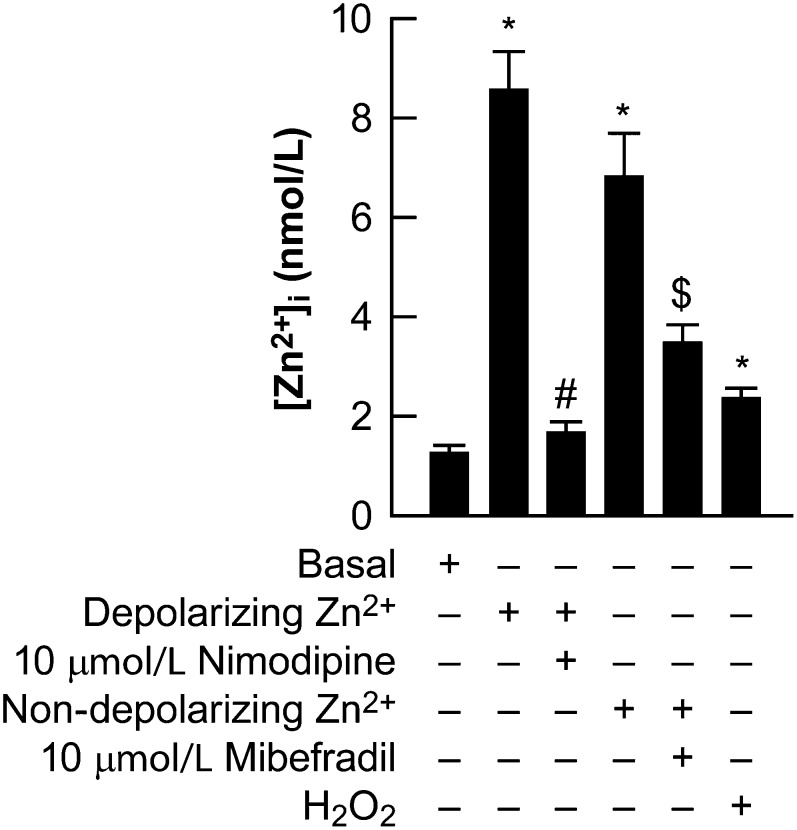
FIGURE 1.
[Zn2+]i in MIN6 cultures exposed to Zn2+, H2O2, and Ca2+ channel antagonists (Expt. 6). MIN6 cultures were loaded with 5 μmol/L FluoZin3 for 30 min and the cells were washed (basal) and then exposed as indicated. The effects of a depolarizing Zn2+ exposure on [Zn2+]i were determined, as was the antagonism of nimodipine. The effects of a nondepolarizing Zn2+ exposure on [Zn2+]i was determined, as was the antagonism of mibefradil. The effects of 100 μmol/L H2O2 on [Zn2+]i was determined. These experiments are from at least 3 independent experiments, n = 45–60 cells/condition. *Different from basal levels; #different from a depolarizing Zn2+ exposure; $different from a nondepolarizing Zn2+ exposure, P < 0.05. [Zn2+]i, intracellular zinc concentration.
Pyruvate, nicotinamide, NAD+, and sirtuin inhibitors reduced Zn2+, streptozotocin, and cytokine toxicities.
Compounds that attenuate Zn2+ neurotoxicity were tested in β-cells. MIN6 cultures were exposed to Zn2+ and GD under normal-density conditions or were exposed under high-density conditions to streptozotocin and cytokines as indicated; cell viability was determined by MTT staining (Fig. 2). High-density insulinoma culture conditions mimic the islet structure, allowing release of endogenous Zn2+ to attain toxic levels (3). Cytokine and streptozotocin toxicities were potentiated by preloading MIN6 cells with 10 μmol/L Zn2+, which increases [Zn2+] to physiologic concentrations (15–20 μmol/L = [Zn2+] in plasma) (36, 37). Zn2+ is present in the growth medium at 3–6 μmol/L, because it is present in FBS at 25–40 μmol/L (38). Optimized concentrations of compounds that reduce [Zn2+]i (Zn2+ chelation and L- and T-type Ca2+ channel antagonists) or restore NAD+ (pyruvate, nicotinamide, and NAD+) attenuated Zn2+, cytokine, and streptozotocin-mediated deaths. Pyruvate was most effective against Zn2+ toxicity, whereas nicotinamide and NAD+ were most effective against streptozotocin toxicity. The partial efficacy of the Ca2+ channel antagonists (nimodipine and mibefradil) mimics the partial efficacy of the Zn2+ chelators (CaEDTA or TPEN) (3). This suggests that ∼50% of these injuries are attributable to Zn2+ toxicity. In addition, optimized concentrations of the sirtuin pathway inhibitors sirtinol and Naph attenuated these injuries. The sirtuin pathway activator, resveratrol, or the NAD+ antagonist 3-AP potentiated these injuries. Pyruvate and lactate prevented GD-mediated death; however, lactate, which cannot regenerate NAD+, was ineffective against streptozotocin, Zn2+, and cytokine exposures. NAD+ and nicotinamide did not prevent GD-mediated death but did attenuate the toxin exposures. Mouse isolated islets were sensitive to Zn2+- or cytokine-mediated death and pyruvate, nicotinamide, and NAD+ attenuated these islet injuries with efficacies similar to those reported for MIN6 cells (Table 1). Streptozotocin caused mouse islet membrane dissolution after 4.5 h, preventing determination of the toxicity of streptozotocin-released Zn2+. Control islets had 7 ± 2% dissolution and β-cell release, whereas streptozotocin-exposed islets had 74 ± 8% dissolution, which is significantly different (n = 10 wells).
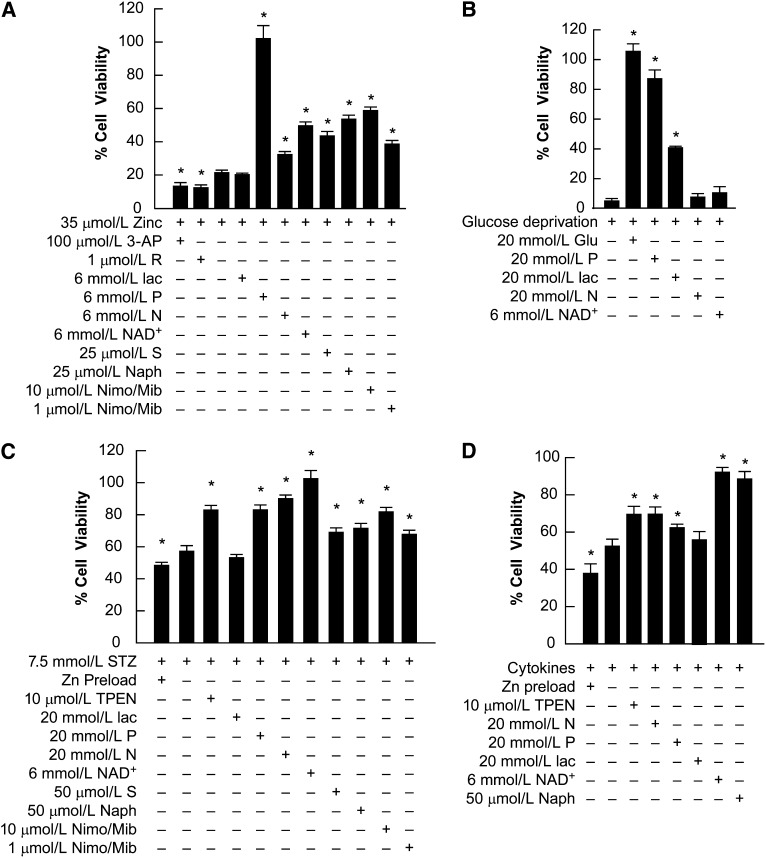
FIGURE 2.
ΜΙΝ6 cell death in cultures exposed to Zn2+ (A), GD (B), streptozotocin (C), or cytokines (D) and treated with therapeutic compounds (Expts. 1 and 2). MIN6 cultures were exposed to 35 μmol/L Zn2+ (A) or GD at normal density (B) and 7.5 mmol/L STZ (C) or mixed cytokines at high density (D) and the therapeutic compounds were present as indicated. Nim/Mib indicates the presence of 10 μmol/L each of nimodipine and mibefradil. Cell survival was assayed by MTT or propidium iodide staining (data not shown) after 24 h (A,B) and by MTT staining after 6 h (C,D) from at least 3 independent experiments. Cell viability was scaled to control untreated cultures (100% viability) after subtraction of the signal associated with near-complete death produced by exposure to 20 μmol/L A23187 for 24 h = 0 (mean ± SEM, n = 8–20). *Different from respective toxin alone at P < 0.05. 3-AP, 3-acetylpyridine; GD, glucose deprivation; Glu, glucose; lac, lactate; MTT, 3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide; N, nicotinamide; Naph, 2-hydroxynaphthaldehyde; Nimo/Mib, nimodipine/mibefradil; P, pyruvate; R, resveratrol; S, sirtinol; STZ, streptozotocin; TPEN, N,N,N'N’-tetrakis(-)[2-pyridylmethyl]-ethylenediamine.
TABLE 1.
Islet viability after Zn2+ or mixed cytokine exposures treated with pyruvate, NAD+, and nicotinamide (Expt. 3)1
| Exposure condition | Islet viability, % |
| Control | 100 ± 5.7 |
| 300 μmol/L Zn2+ | 18.5 ± 5.4* |
| Zn2+ + 10 mmol/L pyruvate | 103 ± 7.9# |
| Zn2+ + 10 mmol/L NAD+ | 108 ± 8.6# |
| Zn2+ + 10 mmol/L nicotinamide | 40.1 ± 8.3*# |
| Zn2+ + 10 mmol/L lactate | 22.5 ± 5.8* |
| Mixed cytokines | 10.1 ± 8.1* |
| Mixed cytokines + 10 mmol/L pyruvate | 80.3 ± 8.9† |
Values are means ± SEM, = 5–6. *Different from control, P < 0.05; #different from Zn2+ exposure alone, P < 0.05; †different from mixed cytokine exposure alone, P < 0.05.
In isolated islets and/or MIN6 cultures, pyruvate, nicotinamide, NAD+, and sirtinol attenuated the loss of NAD+ induced by Zn2+ and streptozotocin.
The effects of β-cell toxicities on NAD+ concentrations were examined, because Zn2+ neurotoxicity is mediated by the loss of NAD+. Both Zn2+ and streptozotocin exposure induced significant losses of NAD+ in MIN6 cells and Zn2+ induced significant NAD+ loss in isolated islets at 3 h which is before cell death (Table 2). Cytokines had a lesser effect on NAD+ concentrations (data not shown). Nicotinamide and NAD+ were the most effective at restoring NAD+ concentrations, followed by pyruvate and sirtinol, each of which had a significant effect on restoring NAD+ concentrations.
TABLE 2.
[NAD+]i in MIN6 or islets exposed to Zn2+ or streptozotocin and therapeutic compounds (Expt. 4)1
| Condition | NAD+ | NAD+ |
| nmol/107 viable cells | nmol/100 islets | |
| Control (3 h) | 3.64 ± 0.10 | 1.0 ± 0.06 |
| 300 μmol/L Zn2+ | 1.62 ± 0.22* | 0.48 ± 0.10* |
| Zn2+ + 10 mmol/L pyruvate | 2.80 ± 0.2*# | 0.80 ± 0.10# |
| Zn2+ + 10 mmol/L nicotinamide | 3.60 ± 0.28# | 1.00 ± 0.09# |
| Zn2+ + 6 mmol/L NAD+ | 5.90 ± 0.50*# | 2.35 ± 0.30*# |
| Zn2+ + 25 μmol/L sirtinol | 2.20 ± 0.23*# | 0.74 ± 0.09*# |
| Control (HD, 3 h) | 2.00 ± 0.39 | NP2 |
| 15 mmol/L streptozotocin | 1.14 ± 0.24* | NP2 |
| Streptozotocin + 10 mmol/L pyruvate | 1.65 ± 0.09*# | NP2 |
| Streptozotocin + 10 mmol/L nicotinamide | 2.87 ± 0.94*# | NP2 |
| Streptozotocin + 6 mmol/L NAD+ | 6.10 ± 1.70*# | NP2 |
| Streptozotocin + 25 μmol/L sirtinol | 1.73 ± 0.15# | NP2 |
Values are means ± SEM, = 6–7. *Different from respective control concentrations; #different from toxin exposure alone, P < 0.05. HD, high-density culture; [NAD+]i, intracellular NAD concentration; NP, not performed.
Quantification not possible due to streptozotocin mediated dissociation of islets.
Islet Zn2+ staining increased with age in NOD mice and was attenuated by reduced-zinc diet or knockout of ZnT5.
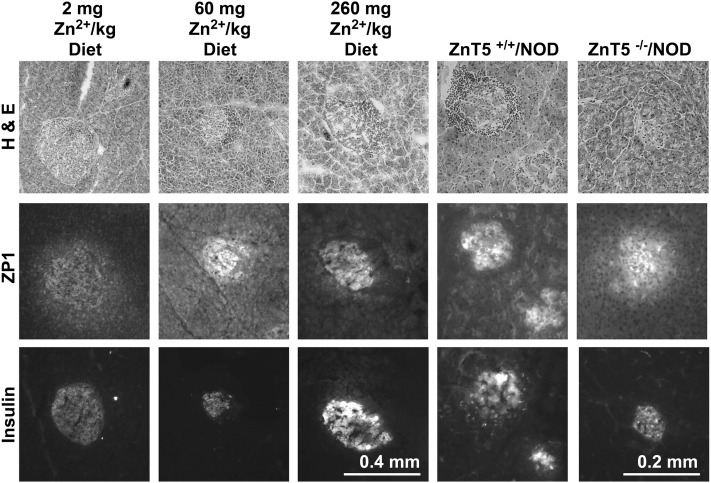
Zn2+ staining precedes death induced by Zn2+ accumulation and was therefore tested in the islets of NOD mice. At 12, 15, 18, 21, and 24 wk, representative pancreatic sections were stained with hematoxylin/eosin, TSQ, ZP1, α-insulin, or anti-polyADP ribose (Fig. 3; Table 3; Supplemental Fig. 2). Punctate Zn2+ staining increased in islets in conjunction with lymphocyte infiltration in an age-dependent fashion starting at 15 wk. Some islets (especially minus pyruvate treatment) appeared to be degranulated, suggesting ongoing injury. Islets that were densely infiltrated with lymphocytes had punctate Zn2+ staining and had lost insulin immunostaining (Supplemental Fig. 2). Anti-polyADP-ribose immunohistochemistry was not dramatically changed (data not shown). The reduced-zinc diet (ZnR) (2 mg Zn2+/kg diet) or ZnT5 knockout (KO) in NOD decreased punctate and total pancreatic Zn2+ staining and increased insulin staining (Fig. 3;Table 3). Quantitation of the total Zn2+ staining of all the conditions shown (Fig. 3) gave the following results. The Zn2+ staining of pancreas from mice fed 60 mg Zn2+/kg diet had 93.1 ± 19.2 relative fluorescence units (RFU)/mm2, whereas those from mice fed 2 mg Zn2+/kg diet had 38.2 ± 11.2 RFU/mm2, which is significantly different. The Zn2+ staining of pancreas from ZnT5 WT in NOD mice had 99.4 ± 15 RFU/mm2, whereas those from ZnT5 KO in NOD mice had 70.8 ± 14 (n = 8–10), which differs (P < 0.05 by a Student’s t test).
FIGURE 3.
Pancreatic Zn2+ and insulin staining in NOD mice exposed to ZnR and ZnT5 KO (Expt. 12). Fresh frozen and dried or fixed sections of pancreas from NOD mice at 21 wk of age that were fed the Zn2+ diets indicated were stained with the Zn2+-specific fluorescent dye ZP1 or with anti-insulin. ZnT5 KO and WT littermates were fed a nonpurified diet. Representative epifluorescence photomicrographs of identical exposure were taken (n = 5–6). H & E, hematoxylin/eosin; NOD, nonobese diabetic; ZnR, reduced-zinc diet; ZnT, zinc transporter; ZP1, ZinPyr-1.
TABLE 3.
Punctate Zn2+ staining in aged NOD mice exposed to 60 or 2 mg/kg Zn2+ diet and ZnT5 KO (Expt. 11)1
| Age, wk | 60 mg Zn2+/kg diet | 60 mg Zn2+/kg diet + pyruvate | −/− in NOD | 2 mg Zn2+/kg diet |
| 15 | 90 ± 12 | 110 ± 19 | NP | 52 ± 11*# |
| 18 | 210 ± 25* | 223 ± 28* | NP | NP |
| 21 | 290 ± 37*#x2020 | 271 ± 30* | 190 ± 21*# | 141 ± 25*# |
| 24 | 205 ± 39* | 245 ± 26* | NP | NP |
Values are means ± SEM, = 5–6, of total Zn2+ puncta from 20 sections (per 105 μm2 of pancreas). *Different from 15-wk NOD, 60 mg Zn2+/kg diet mice, P < 0.05; #different from untreated, age-matched NOD mice, P < 0.05; †different from 18-wk NOD, 60 mg Zn2+/kg diet mice, P < 0.05. NOD, nonobese diabetic; NP, not performed.
Pyruvate or ZnR increased lifespan and #x03B2-cell mass and decreased diabetes incidence.
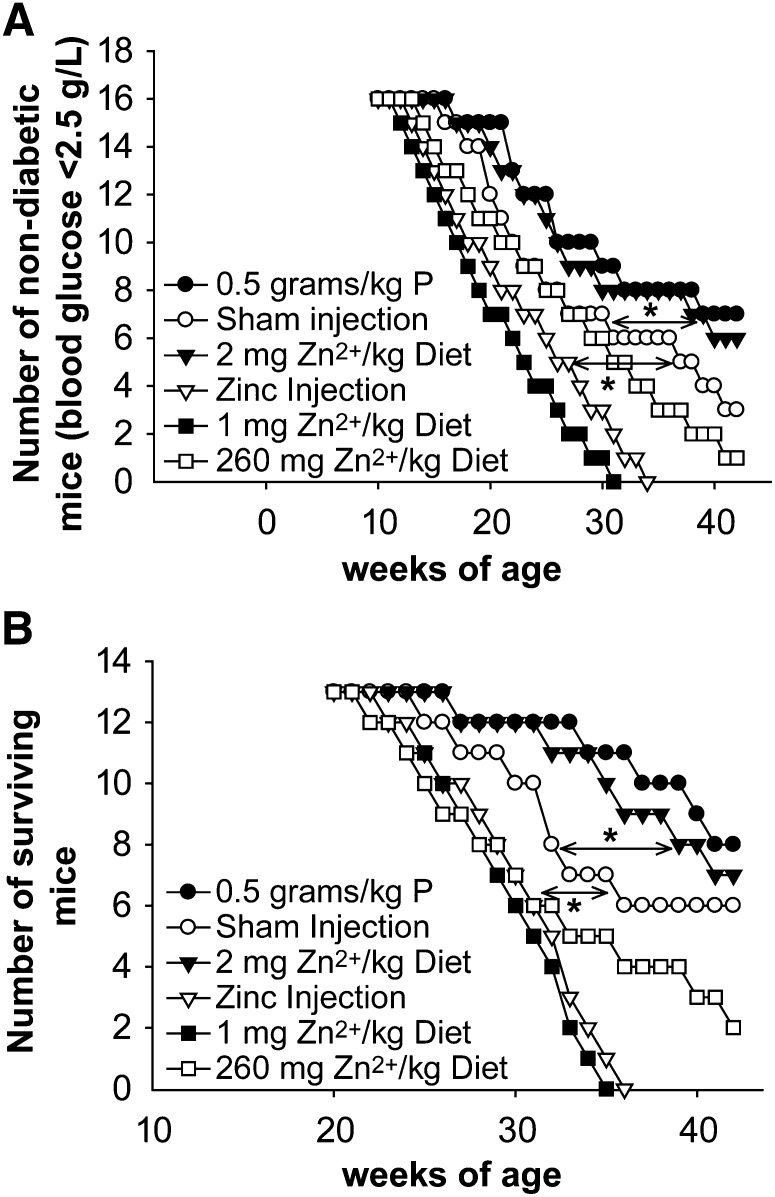
Reducing Zn2+ or pyruvate or nicotinamide exposure has been shown to attenuate Zn2+ neurotoxicity in vivo (10, 39) and so was tested in NOD mice. Female NOD mice were treated with 0.5 g/kg body weight (3×/wk i.p.) of pyruvate, nicotinamide, or saline starting at 6 wk. An s.c. injection of pyruvate was also effective (data not shown). The β-cell mass in the pyruvate-treated or ZnR (2 mg Zn2+/kg diet) mice was greater than nonpurified diet or 60 mg Zn2+/kg purified diet controls, primarily because the islet number increased (Fig. 3; Table 4; Supplemental Fig. 2). The insulin staining intensity also increased. Pyruvate treatment did not affect plasma cytokine concentrations, although a reduction was demonstrated for ZnR at 21 wk using a Bio-Rad multiplex. NOD mice without treatment and fed a normal Zn2+ diet had 295 ± 95 ng/L TNFα and 15,000 ± 8000 U/L IL-1β. NOD + pyruvate-treated mice had 250 ± 100 ng/L TNFα and 14,000 ± 9000 U/L IL-1β, which is not different. NOD + ZnR-treated mice had 200 ± 70 ng/L TNFα and 10,000 ± 5000 U/L IL-1β, which is significantly different. Pyruvate did not affect lymphocyte infiltration or Zn2+ staining, whereas ZnR significantly decreased Zn2+ staining and reduced lymphocyte infiltration (Fig. 3; Table 3; Supplemental Fig. 2). Diabetes incidence (glucose >2.5g/L for 2 consecutive weeks) was significantly reduced and delayed in pyruvate or 2–3 mg Zn2+/kg diet-treated NOD mice, as was animal mortality [2 mg Zn2+/kg diet > 3 mg Zn2+/kg diet (data not shown)]. The i.p. ZnSO4 injections or 1 mg Zn2+/kg diet (no added Zn2+) increased diabetes incidence and caused very rapid subsequent mortality (Fig. 4; Table 4). Furthermore, 260 mg Zn2+/kg diet potentiated diabetes incidence compared with a nonpurified diet that contained 60 mg Zn2+/kg (Fig. 4). Quantitation of the total insulin staining of sections from conditions as in Figure 3 gave the following results. The insulin staining of pancreas from NOD mice fed a 60 mg Zn2+/kg diet had 13.3 ± 3.8 RFU/mm2, whereas NOD mice fed 2 mg Zn2+/kg diet had 18.3 ± 3.2, which is significantly different. The insulin staining of pancreas from ZnT5 WT/NOD mice had 25.7 ± 11 RFU/mm2, whereas pancreas from ZnT5 KO/NOD mice had 40.3 ± 19 (n = 8–10), which is different at P < 0.05 by a Student’s t test. Quantitation of the insulin or Zn2+ staining for 1 or 260 mg Zn2+/kg diets and the ZnSO4 injections was not possible, because very low numbers of islets remained. Pyruvate injection or 2 mg Zn2+/kg diet did not reduce blood glucose in C57/BL6 or young NOD mice and Zn2+ injections had no effect on blood glucose or mortality in C57/BL6 mice. Pyruvate also attenuated diabetes incidence in the multiple low-dose streptozotocin model of diabetes (Supplemental Table 2).
TABLE 4.
β-Cell mass in aged NOD mice exposed to pyruvate, ZnR, and ZnT5 KO (Expt. 12)1
| Age, wk | 60 mg Zn2+/kg diet (NOD) | NOD + pyruvate | −/− in NOD | 2 mg Zn2+/kg diet (NOD) | 260 mg Zn2+/kg diet (NOD) | NOD + ZnSO4 (i.p.) |
| 15 | 3.52 ± 0.3 | 4.10 ± 0.34 | NP | 3.67 ± 0.28 | NP | 2.07 ± 0.31*# |
| 18 | 3.00 ± 0.24 | 3.92 ± 0.30 | NP | NP | NP | NP |
| 21 | 1.00 ± 0.31* | 3.49 ± 0.37# | 2.9 ± 0.2*# | 2.03 ± 0.31*# | 0.50 ± 0.20*# | 0.47 ± 0.22*# |
| 24 | 0.37 ± 0.18* | 2.67 ± 0.42*# | NP | NP | NP | NP |
Values are means ± SEM, = 5–6, of total β-cell mass from 20 sections (per 105 μm2 of pancreas). *Different from 15-wk NOD, 60 mg Zn2+/kg diet mice, P < 0.05; #different from untreated, 60 mg Zn2+/kg diet, age-matched NOD mice, P < 0.05. NOD, nonobese diabetic, NP, not performed.
FIGURE 4.
Diabetes incidence (A) and mortality (B) in NOD mice exposed to P and ZnR or ZnE (Expt. 7). NOD mice were i.p. injected 3 times/wk with 0.5 g/kg P or an equal volume of saline or were given 100 μmol/(kg · d) i.p. ZnSO4 injection and were fed a nonpurified diet. NOD mice were also fed 1, 60, or 260 mg Zn2+/kg purified diet (60 mg Zn2+/kg diet = nonpurified diet). Blood glucose was monitored weekly and mice that demonstrated akinesia with continued prodding by an observer unaware of the treatment conditions were killed. (A) The number of mice whose blood glucose remained <2.5 g/L for 2 consecutive weeks was plotted as a function of weeks of age. (B) The number of surviving mice was plotted as a function of weeks of age. *Different from sham injected mice, P < 0.05, n = 16. NOD, nonobese diabetic; P, pyruvate; ZnE, excess-zinc diet; ZnR, reduced-zinc diet.
ZnT5 KO mice in an NOD background had reduced diabetes incidence, mortality, and Zn2+ staining.
Knockout of the neuronal Zn2+ transporter, ZnT3, has been shown to reduce Zn2+ neurotoxicity, so knockout of a β-cell-enriched Zn2+ transporter, ZnT5, was tested. Backcrossing of ZnT5 KO to NOD mice for 10 generations resulted in syngeneity such that diabetes incidence and mortality for female ZnT5 +/+(WT)/NOD was identical to the parental NOD females. We performed a similar analysis of blood glucose, insulin and Zn2+ staining, and histology showing that ZnT5 −/−(KO)/NOD mice had attenuated diabetes incidence, mortality, and Zn2+ staining while maintaining insulin staining compared with ZnT5 +/+(WT)/NOD littermates (Figs. 3 and 5). The efficacy of ZnT5 +/−/NOD heterozygous littermates was intermediate, demonstrating a gene dosage effect.
FIGURE 5.
Diabetes incidence (A) and mortality (B) in NOD mice with ZnT5 KO (Expt. 8). ZnT5 KO/NOD mice were generated and intercrossed to provide ZnT5 +/+/NOD, ZnT5 +/−/NOD, and ZnT5 −/−/NOD littermates. Blood glucose was monitored weekly and mice that demonstrated akinesia with continued prodding by an observer unaware of the treatment conditions were killed. (A) The number of mice whose blood glucose remained <2.5 g/L for 2 consecutive weeks was plotted as a function of weeks of age. (B) The number of surviving mice was plotted as a function of weeks of age. *Different from ZnT5 +/+/NOD mice, P < 0.05, n = 16. NOD, nonobese diabetic.
Discussion
In this study, we have shown that: 1) Zn2+ entered insulinoma cells primarily through voltage-gated Ca2+ channels, increasing [Zn2+]i, and H2O2 also increased [Zn2+]i; 2) blocking L- and T-type Ca2+ channels attenuated Zn2+ entry and Zn2+ or streptozotocin toxicity; 3) Zn2+ chelation, sirtuin inhibition, pyruvate, nicotinamide, and NAD+ prevented NAD+ loss and toxicity associated with Zn2+, streptozotocin, or cytokine exposures in insulinoma cultures or isolated islets; 4) punctate Zn2+ staining increased in the islets of aged NOD mice; 5) pyruvate attenuated the loss of β-cell mass, diabetes incidence, and death in this model, without changing lymphocyte infiltration, plasma cytokine concentrations, or Zn2+ staining; 6) ZnR and ZnT5 KO mice on an NOD background had decreased pancreatic Zn2+ staining, β-cell loss, diabetes incidence, and/or the immune response, and death in the NOD mouse model; and 7) oral or i.p. Zn2+ supplementation potentiated diabetes incidence and mortality.
Mechanisms of Zn2+ toxicity and amelioration by nicotinamide and pyruvate.
In neurons, an increase in [Zn2+]i causes a loss of NAD+ concentrations, resulting in a decrease in the NAD+:NADH ratio and inhibition of the ratio-sensitive enzymes GAPDH and pyruvate dehydrogenase, which causes triosephosphate accumulation. Pyruvate, nicotinamide, or exogenous NAD+ restore intracellular NAD concentrations ([NAD+]i) and glycolytic flux and thereby attenuate death. Pyruvate is converted to lactate, regenerating NAD+ at the expense of NADH (9, 10). A reduced Zn2+ diet, Zn2+ chelation, pyruvate, nicotinamide, or exogenous NAD+ prevent Zn2+ neurotoxicity in vitro or in vivo (where tested) after many injuries. These include: serum deprivation; Zn2+ or ROS exposure; global, focal, and retinal ischemias; hypoglycemia; head trauma; or target deprivation (9–11, 20, 39–41).
Here, we demonstrated a similar mechanism in β-cells. Pyruvate and lactate, but not nicotinamide or NAD+, can be used as the sole energy source in MIN6 cells. Pyruvate regenerates NAD+ in β-cells and is metabolized by their mitochondria (42). The inability of lactate and the ability of pyruvate, nicotinamide, or NAD+ to attenuate Zn2+, cytokine, and streptozotocin toxicities in insulinoma cultures, coupled with the potentiation of toxicities by the NAD+ antagonist 3-acetylpyridine, showed that intracellular NAD+ concentrations are involved in the mechanism. Loss of NAD+ resulting in decreased glycolysis occurs in islets exposed to streptozotocin (12). The changes in [NAD+]i were not always proportional to the changes in cell survival with each therapeutic compound, indicating that additional pathways were important for the therapeutic effects of pyruvate and sirtinol (Fig. 2A,C;Table 1). Pyruvate also inactivates H2O2 and activates mitochondria (43), and Sir2 proteins can mediate transcriptional modulation (44). The reduction in NOD-induced hyperglycemia by systemic pyruvate is striking, because it is the substrate and signal for gluconeogenesis in the liver and induces glucagon release from α-cells (45). Pyruvate did not affect the immune response, as evidenced by equivalent lymphocyte infiltration and the absence of an effect on plasma cytokine concentrations. Pyruvate also did not affect punctate islet Zn2+ staining, together suggesting that it acted downstream of the immune response and the increase in Zn2+ staining.
Nicotinamide can induce increased synthesis of NAD+ or decrease its degradation by NAD+-catabolizing enzymes (10). It is effective in the streptozotocin and NOD mouse models of T1DM (46), with partial therapeutic effects observed only if it is given to recently diagnosed T1DM patients (47). Both nicotinamide and NAD+ attenuated Zn2+, cytokine, or streptozotocin toxicities and restored [NAD+]i. Pharmacologic, genetic, diabetic, or aging induced inhibition of the rate-limiting enzyme in NAD+ synthesis, nicotinamide phosphoribosyl transferase, in islets or in vivo also causes reduced NAD+ concentrations, reduced glucose tolerance, and reduced glucose-induced insulin secretion. These deficits could be restored by giving the product of the nicotinamide phosphoribosyl transferase enzyme (48, 49). The protective effects of sirtinol and Naph and the antagonistic effects of resveratrol suggested that the NAD+-catabolic sirtuin pathway was involved.
Sirtuins are NAD+-dependent protein deacetylases that transfer modulatory acetyl groups from lysine residues of histones or transcription factors to NAD+, resulting in NAD+ catabolism and transcriptional regulation [for review, see (44)]. Overexpression of SIRT-1 in β-cells causes increased basal levels of glucose-induced insulin secretion (50). We showed that overexpression of SIRT-1 in β-cells in vitro made them more susceptible to Zn2+, streptozotocin, and cytokine toxicity (51). We have implicated the sirtuin pathway in Zn2+ and ROS-mediated neurotoxicity (10, 11). Sirtuins appeared to mediate part of the NAD+ loss after Zn2+ and streptozotocin exposures of MIN6 cells, because NAD concentrations were partially restored by sirtuin inhibition (Table 2). Transcriptional modulation may be the predominant mechanism of sirtuins against cytokines and Zn2+ neurotoxicity.
Previous studies showed that 2 mg Zn2+/kg diet can significantly decrease the Zn2+ concentration in the serum and brain and switching back to a Zn2+-adequate diet can restore Zn2+ concentrations (52, 53). This ZnR diet, when used chronically, attenuated pancreatic Zn2+ staining and β-cell loss, whereas excess oral or i.p. Zn2+ potentiated diabetes incidence. These results, and those showing the efficacy of Zn2+ chelators against streptozotocin-induced diabetes (5), suggest that a diabetes-induced increase in β-cell Zn2+ accumulation is toxic. Chronic Zn2+ deficiency can also attenuate the immune response, depending on cell type, concentration, and conditions [for review, see (54)]. We observed a reduction in lymphocyte infiltration and serum cytokine concentrations, suggesting this mechanism is involved in the beneficial effects of ZnR on diabetes. In contrast, oral Zn2+ supplementation studies previously suggested that Zn2+ may be beneficial against diabetic models. Short-term Zn2+ exposures may mediate beneficial effects in the periphery, rather than in the pancreas, through the insulin receptor or by induction of metallothionein I/II preconditioning (55, 56). That i.p. ZnSO4 supplementation had a dramatic detrimental effect only in NOD mice suggested that i.p. Zn2+ is more potent than dietary Zn2+ excess. Pancreatic Zn2+ concentrations are required for insulin packaging (1 mg Zn2+/kg diet potentiated NOD diabetes incidence). This explains why the β-cell Zn2+ pool is one of the last labile, stainable Zn2+ pools to be depleted during ZnR [for review, see (57)]. However, 60 mg Zn2+/kg diet increased NOD diabetes incidence compared with 2 mg Zn2+/kg diet, demonstrating a sharp susceptibility curve for dietary Zn2+ and ongoing T1DM.
Physiologically, it is beneficial to have ∼60 mg Zn2+/kg diet to support the immune system, gut, and tissues that have cellular turnover [for review, see (54)]. However, patho-physiologically, in tissues that have an abundance of releasable Zn2+ and do not require Zn2+ for cellular turnover (pancreas, brain, eye), reducing dietary Zn2+ to just above that which is required for cell survival causes a reduction in the releasable labile Zn2+. Most Zn2+ in the body is tightly bound to proteins serving a structural or catalytic role, and this Zn2+ does not appreciably stain using dyes. We think the loss of labile, stainable, releasable Zn2+ reduces the Zn2+ concentration achieved in the susceptible tissues during pathological attack below that required for further toxicity (11, 53, 58). Dietary Zn2+ deficiency occurs in many elderly adults (59), but T1DM affects primarily adolescents and young adults. Therefore, we suggest that ZnR can attenuate T1DM by reducing both the immune attack and the noninsulin-bound labile Zn2+ released from β-cells causing toxicity. ZnR may also be effective against other autoimmune diseases.
We wanted to genetically reduce pancreatic Zn2+ concentrations. The effects of ZnT5 KO on NOD-induced diabetes were to reduce diabetes incidence, mortality, and pancreatic Zn2+ staining. The effects of ZnT5 KO on the immune response are under study. ZNT5 is ubiquitously expressed in many cell types but is highly expressed in the pancreatic Golgi. Here, it is likely involved in maintaining Zn2+ concentrations sufficient to allow loading into newly synthesized proteins and granular packaging. ZnT5 KO mice do not develop diabetes spontaneously, though a percentage of male mice have sudden cardiac arrest and mortality. ZNT5 expression is ubiquitous but highest in pancreas (24). However, neither ZnT5 KO nor ZnT8 KO induces diabetes and both demonstrated a modest decrease in granular Zn2+ staining, perhaps reflecting a loss of labile Zn2+ (28, 29). Therefore, multiple Zn2+ transporters maintain the Zn2+ concentrations necessary for sufficient insulin processing and packaging.
Our studies have implicated novel therapeutic targets for the prevention of ongoing diabetes: reduction of dietary Zn2+, partial Zn2+ chelation, modulation of Zn2+ transporters, inhibition of Ca2+ channels, maintenance of [NAD+]i, and pyruvate supplementation. This is especially relevant, because pyruvate, Zn2+ chelation, and modulation of Zn2+ transporters also prevent the Zn2+-dependent neuronal injury associated with hypoglycemia resulting from aggressive insulin therapy in animal models (41, 60). Oral pyruvate is used routinely in foods and as a body-building supplement without symptoms and i.p. pyruvate has been used in multiple animal models of injuries without off-target effects (11, 39, 41). Pyruvate is not effective orally in attenuating injury; therefore, a pyruvate/insulin s.c. injection should be clinically tested.
Acknowledgments
The authors thank Dr. Hilary Thompson (Louisiana State University Health Sciences Center) for help with statistical analyses, Connie Marshall (Washington University) for help with islet isolation, Dr. Toshihiro Tanaka (RIKEN Institute, Japan) for the ZnT5 KO animal line, and Dr. John Corbett (Washington University) for the MIN6 cell line. C.T.S. is the guarantor of this manuscript and had primary responsibility for research design and conduct, writing, and final content; T.T. performed the [Zn2+]i measurements and analyzed data; J.Z. and W.Z. performed the Zn2+ reduced and Zn2+ excess NOD trials and analyzed data; P.J.S. performed some of the MIN6 cell culture experiments and analyzed data; A.-L.C. performed the isolated islet and NAD+ measurement experiments and analyzed data; and L.L. performed the ZnT5 KO/NOD experiments and analyzed data. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: GD, glucose deprivation; HD, high-density culture; KO, knockout; MTT, 3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide; [NAD+]i, intracellular NAD concentration; Naph, 2-hydroxynaphthaldehyde; NOD, nonobese diabetic; RFU, relative fluorescence unit; ROS, reactive oxygen species; T1DM, type 1 diabetes; T2DM, type 2 diabetes; TPEN, N,N,N'N’-tetrakis(-)[2-pyridylmethyl]-ethylenediamine; TSQ, N-(6-methoxy-quinolyl)-para-toluenesulfonamide; WT, wildtype; [Zn2+]i, intracellular zinc concentration; ZnR, reduced-zinc diet; ZnT, zinc transporter; ZP1, ZinPyr-1.
Literature Cited
- 1.Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–7 [DOI] [PubMed] [Google Scholar]
- 2.Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:481–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim BJ, Kim YH, Kim S, Kim JW, Koh JY, Oh SH, Lee MK. Kim KW, Lee MS. Zinc as a paracrine effector in pancreatic islet cell death. Diabetes. 2000;49:367–72 [DOI] [PubMed] [Google Scholar]
- 4.Chang I, Cho N. Koh JY, Lee MS. Pyruvate inhibits zinc-mediated pancreatic islet cell death and diabetes. Diabetologia. 2003;46:1220–7 [DOI] [PubMed] [Google Scholar]
- 5.Priel T. Aricha-Tamir B, Sekler I. Clioquinol attenuates zinc-dependent beta-cell death and the onset of insulitis and hyperglycemia associated with experimental type I diabetes in mice. Eur J Pharmacol. 2007;565:232–9 [DOI] [PubMed] [Google Scholar]
- 6.Mathis D. Vence L, Benoist C. beta-Cell death during progression to diabetes. Nature. 2001;414:792–8 [DOI] [PubMed] [Google Scholar]
- 7.Bunik VI. 2-Oxo acid dehydrogenase complexes in redox regulation. Eur J Biochem. 2003;270:1036–42 [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez C, Menissier De Murcia J, Janiak P, Bidouard JP, Beauvais C, Karray S. Garchon HJ, Levi-Strauss M. Unexpected sensitivity of nonobese diabetic mice with a disrupted poly(ADP-Ribose) polymerase-1 gene to streptozotocin-induced and spontaneous diabetes. Diabetes. 2002;51:1470–6 [DOI] [PubMed] [Google Scholar]
- 9.Sheline CT. Behrens MM, Choi DW. Zinc-induced cortical neuronal death: contribution of energy failure attributable to loss of NAD(+) and inhibition of glycolysis. J Neurosci. 2000;20:3139–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai AL. Zipfel GJ, Sheline CT. Zinc neurotoxicity is dependent on intracellular NAD levels and the sirtuin pathway. Eur J Neurosci. 2006;24:2169–76 [DOI] [PubMed] [Google Scholar]
- 11.Sheline CT, Cai AL. Zhu J, Shi C. Serum or target deprivation-induced neuronal death causes oxidative neuronal accumulation of Zn2+ and loss of NAD+. Eur J Neurosci. 2010;32:894–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strandell E, Eizirik DL. Korsgren O, Sandler S. Functional characteristics of cultured mouse pancreatic islets following exposure to different streptozotocin concentrations. Mol Cell Endocrinol. 1988;59:83–91 [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto H. Uchigata Y, Okamoto H. Streptozotocin and alloxan induce DNA strand breaks and poly(ADP- ribose) synthetase in pancreatic islets. Nature. 1981;294:284–6 [DOI] [PubMed] [Google Scholar]
- 14.Mabley JG, Hasko G, Liaudet L, Soriano FG, Southan GJ. Salzman AL, Szabo C. NFkappaB1 (p50)-deficient mice are not susceptible to multiple low-dose streptozotocin-induced diabetes. J Endocrinol. 2002;173:457–64 [DOI] [PubMed] [Google Scholar]
- 15.Mendola J, Wright JR, Jr, Lacy PE. Oxygen free-radical scavengers and immune destruction of murine islets in allograft rejection and multiple low-dose streptozocin-induced insulitis. Diabetes. 1989;38:379–85 [DOI] [PubMed] [Google Scholar]
- 16.Formby B. Schmid-Formby F, Grodsky GM. Relationship between insulin release and 65zinc efflux from rat pancreatic islets maintained in tissue culture. Diabetes. 1984;33:229–34 [DOI] [PubMed] [Google Scholar]
- 17.Schott-Ohly P, Lgssiar A, Partke HJ, Hassan M. Friesen N, Gleichmann H. Prevention of spontaneous and experimentally induced diabetes in mice with zinc sulfate-enriched drinking water is associated with activation and reduction of NF-kappa B and AP-1 in islets, respectively. Exp Biol Med (Maywood). 2004;229:1177–85 [DOI] [PubMed] [Google Scholar]
- 18.Taylor CG. Zinc, the pancreas, and diabetes: insights from rodent studies and future directions. Biometals. 2005;18:305–12 [DOI] [PubMed] [Google Scholar]
- 19.Ahn YH, Kim YH. Hong SH, Koh JY. Depletion of intracellular zinc induces protein synthesis-dependent neuronal apoptosis in mouse cortical culture. Exp Neurol. 1998;154:47–56 [DOI] [PubMed] [Google Scholar]
- 20.Koh JY, Suh SW, Gwag BJ, He YY. Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–6 [DOI] [PubMed] [Google Scholar]
- 21.Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr. 2009;29:153–76 [DOI] [PubMed] [Google Scholar]
- 22.Liuzzi JP, Bobo JA, Lichten LA. Samuelson DA, Cousins RJ. Responsive transporter genes within the murine intestinal-pancreatic axis form a basis of zinc homeostasis. Proc Natl Acad Sci USA. 2004;101:14355–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gyulkhandanyan AV, Lee SC, Bikopoulos G. Dai F, Wheeler MB. The Zn2+-transporting pathways in pancreatic beta-cells: a role for the L-type voltage-gated Ca2+ channel. J Biol Chem. 2006;281:9361–72 [DOI] [PubMed] [Google Scholar]
- 24.Kambe T, Narita H, Yamaguchi-Iwai Y, Hirose J, Amano T, Sugiura N, Sasaki R, Mori K. Iwanaga T, Nagao M. Cloning and characterization of a novel mammalian zinc transporter, zinc transporter 5, abundantly expressed in pancreatic beta cells. J Biol Chem. 2002;277:19049–55 [DOI] [PubMed] [Google Scholar]
- 25.Chimienti F, Devergnas S. Favier A, Seve M. Identification and cloning of a beta-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes. 2004;53:2330–7 [DOI] [PubMed] [Google Scholar]
- 26.Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA. 2007;104:17040–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–5 [DOI] [PubMed] [Google Scholar]
- 28.Nicolson TJ, Bellomo EA, Wijesekara N, Loder MK, Baldwin JM, Gyulkhandanyan AV, Koshkin V, Tarasov AI, Carzaniga R, Kronenberger K, et al. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes. 2009;58:2070–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wijesekara N, Dai FF, Hardy AB, Giglou PR, Bhattacharjee A, Koshkin V, Chimienti F, Gaisano HY. Rutter GA, Wheeler MB. Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia. 2010;53:1656–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheline CT, Takata T, Ying H, Canzoniero LM, Yang A. Yu SP, Choi DW. Potassium attenuates zinc-induced death of cultured cortical astrocytes. Glia. 2004;46:18–27 [DOI] [PubMed] [Google Scholar]
- 31.McDaniel ML, Colca JR. Kotagal N, Lacy PE. A subcellular fractionation approach for studying insulin release mechanisms and calcium metabolism in islets of Langerhans. Methods Enzymol. 1983;98:182–200 [DOI] [PubMed] [Google Scholar]
- 32.Li X. Chen H, Epstein PN. Metallothionein protects islets from hypoxia and extends islet graft survival by scavenging most kinds of reactive oxygen species. J Biol Chem. 2004;279:765–71 [DOI] [PubMed] [Google Scholar]
- 33.Haugland RP. Handbook of fluorescent probes and research chemicals. Eugene (OR): Molecular Probes; 2005 [Google Scholar]
- 34.Dalton T, Fu K. Palmiter RD, Andrews GK. Transgenic mice that overexpress metallothionein-I resist dietary zinc deficiency. J Nutr. 1996;126:825–33 [DOI] [PubMed] [Google Scholar]
- 35.Klein JP, Moeschberger ML. Survival analysis: techniques for censored and truncated data. New York: Springer-Verlag; 1997 [Google Scholar]
- 36.Davis SR. McMahon RJ, Cousins RJ. Metallothionein knockout and transgenic mice exhibit altered intestinal processing of zinc with uniform zinc-dependent zinc transporter-1 expression. J Nutr. 1998;128:825–31 [DOI] [PubMed] [Google Scholar]
- 37.Cesur S, Kocaturk PA, Kavas GO, Aksaray S. Tezeren D, Ciftci U. Serum copper and zinc concentrations in patients with brucellosis. J Infect. 2005;50:31–3 [DOI] [PubMed] [Google Scholar]
- 38.Messer HH. Murray EJ, Goebel NK. Removal of trace metals from culture media and sera for in vitro deficiency studies. J Nutr. 1982;112:652–7 [DOI] [PubMed] [Google Scholar]
- 39.Lee JY. Kim YH, Koh JY. Protection by pyruvate against transient forebrain ischemia in rats. J Neurosci. 2001;21:RC171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suh SW, Garnier P, Aoyama K. Chen Y, Swanson RA. Zinc release contributes to hypoglycemia-induced neuronal death. Neurobiol Dis. 2004;16:538–45 [DOI] [PubMed] [Google Scholar]
- 41.Suh SW, Aoyama K, Matsumori Y. Liu J, Swanson RA. Pyruvate administered after severe hypoglycemia reduces neuronal death and cognitive impairment. Diabetes. 2005;54:1452–8 [DOI] [PubMed] [Google Scholar]
- 42.Rocheleau JV, Head WS, Nicholson WE. Powers AC, Piston DW. Pancreatic islet beta-cells transiently metabolize pyruvate. J Biol Chem. 2002;277:30914–20 [DOI] [PubMed] [Google Scholar]
- 43.Kauppinen RA, Nicholls DG. Synaptosomal bioenergetics. The role of glycolysis, pyruvate oxidation and responses to hypoglycaemia. Eur J Biochem. 1986;158:159–65 [DOI] [PubMed] [Google Scholar]
- 44.Blander G, Guarente L. The sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–35 [DOI] [PubMed] [Google Scholar]
- 45.Ishihara H, Maechler P, Gjinovci A. Herrera PL, Wollheim CB. Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nat Cell Biol. 2003;5:330–5 [DOI] [PubMed] [Google Scholar]
- 46.O'Brien BA, Harmon BV. Cameron DP, Allan DJ. Nicotinamide prevents the development of diabetes in the cyclophosphamide-induced NOD mouse model by reducing beta-cell apoptosis. J Pathol. 2000;191:86–92 [DOI] [PubMed] [Google Scholar]
- 47.Pozzilli P. Browne PD, Kolb H. Meta-analysis of nicotinamide treatment in patients with recent-onset IDDM. The nicotinamide trialists. Diabetes Care. 1996;19:1357–63 [DOI] [PubMed] [Google Scholar]
- 48.Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14:528–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Meneur C. Permutt MA, Imai S. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–17 [DOI] [PubMed] [Google Scholar]
- 51.Sheline C. Involvement of SIRT1 in Zn2+, steptozotocin, non-obese diabetic, and cytokine-mediated toxicities of beta-cells. J Diab Metab. 2012;3:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takeda A, Hirate M, Tamano H, Nisibaba D, Oku N. Susceptibility to kainate-induced seizures under dietary zinc deficiency. J Neurochem. 2003;85:1575–80 [DOI] [PubMed] [Google Scholar]
- 53.Suh SW, Won SJ, Hamby AM, Yoo BH, Fan Y, Sheline CT, Tamano H. Takeda A, Liu J. Decreased brain zinc availability reduces hippocampal neurogenesis in mice and rats. J Cereb Blood Flow Metab. 2009;29:1579–88 [DOI] [PubMed] [Google Scholar]
- 54.Haase H, Rink L. Functional significance of zinc-related signaling pathways in immune cells. Annu Rev Nutr. 2009;29:133–52 [DOI] [PubMed] [Google Scholar]
- 55.Ilouz R, Kaidanovich O. Gurwitz D, Eldar-Finkelman H. Inhibition of glycogen synthase kinase-3beta by bivalent zinc ions: insight into the insulin-mimetic action of zinc. Biochem Biophys Res Commun. 2002;295:102–6 [DOI] [PubMed] [Google Scholar]
- 56.Sitasawad S, Deshpande M, Katdare M. Tirth S, Parab P. Beneficial effect of supplementation with copper sulfate on STZ- diabetic mice (IDDM). Diabetes Res Clin Pract. 2001;52:77–84 [DOI] [PubMed] [Google Scholar]
- 57.Wastney ME, House WA. Development of a compartmental model of zinc kinetics in mice. J Nutr. 2008;138:2148–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi DW, Koh JY. Zinc and brain injury. Annu Rev Neurosci. 1998;21:347–75 [DOI] [PubMed] [Google Scholar]
- 59.Ervin RB, Kennedy-Stephenson J. Mineral intakes of elderly adult supplement and non-supplement users in the third national health and nutrition examination survey. J Nutr. 2002;132:3422–7 [DOI] [PubMed] [Google Scholar]
- 60.Suh SW, Hamby AM, Gum ET, Shin BS, Won SJ, Sheline CT. Chan PH, Swanson RA. Sequential release of nitric oxide, zinc, and superoxide in hypoglycemic neuronal death. J Cereb Blood Flow Metab. 2008;28:1697–706 [DOI] [PubMed] [Google Scholar]