Abstract
Linoleic acid (LA) and α-linolenic acid (ALA) are essential fatty acids that play an important role in modulation of T cell proliferation. The effects of consuming novel soybean oils varying in LA:ALA ratios on T cell proliferation and inflammatory responses were assessed in older adults. Eighteen participants (>50 y old) with elevated cholesterol concentrations (3.37–4.14 mmol/L LDL cholesterol) consumed 5 experimental diets in random order for periods of 35 d. Each diet contained 30% of energy as fat, two-thirds of which was high-oleic acid soybean oil (HiOleic-SO), soybean oil (SO), low-SFA soybean oil (LoSFA-SO), hydrogenated soybean oil (Hydrog-SO), or low-ALA soybean oil (LoALA-SO), resulting in LA:ALA ratios of 2.98, 8.70, 9.69, 15.2, and 18.3, respectively. Participants had higher proliferative responses to phytohemagglutinin (PHA) compared with baseline following consumption of SO (26%; P < 0.05), LoSFA-SO (22%; P < 0.05), or HiOleic-SO (24%; P < 0.05) diets. Proliferative response was similar to the baseline after participants consumed diets with an LA:ALA ratio >10 (Hydrog-SO and LoALA-SO). Post-diet intervention, LA:ALA ratios correlated with proliferative responses to PHA (r = −0.87; P = 0.05). An optimal proliferative response was observed at an LA:ALA ratio of 8.70, with an inverse correlation between proliferative response and LA:ALA ratios >8.70. These effects were independent of changes in the production of PGE2, inflammatory cytokines, or cytokines involved in growth of lymphocytes. These data suggest that the LA:ALA ratio modulates the proliferative ability of T lymphocytes, which may be due to subtle changes in fatty acid composition of the phospholipids in immune cells.
Introduction
In the United States, the dietary (n-6):(n-3) fatty acid ratio is estimated to be ∼10:1, with an (n-3) fatty acid intake of ~1.6 g/d (0.7% of energy) (1). Some investigators have suggested an optimal (n-6):(n-3) fatty acid ratio of 6:1 (2). Higher ratios of (n-6):(n-3) fatty acid were associated with adverse effects on bone mineral density (3) and risk of prostate cancer (4). However, the FAO recently reported that there is no rationale for a specific recommendation for the (n-6):(n-3) fatty acid ratio if the (n-6) fatty acid intake is 2.5–9% energy and the (n-3) fatty acid intake is 0.5–2% energy (5). Likewise, the 2010 Dietary Guidelines for Americans (6) concluded that there are insufficient data to justify a specific recommendation for a dietary (n-6):(n-3) fatty acid ratio. For cardiovascular health, evidence suggests that both linoleic acid [LA7; 18:2(n-6)] and α-linolenic acid [ALA; 18:3(n-3)] have a role in reducing the risk of coronary heart disease (7, 8) and that the absolute amount consumed (of these essential fatty acids) should be emphasized rather than the ratio of (n-6):(n-3) fatty acids (2).
Inflammatory and T cell-mediated responses are dysregulated in older adults (9). In older adults with hypercholesterolemia, modulation of inflammatory and immune responses may be of more importance, because hypercholesterolemia has been associated with higher risk of infection in animal models (10, 11). The types and amounts of dietary fatty acids can modulate inflammation and T cell-mediated immune response; however, the reported results have not been always consistent. A diet high in ALA [ALA, 6.5% of energy and an (n-6):(n-3) fatty acid ratio of 2:1] decreased the production of the proinflammatory cytokines IL-6, IL-1β, and TNF -α by peripheral blood mononuclear cells (PBMC) in hypercholesterolemic participants compared with a diet high in LA [LA, 12.6% of energy and an (n-6):(n-3) fatty acid ratio of 4:1] or the average American diet [LA, 7.7% and ALA, 0.8% of energy and an (n-6):(n-3) fatty acid ratio of 10:1] (12). A diet containing flaxseed oil, which is high in ALA, suppressed the proliferation of PBMC and the delayed type hypersensitivity (DTH) response in healthy adults (13). Therefore, a high intake of ALA might have an inhibitory effect on inflammatory and T cell-mediated responses. However, the high ALA diets used in the above studies are impractical, because the amount of ALA or oils used in the study are higher than the average intake of the U.S. population. In a placebo-controlled, double-blind study that involved participants 25–72 y old, supplementation of 4.5 or 9.5 g/d of ALA or 0.77 or 1.7 g/d EPA + DHA for 6 mo did not alter immune functions as determined by the proliferative response of PBMC and the in vivo DTH response (14). Furthermore, results from an observational study by Merchant et al. (15) reported that participants consuming higher levels of ALA had a lower risk of pneumonia.
Newer plant breeding techniques have made it possible to alter the fatty acid composition of vegetable oils. In this study, we used selectively bred and genetically modified soybean oils distinguished by various fatty acid compositions, resulting in different dietary LA:ALA ratios. The diets used in this study contained ALA close to the average intake of the U.S. population (1). We assessed the impact of varying LA:ALA ratios on inflammatory and T cell-mediated immune responses in hypercholesterolemic older adults using a double-blind, randomized design.
Participants and Methods
Participants.
Eleven women and 7 men older than 50 y with moderately elevated LDL cholesterol concentrations (3.37–4.14 mmol/L) were included in the study. The number of participants used was based on the previous study with a similar design (16). Consistent with the inclusion criteria, none of the study participants had evidence of chronic illnesses, including endocrine, hepatic, renal, thyroid, or cardiac dysfunction, and all had normal fasting serum glucose concentrations. None of the participants smoked. Further, no participant was taking medications known to affect serum lipid concentrations, nonsteroidal antiinflammatory drugs such as aspirin, or dietary supplements known to affect immune functions. All women were postmenopausal and none were taking hormone replacement therapy. The characteristics of the participants at the time of screening are shown in Table 1. This protocol was approved by the Human Investigation Review Committee of Tufts Medical Center and Tufts University and all participants gave written informed consent. A portion of these data, addressing a different experimental question, was previously published (17).
TABLE 1.
Anthropometrics and serum lipid profiles of the participants at the time of screening1
| Characteristics | Women | Men | All participants |
| (n = 11) | (n = 7) | (n = 18) | |
| Age, y | 62.4 ± 2.4 | 64.2 ± 4.9 | 63.1 ± 2.3 |
| BMI, kg/m2 | 26.8 ± 1.3 | 26.4 ± 1.4 | 26.7 ± 0.9 |
| Total cholesterol, mmol/L | 6.01 ± 0.23 | 5.91 ± 0.31 | 5.96 ± 0.18 |
| VLDL cholesterol, mmol/L | 0.78 ± 0.08 | 0.85 ± 0.13 | 0.80 ± 0.08 |
| LDL cholesterol, mmol/L | 3.81 ± 0.23 | 3.81 ± 0.39 | 3.81 ± 0.21 |
| HDL cholesterol, mmol/L | 1.40 ± 0.13 | 1.24 ± 0.08 | 1.34 ± 0.08 |
| TG, mmol/L | 1.56 ± 0.20 | 1.64 ± 0.23 | 1.58 ± 0.15 |
Values are mean ± SE, n = 18.
Study design and diets.
Study participants were provided in random order with each of the 5 experimental diets varying in the major source of fat for periods of 35 d/diet phase. The participants, investigators, and laboratory personnel were unaware of the order and identification of the diet phases. A minimum interval of 2 wk occurred between each diet phase. During these periods, the participants consumed their habitual diets. Additionally, the participants were encouraged to maintain their customary level of physical activity throughout the study period.
Each diet was designed to provide 30% of energy as fat in which two-thirds of the fat was contributed by the experimental oils. The experimental oils, provided by Solae Company, were: soybean oil (SO), low SFA soybean oil (LoSFA-SO) developed by selective breeding, high oleic acid soybean oil (HiOleic-SO) developed by genetic modification, and low-ALA soybean oil (LoALA-SO) developed by selective breeding. Hydrogenated soybean oil (Hydrog-SO) was a commercially available product (Whirl, Proctor and Gamble). The nutrient composition of the experimental diets is presented in Table 2. All food and drink, provided by the Metabolic Research Unit of the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, were consumed on site or packaged for take-out. Participants were required to consume all food and drink provided to them and were not allowed to supplement their diets with anything other than water and noncaloric beverages. Initial energy levels were estimated with use of the Harris-Benedict formula and were adjusted, when necessary, to maintain body weight. Daily energy intakes (mean ± SE) were 2110 ± 60 kcal for the women and 2860 ± 260 kcal for the men. Analysis of protein, carbohydrate, fatty acid, and cholesterol contents of the diets were performed by Covance Laboratories (17).
TABLE 2.
Nutrient composition of the experimental diets1
| Nutrient composition | SO | HiOleic-SO | LoSFA-SO | Hydrog-SO | LoALA-SO |
| % of Energy | |||||
| Carbohydrate | 52.1 | 54.6 | 53.5 | 53.9 | 52.1 |
| Protein | 16.7 | 17.7 | 17.0 | 17.2 | 16.8 |
| Total fat | 31.2 | 29.0 | 29.5 | 28.9 | 31.1 |
| SFA | 6.52 | 5.76 | 4.91 | 7.25 | 6.75 |
| 14:0 | 0.46 | 0.50 | 0.45 | 0.48 | 0.49 |
| 16:0 | 3.48 | 2.78 | 2.10 | 3.48 | 3.58 |
| 18:0 | 1.34 | 1.17 | 1.16 | 2.01 | 1.41 |
| MUFA | 6.48 | 18.9 | 6.19 | 9.95 | 6.96 |
| 16:1(n-9) (cis) | 0.15 | 0.13 | 0.10 | 0.12 | 0.14 |
| 18:1(n-9) (cis) | 6.06 | 18.6 | 5.91 | 7.88 | 6.52 |
| 18:1(n-9) (trans) | 0.18 | 0.08 | 0.09 | 1.85 | 0.23 |
| PUFA | 12.7 | 2.82 | 14.6 | 8.66 | 13.5 |
| 18:2(n-6) (cis) | 11.0 | 1.91 | 12.7 | 7.46 | 12.5 |
| 18:2(n-6) (trans) | 0.25 | 0.14 | 0.21 | 0.47 | 0.16 |
| 18:3(n-3) (cis) | 1.26 | 0.64 | 1.31 | 0.49 | 0.68 |
| 18:3(n-3) (trans) | 0.18 | 0.11 | 0.34 | 0.20 | 0.13 |
| Trans fatty acid | 0.61 | 0.33 | 0.64 | 2.45 | 0.52 |
| LA:ALA | 8.70 | 2.98 | 9.69 | 15.2 | 18.3 |
| Cholesterol, mg/1000 kcal | 57.0 | 60.0 | 65.0 | 63.0 | 66.0 |
| Fiber, g/1000 kcal | 13.6 | 14.1 | 14.6 | 13.4 | 14.6 |
HiOleic-SO, high-oleic acid soybean oil; Hydrog-SO, hydrogenated soybean oil; LoALA-SO, low-α-linolenic acid soybean oil; LoSFA-SO, low-SFA soybean oil; SO, soybean oil.
Blood samples.
Fasting blood samples (12 h) were collected at the beginning of the study (baseline) prior to the start of the randomized diet phases and at the end of each diet phase in a serum collection tube or heparin-containing tube [Vacutainer, Becton Dickinson, containing sodium heparin (143 USP units)]. Blood samples were kept at room temperature and used within 6 h.
Lymphocyte proliferation.
Lymphocyte proliferation was measured by [3H] thymidine incorporation after stimulation with T cell mitogens using a modified whole blood assay (18). Heparinized whole blood was diluted 1:5 (v:v) with complete RPMI (RPMI 1640 supplemented with 100 kU/L penicillin, 100 mg/L streptomycin, 2 mmol/L l-glutamine, and 25 mmol/L HEPES; Gibco Laboratories). Then 100 μL of the diluted blood was stimulated with concanavalin A (ConA) or phytohemagglutinin (PHA) and incubated in 96-well, round-bottom plates (200 μL of final culture volume) for 72 h at 37°C in an atmosphere of 5% CO2 and 95% humidity. The final mitogen concentrations were 5, 10, 25, 50, and 100 mg/L for ConA (Sigma Chemical) and 1, 5, 10, and 50 mg/L for PHA (Difco Laboratories). Each well was pulsed with 0.5 Ci of [3H] thymidine (New England Nuclear) in 20 μL for the last 4 h of 72 h of incubation. Cells were harvested onto glass microtiter filter paper using a cell harvester (Cambridge Technologies). Radioactivity incorporation was counted in a liquid scintillation counter (Beckman Instruments). The results are reported as corrected disintegrations per minute (dpm), which is the mean dpm of mitogen-stimulated cultures minus the mean dpm of cultures without mitogens.
IL-2 and GM-CSF production.
Heparinized whole blood was diluted 1:3.6 (v:v) with complete RPMI. Diluted whole blood (900 μL) was stimulated with 100 μL of ConA (40 mg/L final concentration) or PHA (20 mg/L final concentration) solutions and incubated in 24-well, flat-bottom plates for 48 h at 37°C in an atmosphere of 5% CO2 and 95% humidity. Levels of IL-2 and granulocyte-macrophage colony-stimulating factor (GM-CSF) were measured from cell-free supernatants using ELISA according to the manufacturer’s instructions, with mouse anti-human IL-2 and GM-CSF monoclonal antibodies (mAbs) (PharMingen) and biotinylated anti-human IL-2 and GM-CSF mAbs.
PGE2 production.
Heparinized whole blood was diluted 1:3.6 (v:v) with complete RPMI. Diluted whole blood (900 μL) was stimulated with 100 μL of LPS (0.01 or 1 mg/L final concentrations) solution and incubated in 24-well, flat-bottom plates for 24 h at 37°C in an atmosphere of 5% CO2 and 95% humidity. PGE2 was measured from the cell-free supernatants by RIA as previously described (19).
IL-1β, IL-6, and TNFα production.
The culture condition for IL-1β, IL-6, and TNFα was the same as the condition for PGE2 production. IL-1β, IL-6, and TNFα were measured from cell-free supernatants by ELISA according to the manufacturer’s instructions, with mouse anti-human IL-1β (R&D Systems), rat anti-human IL-6, or mouse anti-human TNFα mAbs (PharMingen) and biotinylated goat anti-human IL-1β, biotinilyated rat anti-human IL-6, or biotinylated mouse anti-human TNFα.
Serum lipoprotein cholesterol, CRP concentration, and plasma phospholipid fatty acid analysis.
Serum was separated by centrifugation at 1100 × g at 4°C and assayed for total, HDL, and LDL cholesterol and TG (Roche Diagnostics) on a Hitachi 911 automated analyzer using colorimetric reagents. VLDL cholesterol was calculated as the difference between total cholesterol minus LDL plus HDL cholesterol. The assays were standardized through the Lipid Standardization Program of the CDC. The serum CRP concentration was measured by a Tinaquant CRP (Latex) high-sensitive immunoturbidimetric assay (Roche Diagnostics) using a Hitachi 911 automated analyzer. Plasma phospholipid fatty acid composition was determined as previously described using GC (20).
Statistical analysis.
Data were analyzed using the SYSTAT statistical package (SYSTAT 10.0, 2000; SYSTAT). An ANOVA, with the diet as main effect and participant as the repeated measure, was performed for each outcome, using the General Linear Models procedure, followed by the Fisher’s Least Significant difference test for pairwise comparisons of the 5 experimental diets and baseline. Measures not normally distributed were log-transformed to achieve normal distribution prior to statistical analysis. Pearson correlation was used to determine the association between mean lymphocyte proliferation and the LA:ALA ratio. Significance was set at P < 0.05.
Results
Serum lipoprotein cholesterol and CRP concentration.
Overall, there was an effect of dietary fat on HDL cholesterol concentrations (P < 0.05) (Table 3). The HDL cholesterol concentration was lower after consumption of the LoSFA-SO diet compared with the HiOleic-SO (P < 0.05) and LoALA-SO (P < 0.05) diets. The effect of the diets differing in the experimental oils was not significant for total cholesterol, LDL cholesterol, VLDL cholesterol, TG, or CRP concentrations in this group of study participants.
TABLE 3.
Serum lipid profiles and CRP concentrations at the end of each diet phase in moderately hypercholesterolemic older adults who consumed diets containing novel soybean oils differing in fatty acid composition1
| Variable | SO | HiOleic-SO | LoSFA-SO | Hydrog-SO | LoALA-SO | P value2 |
| Total cholesterol, mmol/L | 5.98 ± 0.21 | 5.93 ± 0.21 | 5.91 ± 0.23 | 6.16 ± 0.21 | 6.01 ± 0.18 | 0.10 |
| LDL cholesterol, mmol/L | 3.81 ± 0.16 | 3.81 ± 0.18 | 3.73 ± 0.21 | 3.91 ± 0.16 | 3.81 ± 0.16 | 0.45 |
| VLDL cholesterol, mmol/L | 0.80 ± 0.13 | 0.70 ± 0.10 | 0.80 ± 0.16 | 0.85 ± 0.13 | 0.80 ± 0.18 | 0.37 |
| HDL cholesterol, mmol/L | 1.40 ± 0.08ab | 1.42 ± 0.08a | 1.37 ± 0.10b | 1.40 ± 0.08ab | 1.42 ± 0.08a | 0.03 |
| TG, mmol/L | 1.75 ± 0.23 | 1.66 ± 0.19 | 1.79 ± 0.25 | 1.76 ± 0.21 | 1.77 ± 0.28 | 0.84 |
| CRP, mg/L | 4.20 ± 1.30 | 5.40 ± 2.00 | 4.50 ± 1.60 | 5.50 ± 1.90 | 4.00 ± 1.30 | 0.56 |
Values are mean ± SE, n = 18. Means in a row without a common letter differ, P < 0.05. HiOleic-SO, high-oleic acid soybean oil; Hydrog-SO, hydrogenated soybean oil; LoALA-SO, low-α-linolenic acid soybean oil; LoSFA-SO, low-SFA soybean oil; SO, soybean oil.
P values are for the effect of the diet from ANOVA with diet as main effect and participant as repeated measures.
Plasma phospholipid fatty acid composition.
Consumption of the HiOleic-SO diet resulted in a significantly higher proportion of MUFA in the phospholipids and consumption of the Hydrog-SO diet resulted in significantly higher incorporation of trans fatty acids (Table 4). Although the differences in the plasma phospholipid (n-6):(n-3) fatty acid ratio were subtle, the varied dietary ratio of LA:ALA was reflected in the plasma fatty acid composition, because a positive correlation between dietary LA:ALA ratio and plasma phospholipid (n-6):(n-3) fatty acid ratio (r = 0.53; P < 0.01) was observed.
TABLE 4.
Plasma phospholipid fatty acid composition at the end of each diet phase in moderately hypercholesterolemic older adults who consumed diets containing novel soybean oils differing in fatty acid composition1
| Fatty acid | SO | HiOleic-SO | LoSFA-SO | Hydrog-SO | LoALA-SO | P value2 |
| mol % | ||||||
| SFA | 49.1 ± 0.47a | 48.3 ± 0.54b | 49.0 ± 0.52ab | 46.6 ± 0.46c | 48.8 ± 0.46ab | 0.01 |
| MUFA | 9.98 ± 0.49c | 16.6 ± 0.72a | 9.72 ± 0.44c | 14.2 ± 0.44b | 9.61 ± 0.40c | 0.01 |
| 18:1(n-9) (cis) | 6.20 ± 0.19b | 12.7 ± 0.69a | 6.09 ± 0.21b | 6.55 ± 0.22b | 6.15 ± 0.16b | 0.01 |
| 18:1(n-9) (trans) | 1.06 ± 0.42b | 0.82 ± 0.14b | 0.97 ± 0.28b | 5.04 ± 0.30a | 0.89 ± 0.33b | 0.01 |
| PUFA | 40.9 ± 0.41a | 35.1 ± 0.64c | 41.2 ± 0.47a | 39.1 ± 0.24b | 41.6 ± 0.42a | 0.01 |
| 18:2(n-6) (cis) | 23.7 ± 0.67ab | 17.3 ± 0.53d | 23.6 ± 0.74b | 22.1 ± 0.54c | 24.6 ± 0.77a | 0.01 |
| 18:2(n-6) (trans) | 0.48 ± 0.07b | 0.53 ± 0.06b | 0.94 ± 0.27a | 1.07 ± 0.05a | 0.53 ± 0.12b | 0.01 |
| 18:3(n-6) (cis) | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.09 ± 0.02 | 0.15 |
| 18:3(n-3) (cis) | 0.24 ± 0.01b | 0.30 ± 0.02a | 0.24 ± 0.01b | 0.21 ± 0.02c | 0.20 ± 0.02c | 0.01 |
| 20:4(n-6) (cis) | 9.17 ± 0.38a | 8.96 ± 0.32a | 9.12 ± 0.37a | 8.43 ± 0.33b | 9.12 ± 0.37a | 0.01 |
| 20:5(n-3) (cis) | 0.54 ± 0.03b | 0.80 ± 0.06a | 0.58 ± 0.04b | 0.51 ± 0.06bc | 0.43 ± 0.04c | 0.01 |
| 22:6(n-3) (cis) | 2.68 ± 0.16ab | 2.75 ± 0.17a | 2.58 ± 0.13abc | 2.50 ± 0.18c | 2.52 ± 0.15bc | 0.03 |
| Trans fatty acid | 1.54 ± 0.48b | 1.34 ± 0.16b | 1.91 ± 0.38b | 6.11 ± 0.34a | 1.42 ± 0.38b | 0.01 |
| (n-6) | 36.3 ± 0.46b | 30.1 ± 0.58d | 36.3 ± 0.49b | 34.2 ± 0.34c | 37.4 ± 0.48a | 0.01 |
| (n-3) | 4.07 ± 0.16b | 4.46 ± 0.20a | 4.03 ± 0.13b | 3.79 ± 0.24c | 3.69 ± 0.17c | 0.01 |
| (n-6):(n-3) ratio | 9.15 ± 0.37b | 6.95 ± 0.30c | 9.19 ± 0.35b | 9.54 ± 0.53b | 10.5 ± 0.50a | 0.01 |
Values are mean ± SE, n = 18. Labeled means in a row without a common letter differ, P < 0.05. HiOleic-SO, high-oleic acid soybean oil; Hydrog-SO, hydrogenated soybean oil; LoALA-SO, low-α-linolenic acid soybean oil; LoSFA-SO, low-SFA soybean oil; SO, soybean oil.
P values are for the effect of the diet from ANOVA with diet as main effect and participant as repeated measures.
Lymphocyte proliferation.
Overall, dietary fat had a significant effect on lymphocyte proliferative response to optimal levels of T cell mitogens (Table 5). The highest proliferative response was observed with 5 mg/L of PHA stimulation among the doses tested (1, 5, 10, and 50 mg/L) and dietary fat had an impact on proliferative response (P < 0.05) to PHA at this level. The proliferative response to 5 mg/L PHA increased after consumption of the SO diet (26%; P < 0.05), HiOleic-SO diet (24%; P < 0.05), and LoSFA-SO diet (22%; P < 0.05) compared with baseline. Following consumption of the LoALA-SO diet, the proliferative response to 5 mg/L PHA was lower compared with the proliferative response after consumption of the SO diet (17%; P < 0.05) and it tended to be lower compared with the responses after consumption of the Hi-Oleic SO diet (15%; P = 0.07) or LoSFA-SO diet (14%; P = 0.09). The proliferative responses to 5 mg/L PHA after consumption of the Hydrog-SO and LoALA SO diets did not differ.
TABLE 5.
Proliferative responses to different concentrations of PHA at baseline and at the end of each diet phase of whole blood from older adults who consumed diets containing novel soybean oils differing in fatty acid composition1
| PHA | Baseline | SO | HiOleic-SO | LoSFA-SO | Hydrog-SO | LoALA-SO | P value2 |
| dpm × 103 | |||||||
| 5 mg/L | 68.6 ± 5.6c | 86.1 ± 7.2a | 84.2 ± 8.3ab | 83.2 ± 6.0ab | 73.4 ± 4.5abc | 71.4 ± 4.5bc | 0.04 |
| 10 mg/L | 59.7 ± 5.1 | 75.0 ± 6.5 | 69.7 ± 6.0 | 73.8 ± 5.6 | 68.3 ± 4.4 | 61.7 ± 2.8 | 0.10 |
| 50 mg/L | 34.3 ± 3.8 | 39.1 ± 5.2 | 35.5 ± 3.5 | 36.1 ± 2.9 | 33.4 ± 2.9 | 30.0 ± 2.0 | 0.40 |
Values are mean ± SE, n = 18. Means in a row without a common letter differ, P < 0.05. Data are corrected dpm, which is the mean dpm of mitogen-stimulated cultures minus the mean dpm of cultures without mitogens. dpm, disintegrations per minute; HiOleic-SO, high-oleic acid soybean oil; Hydrog-SO, hydrogenated soybean oil; LoALA-SO, low-α-linolenic acid soybean oil; LoSFA-SO, low-SFA soybean oil; SO, soybean oil.
P values are for the effect of the diet from ANOVA with diet as main effect and participant as repeated measure.
Lymphocyte proliferative responses to optimal levels of ConA (25 and 50 mg/L) also increased following consumption of the SO diet (21 and 25%, P = 0.08 and P < 0.05, respectively), Hi-Oleic SO diet (31 and 29%, respectively, P < 0.05), and LoSFA SO diet (28 and 25%, respectively, P < 0.05) compared with the baseline (data not shown).
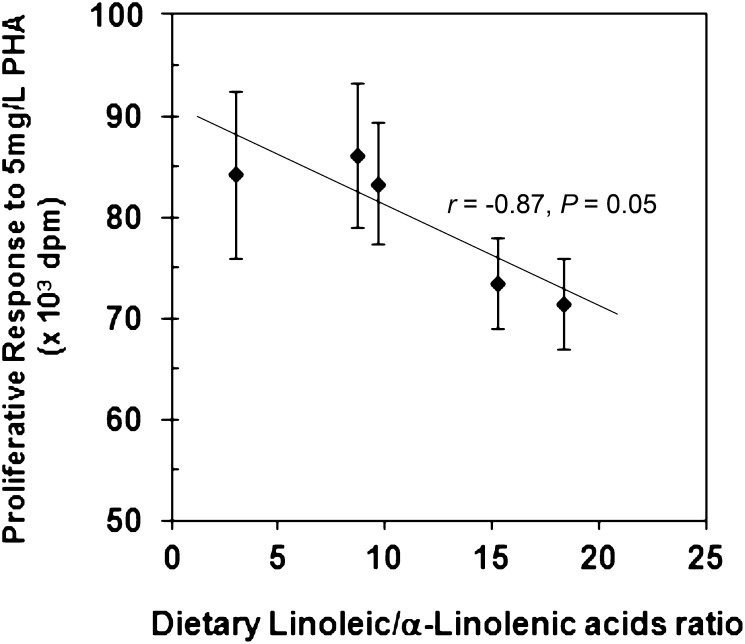
An inverse correlation between the LA:ALA ratio based on the fatty acid composition of the diet and the means of whole blood proliferative response to PHA was observed (r = −0.87; P = 0.05; n = 5) (Fig. 1). The highest proliferative response was observed with consumption of the SO diet, which had the LA:ALA ratio of 8.70, among all the experimental diets. The proliferative response decreased following consumption of diets with an LA:ALA ratio higher than 8.70.
FIGURE 1.
Relationship between the ratio of LA:ALA in the diet and the lymphocyte proliferative response of whole blood culture to 5 mg/L of PHA. Data represent corrected dpm, which are the mean dpm of mitogen-stimulated cultures minus the mean dpm of cultures without mitogens. Values are mean ± SE, n = 18. An inverse correlation between the LA:ALA ratio based on fatty acid composition of the diet and means of whole blood proliferative response to PHA was observed (n = 5). ALA, α-linolenic acid; dpm, disintegrations per minute; LA, linoleic acid; PHA, phytohemagglutinin.
IL-2, GM-CSF, PGE2, IL-1β, IL-6, and TNF#x03B1; production.
No significant differences in IL-2 and GM-CSF production by whole blood culture stimulated with ConA or PHA were observed after consumption of the experimental diets. Likewise, no significant differences in LPS-stimulated PGE2 production from whole blood cultures were observed after consumption of the experimental diets (Supplemental Table 1). Further, there was no significant effect of the experimental diets on the production of proinflammatory cytokines IL-1β, IL-6, and TNFα by whole blood culture stimulated with LPS (Supplemental Table 2).
Discussion
The major finding of this study is that a significant correlation between the dietary LA:ALA ratio and T cell-mediated responses occurs as a result of consuming diets enriched with SO differing in fatty acid compositions. The ability of lymphocytes to proliferate is a key measure of their functionality and ability to fight against invading pathogens. Given that a main biological change associated with aging is a decline in T cell-mediated responses, this finding has important implications for determining the fatty acid requirements of older adults.
Animal and human studies have shown that the type of dietary fatty acids could influence the proliferative response of lymphocytes (13, 21, 22). Increased amounts of ALA in the diet decreased splenocyte proliferation in rodents and PBMC proliferation in humans. In contrast, other research has reported no change in lymphocyte proliferation after 6 mo of 4.5 or 9.5 g/d of ALA intake (14). In our study, consumption of the LoALA-SO diet (ALA, 0.68% of energy in the diet) resulted in a significantly lower proliferative response compared with the response after consumption of SO (ALA, 1.26% of energy in the diet). It is unlikely that this difference was attributable to the low levels of ALA in the diet, because the HiOleic-SO diet with an ALA level of 0.64% of energy had similar levels of proliferative response to the SO diet with an ALA level of 1.26% of energy.
The FAO recently reported that as long as dietary intake of (n-6) fatty acid is 2.5–9% of energy and (n-3) fatty acid is 0.5–2% of energy, there is no rationale for recommending a specific ratio for (n-6):(n-3) fatty acid (5). However, our study results suggest that the (n-6):(n-3) fatty acid ratio may alter T cell-mediated immune responses. The consumption of 2 diets (LoSFA-SO and LoALA-SO) similar in LA content but differing in LA:ALA ratios due to a difference in the ALA content resulted in a significantly different proliferative response. Of note, both diets contained ALA within the recommended FAO level. Hydrogenation of SO resulted in decreases of both LA and ALA, but of a relatively greater magnitude in ALA (from 10.96 to 7.46% of energy from LA and from 1.26 to 0.49% of energy from ALA) and an increase in the LA:ALA ratio. After consumption of the Hydrog-SO diet, the proliferative response was lower compared with the response after SO consumption.
In the present study, an optimal proliferative response was observed at the LA:ALA ratio of 8.70, with an inverse association between proliferative response and the ratio of LA:ALA in the diet at ratios above this level. These effects were independent of changes in PGE2 production or cytokines involved in the growth of lymphocytes, because the dietary fats did not have significant effects on these variables. Dietary ratios of LA:ALA seemed to be reflected in plasma phospholipid (n-6):(n-3) fatty acid ratios, because a positive correlation (r = 0.53; P < 0.01) between dietary LA:ALA ratios and plasma phospholipid (n-6):(n-3) fatty acid ratios was observed. Findings by Kew et al. (14) support the results from our study. A negative correlation was observed between the (n-6):(n-3) fatty acid ratio of PBMC phospholipids and the proliferative response of PBMC to ConA in healthy participants aged 25–72 y.
The dietary LA:ALA ratio was unrelated to the serum lipid profiles in our participants, which is consistent with previously reported results (23). However, the influence of the dietary LA:ALA ratio on proliferative response of the hypercholesterolemic older adults in this study may have important implications. Both hypercholesterolemia and advancing age can contribute to impaired immune response against infections. Higher susceptibility to coxackievirus B, Listeria monocytogenes, and Candida albicans has been reported in animal models of hypercholesterolemia induced by diet or genetic modification (10, 11, 24). Previously, we showed that lowering the dietary intake of fat by switching from a Western diet with 38% energy from fat to a therapeutic lifestyle change diet with 28% fat energy can enhance the cell-mediated immune response, as evidenced by higher proliferative and DTH responses (19). In this study, we showed that modulation of the dietary LA:ALA ratio can alter cell-mediated immune response in the context of a relatively low-fat diet (30% of energy from fat).
No significant effect of the experimental diets was observed on the production of PGE2 and proinflammatory cytokines IL-1β, IL-6, and TNFα by whole blood culture stimulated with LPS. The impact of (n-6) fatty acid and (n-3) fatty acid levels in the diet on the production of proinflammatory cytokines was inconclusive. Studies have shown that a high consumption of (n-3) fatty acid suppresses the inflammatory response (12, 14, 25, 26). ALA is an important precursor of long-chain (n-3) fatty acid synthesis (27). An increased proportion of (n-3) fatty acid in the diet results in the incorporation of (n-3) fatty acid in immune cell phospholipids at the expense of arachidonic acid and therefore reduces the availability of substrate for PGE2 synthesis (28). Zhao et al. (12) reported that consumption of a diet high in ALA with the ratio of (n-6):(n-3) fatty acids at 2:1 for 6 wk reduced the production of IL-1β, IL-6, and TNFα by PBMC of hypercholesterolemic participants. In addition, TNFα production was inversely correlated with ALA concentrations in PBMC. Caughney et al. (25) showed that a high intake of ALA through the consumption of a flaxseed oil-based diet for 4 wk inhibited the production of TNFα, IL-1β, and PGE2. Participants’ intake of ALA in the above 2 studies differed from the intake by participants in our study. Intake of ALA was 6.5% of energy in the ALA diet (17.3 g/d of ALA for a 2400-kcal diet) (12) and 13.7 ± 5.5 g/d in the flaxseed group (25) in the above 2 studies. In our study, the lowest ALA intake was 1.0–1.5 g/d (in the Hydrog-SO diet, 0.49% of energy) and the highest ALA intake was 3.0–4.0 g/d (in the LoSFA-SO diet, 1.31% of energy). Low levels of ALA in the HiOleic-SO (0.64% of energy), Hydrog-SO (0.49% of energy), and LoALA-SO (0.68% of energy) diets did not result in higher production of PGE2 or proinflammatory cytokines. The lack of a significant effect of ALA in our diets on PGE2 or proinflammatory cytokines may have been due to the relatively low levels of ALA in all our diets compared with the levels at which antiinflammatory effects were observed by others. Higher levels of PUFA did not have a significant impact on the production of PGE2 and proinflammatory cytokines, because the production of these mediators was not significantly different between the HiOleic-SO (2.82% PUFA and 18.90% MUFA of energy) and SO diets (12.74% PUFA and 6.48% MUFA of energy) or the LoSFA diet (14.60% PUFA and 6.19% MUFA of energy).
The data from this intervention study suggest that the dietary LA:ALA ratio significantly affects T cell-mediated function, which may be due to subtle changes in fatty acid composition of the phospholipids of immune cells. Given that both advancing age and hypercholesterolemia are associated with impaired T cell-mediated function and higher susceptibility to infection, this finding has significant implications for determining the PUFA requirements of older adults, particularly those with elevated blood lipids.
Acknowledgments
The authors thank Di Wu for his technical assistance and Stephanie Marco for assistance during the preparation of the revised manuscript. A.H.L. and S.N.M. designed research; S.N.H. conducted research; S.N.H., A.H.L., L.M.A., and S.N.M. analyzed data; and S.N.H., A.H.L., and S.N.M. wrote the paper and had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ALA, α-linolenic acid; ConA, concanavalin A; dpm, disintegrations per minute; DTH, delayed type hypersensitivity; GM-CSF, granulocyte-macrophage colony-stimulating factor; HiOleic-SO, high-oleic acid soybean oil; Hydrog-SO, hydrogenated soybean oil; LA, linoleic acid; LoALA-SO, low-α-linolenic acid soybean oil; LoSFA-SO, low-SFA soybean oil; mAbs, monoclonal antibodies; PBMC, peripheral blood mononuclear cell; PHA, phytohemagglutinin; SO, soybean oil.
Literature Cited
- 1.Kris-Etherton PM, Taylor DS, Yu-Poth S, Huth P, Moriarty K, Fishell V, Hargrove RL, Zhao G, Etherton TD. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71:S179–88 [DOI] [PubMed] [Google Scholar]
- 2.Wijendran V, Hayes KC. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu Rev Nutr. 2004;24:597–615 [DOI] [PubMed] [Google Scholar]
- 3.Weiss LA, Barrett-Connor E, von Muhlen D. Ratio of n-6 to n-3 fatty acids and bone mineral density in older adults: the Rancho Bernardo Study. Am J Clin Nutr. 2005;81:934–8 [DOI] [PubMed] [Google Scholar]
- 4.Williams CD, Whitley BM, Hoyo C, Grant DJ, Iraggi JD, Newman KA, Gerber L, Taylor LA, McKeever MG, Freedland SJ. A high ratio of dietary n-6/n-3 polyunsaturated fatty acids is associated with increased risk of prostate cancer. Nutr Res. 2011;31:1–8 [DOI] [PubMed] [Google Scholar]
- 5. FAO. Fats and fatty acids in human nutrition. Report of an expert consultation. Rome: FAO; 2010. [PubMed]
- 6.USDA and U.S. Department of Health and Human Services Dietary Guidelines for Americans, 2010. 7th ed. Washington, DC: U.S. Government Printing Office; 2010 [DOI] [PMC free article] [PubMed]
- 7.Jakobsen MU, O'Reilly EJ, Heitmann BL, Pereira MA, Bälter K, Fraser GE, Goldbourt U, Hallmans G, Knekt P, Liu S, et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr. 2009;89:1425–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr. 2010;91:502–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–4 [DOI] [PubMed] [Google Scholar]
- 10.Loria RM, Kibrick S, Madge GE. Infection of hypercholesteremic mice with Coxsackievirus B. J Infect Dis. 1976;133:655–62 [DOI] [PubMed] [Google Scholar]
- 11.Kos WL, Loria R, Snodgrass MJ, Cohen D, Thorpe TG, Kaplan AM. Inhibition of host resistance by nutritional hypercholesteremia. Infect Immun. 1979;26:658–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao G, Etherton TD, Martin KR, Gillies PJ, West SG, Kris-Etherton PM. Dietary alpha-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am J Clin Nutr. 2007;85:385–91 [DOI] [PubMed] [Google Scholar]
- 13.Kelley DS, Branch LB, Love JE, Taylor PC, Rivera YM, Iacono JM. Dietary alpha-linolenic acid and immunocompetence in humans. Am J Clin Nutr. 1991;53:40–6 [DOI] [PubMed] [Google Scholar]
- 14.Kew S, Banerjee T, Minihane AM, Finnegan YE, Muggli R, Albers R, Williams CM, Calder PC. Lack of effect of foods enriched with plant- or marine-derived n-3 fatty acids on human immune function. Am J Clin Nutr. 2003;77:1287–95 [DOI] [PubMed] [Google Scholar]
- 15.Merchant AT, Curhan GC, Rimm EB, Willett WC, Fawzi WW. Intake of n-6 and n-3 fatty acids and fish and risk of community-acquired pneumonia in US men. Am J Clin Nutr. 2005;82:668–74 [DOI] [PubMed] [Google Scholar]
- 16.Han SN, Leka LS, Lichtenstein AH, Ausman LM, Schaefer EJ, Meydani SN. Effect of hydrogenated and saturated, relative to polyunsaturated fat on immune and inflammatory responses of adults with moderate hypercholesterolemia. J Lipid Res. 2002;43:445–52 [PubMed] [Google Scholar]
- 17.Lichtenstein AH, Matthan NR, Jalbert SM, Resteghini NA, Schaefer EJ, Ausman LM. Novel soybean oils with different fatty acid profiles alter cardiovascular disease risk factors in moderately hyperlipidemic subjects. Am J Clin Nutr. 2006;84:497–504 [DOI] [PubMed] [Google Scholar]
- 18.Bloemena E, Roos MT, Van Heijst JL, Vossen JM, Schellekens PT. Whole-blood lymphocyte cultures. J Immunol Methods. 1989;122:161–7 [DOI] [PubMed] [Google Scholar]
- 19.Han SN, Leka LS, Lichtenstein AH, Ausman LM, Meydani SN. Effect of a therapeutic lifestyle change diet on immune functions of moderately hypercholesterolemic humans. J Lipid Res. 2003;44:2304–10 [DOI] [PubMed] [Google Scholar]
- 20.Erkkilä AT, Matthan NR, Herrington DM, Lichtenstein AH. Higher plasma docosahexaenoic acid is associated with reduced progression of coronary atherosclerosis in women with CAD. J Lipid Res. 2006;47:2814–9 [DOI] [PubMed] [Google Scholar]
- 21.Yaqoob P, Newsholme EA, Calder PC. The effect of dietary lipid manipulation on rat lymphocyte subsets and proliferation. Immunology. 1994;82:603–10 [PMC free article] [PubMed] [Google Scholar]
- 22.Jolly CA, Jiang YH, Chapkin RS, McMurray DN. Dietary (n-3) polyunsaturated fatty acids suppress murine lymphoproliferation, interleukin-2 secretion, and the formation of diacylglycerol and ceramide. J Nutr. 1997;127:37–43 [DOI] [PubMed] [Google Scholar]
- 23.Goyens PL, Mensink RP. The dietary alpha-linolenic acid to linoleic acid ratio does not affect the serum lipoprotein profile in humans. J Nutr. 2005;135:2799–804 [DOI] [PubMed] [Google Scholar]
- 24.Netea MG, Demacker P, de Bont N, Boerman OC, Stalenhoef AF, van der Meer JW, Kullberg BJ. Hyperlipoproteinemia enhances susceptibility to acute disseminated Candida albicans infection in low-density-lipoprotein-receptor-deficient mice. Infect Immun. 1997;65:2663–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr. 1996;63:116–22 [DOI] [PubMed] [Google Scholar]
- 26.Wu D, Meydani SN, Meydani M, Hayek MG, Huth P, Nicolosi RJ. Immunologic effects of marine- and plant-derived n-3 polyunsaturated fatty acids in nonhuman primates. Am J Clin Nutr. 1996;63:273–80 [DOI] [PubMed] [Google Scholar]
- 27.Burdge GC, Calder PC. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod Nutr Dev. 2005;45:581–97 [DOI] [PubMed] [Google Scholar]
- 28.Calder PC, Grimble RF. Polyunsaturated fatty acids, inflammation and immunity. Eur J Clin Nutr. 2002;56 Suppl 3:S14–9 [DOI] [PubMed] [Google Scholar]