The P-III metalloproteinase from B. moojeni was crystallized and diffraction data were collected to a maximum resolution of 3.3 Å.
Keywords: P-III metalloproteinases, Bothrops moojeni, snake venoms
Abstract
Snake-venom metalloproteinases (SVMPs) comprise a family of haemostatically active toxins which can cause haemorrhage, coagulopathy, inhibition of platelet aggregation and inflammatory response. These effects are attributed to the proteolytic action of SVMPs on extracellular matrix components, plasma proteins and cell-surface proteins. SVMPs are classified into four classes (P-I to P-IV) based on their domain structures. In order to understand the multiple roles played by the domains of P-III SVMPs, a P-III SVMP (BmMP-III) from the venom of Bothrops moojeni was purified, characterized and crystallized. The crystals belonged to space group I4122, with unit-cell parameters a = b = 108.16, c = 196.09 Å. Initially, flash-cooled crystals diffracted poorly to a resolution of about 10 Å. However, a significant improvement in the diffraction resolution was observed upon annealing and a complete data set was collected to 3.3 Å resolution. The asymmetric unit contained one molecule and the structure was determined and partially refined to an R factor of 34%. Structural comparisons indicated that the cysteine-rich domain can adopt different conformations in relation to the catalytic domain, which may modulate the enzyme activity.
1. Introduction
Snake venoms are rich and complex mixtures of biologically active peptides and proteins, principally acetylcholinesterases, l-amino-acid oxidases (LAAOs), serine proteinases (SVSPs), metalloproteinases (SVMPs) and phospholipases A2 (PLA2s) that perturb physiological processes and hence serve as models for biomedical investigations and the development of specific inhibitors (Calvete et al., 2007 ▶; Fox & Serrano, 2008 ▶; Kang et al., 2011 ▶). The proteinase content of snake venoms varies between genera and species, but is generally the most abundant component. It has been estimated that most viperid venoms contain at least 32% SVMPs and 18% SVSPs (Calvete et al., 2007 ▶; Fox & Serrano, 2008 ▶; Serrano & Maroun, 2005 ▶).
SVMPs belong to a subgroup whose leading members are the reprolysins, which participate in the haemorrhagic process by the proteolytic degradation of endothelial cell-surface proteins and extracellular matrix components involved in the maintenance of capillary structure and integrity, leading to the disruption of capillary networks and consequently resulting in oedema and haemorrhage (Escalante et al., 2006 ▶; Fox & Serrano, 2005 ▶).
The molecular weights of SVMPs usually range from 20 to 100 kDa and are divided into four classes (P-I to P-IV) based on their molecular weights and domain organization. The P-I class of SVMPs (20–30 kDa) are the simplest and contain only a metalloproteinase (M) domain, P-II SVMPs (30–50 kDa) contain metalloproteinase (M) and disintegrin-like (D) domains, P-III SVMPs (50–80 kDa) consist of metalloproteinase (M), disintegrin-like (D) and cysteine-rich (C) domains, and P-IV SVMPs (80–100 kDa) additionally enclose two C-type lectin domains connected by disulfide bonds to the cysteine-rich domain (Bjarnason & Fox, 1995 ▶; Fox & Serrano, 2008 ▶). The P-III SVMPs are more haemorrhagic than the P-I SVMPs and this has been attributed to the presence of the D and C domains (Bjarnason & Fox, 1995 ▶; Jeon & Kim, 1999 ▶).
The present work describes the purification, crystallization and preliminary crystallographic analysis of a P-III metalloproteinase (BmMP-III) from the venom of Bothrops moojeni.
2. Materials and methods
2.1. Venom collection and purification
500 mg desiccated crude B. moojeni venom was suspended in 1.5 ml 0.02 M Tris–HCl buffer pH 8.0 and centrifuged at 10 000g for 10 min. The supernatant containing the crude B. moojeni venom was initially fractionated by size-exclusion chromatography using a Sephacryl S-100 column previously equilibrated with 0.02 M Tris–HCl pH 8.0 (buffer A). The fractions corresponding to peak 2 were pooled and submitted to anion-exchange chromatography on a Mono Q 5/50 GL column. Elution was performed using a nonlinear gradient of 0–100% buffer A containing 1.0 M NaCl. All fractions were analyzed by SDS–PAGE. The above chromatographic procedure was repeated five times using a total of 500 mg crude venom. The fractions corresponding to BmMP-III (peak 1) were pooled and were concentrated to 0.5 ml using a microconcentrator (Amicon) with a membrane cutoff of 30 kDa. The concentrated fraction was subjected to anion-exchange chromatography using a Mono Q 5/50 GL column. The column was washed with 0.02 M Tris–HCl pH 8.0 and the bound fractions were eluted with a nonlinear (0–100%) NaCl gradient to 0.02 M Tris–HCl pH 8.0, 1.0 M NaCl. The purity of the BmMP-III fraction was analyzed by 12% SDS–PAGE (Laemmli, 1970 ▶).
2.2. Crystallization
The BmMP-III sample was dialyzed against 0.02 M Tris–HCl pH 8.0 and concentrated to 11 mg ml−1 in microconcentrators (Amicon). Crystallization was performed by the hanging-drop vapour-diffusion method at 291 K using 24-well tissue-culture plates (Jancarik & Kim, 1991 ▶). The crystallization screening kits used were Crystal Screen, Crystal Screen 2, Grid Screen Ammonium Sulfate and Grid Screen PEG 6000 (Hampton Research). Typically, a 1 µl drop of protein solution was mixed with 1 µl screening solution and equilibrated against a reservoir consisting of 0.8 ml of the latter solution. Initially, microcrystals were obtained using 0.1 M HEPES pH 7.0, 0.8 M ammonium sulfate. These conditions were optimized by changing the pH of the buffer and the concentration of ammonium sulfate; large single crystals were obtained when a 2 µl protein droplet was mixed with an equal volume of reservoir solution consisting of 0.1 M HEPES pH 8.0, 1.6 M ammonium sulfate.
2.3. Data collection, processing and structure determination
A single BmMP-III crystal was transferred to cryoprotectant solution [mother solution plus 25%(v/v) glycerol] and flash-cooled in a nitrogen-gas stream at 100 K. X-ray diffraction data were collected on the W01B-MX2 beamline at the Brazilian Synchrotron Light Laboratory, Campinas, Brazil. Crystal annealing consisted of maintaining the cooled crystal at ambient temperature for approximately 1 min and then rapidly transferring it to cryogenic temperature. The data were indexed and integrated using MOSFLM (Leslie & Powell, 2007 ▶) and were scaled using SCALA (Kabsch, 2010 ▶). Data-collection statistics are presented in Table 1 ▶. The structure was solved by molecular replacement with MOLREP (Vagin & Teplyakov, 2010 ▶) using the atomic coordinates of bothropasin from B. jararaca venom (PDB entry 3dsl; Muniz et al., 2008 ▶) and BmooMPα-I from B. moojeni venom (PDB entry 3gbo; Akao et al., 2010 ▶) as search models. Since the primary sequence has not yet been determined, refinement will be completed when the amino-acid sequence is available.
Table 1. Data-collection statistics.
Values in parentheses are for the last resolution shell.
| Data statistics | |
| Temperature (K) | 100 |
| Radiation source | Brazilian Synchrotron Light Laboratory |
| Beamline | W01B-MX2 |
| Wavelength (Å) | 1.458 |
| Detector | MAR Mosaic 225 mm |
| Space group | I4122 |
| Unit-cell parameters (Å, °) | a = 108.16, b = 108.16, c = 196.09 |
| Resolution range (Å) | 50.00–3.30 (3.48–3.30) |
| R merge † (%) | 14.1 (49.5) |
| 〈I/σ(I)〉 | 16.6 (6.3) |
| Data completeness (%) | 100.0 (100.0) |
| No. of measured reflections | 168053 (24441) |
| No. of unique reflections | 9130 (1304) |
| Multiplicity | 18.4 (18.7) |
| Data analysis | |
| V M (Å3 Da−1) | 2.39 |
| Solvent content (%) | 48.56 |
| Molecules per asymmetric unit | 1 |
R
merge = 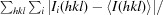
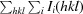 , where Ii(hkl) is the ith observation of reflection hkl and 〈I(hkl)〉 is the weighted average intensity for all observations i of reflection hkl.
, where Ii(hkl) is the ith observation of reflection hkl and 〈I(hkl)〉 is the weighted average intensity for all observations i of reflection hkl.
3. Results and discussion
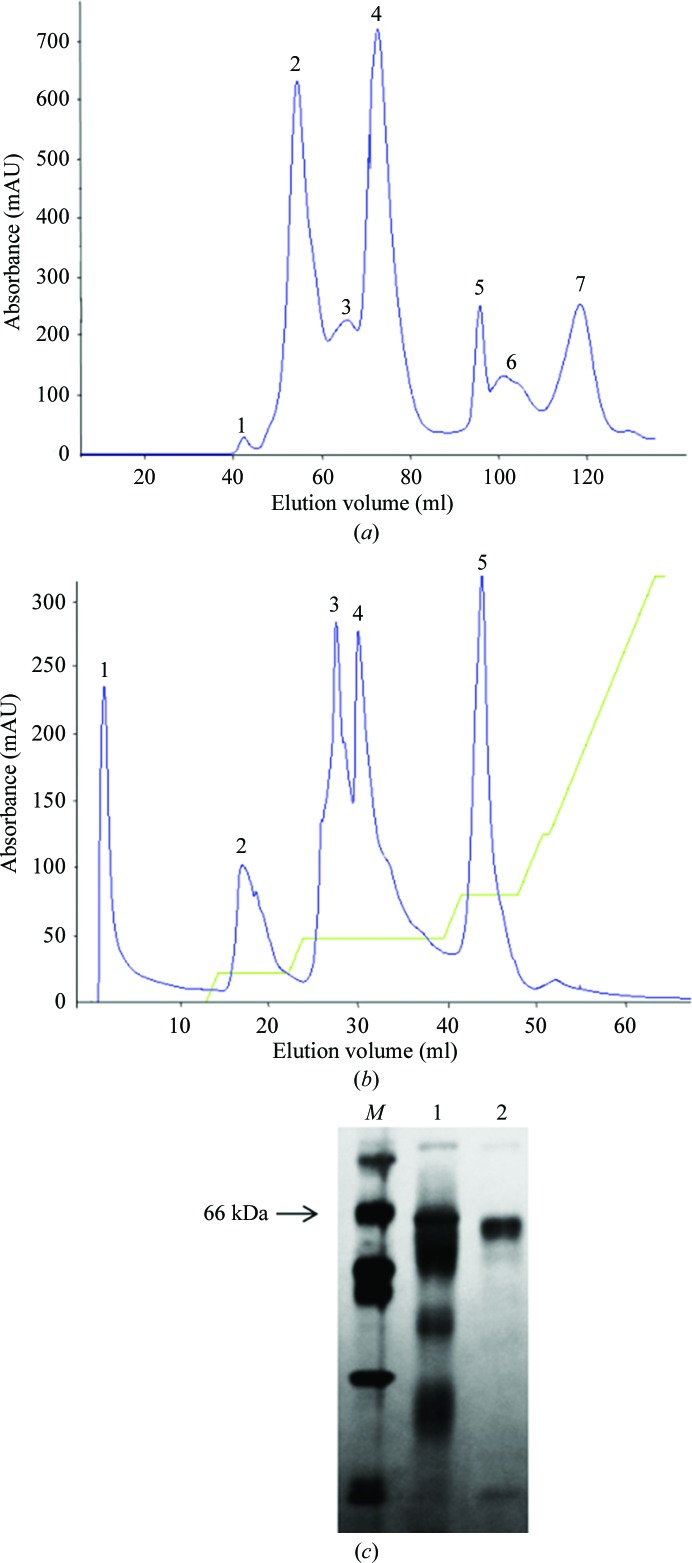
We purified and crystallized a P-III SVMP (BmMP-III) from the venom of B. moojeni by a rapid procedure involving two chromatographic steps. Molecular-exclusion chromatography resulted in seven fractions (Fig. 1 ▶ a). The second peak was subsequently subjected to ion-exchange chromatography and sub-fractioned into six further peaks. Peak 1 corresponds to BmMP-III (Fig. 1 ▶ b) with high purity (>95%) as assessed by SDS–PAGE (Fig. 1 ▶ c). This protocol yielded 4 mg purified protein from 100 mg initial crude venom. The protein was submitted to MS/MS and the fragments found by fingerprint analysis (data not shown) showed sequence similarity to snake-venom metalloproteinases, indicating the isolation of a high-molecular-weight metalloproteinase from crude B. moojeni venom that corresponds to a P-III SVMP protein and is referred to as BmMP-III.
Figure 1.
Purification of BmMP-III. (a) Size-exclusion chromatography profile of crude B. moojeni venom on Sephacryl S-100. (b) Anion-exchange chromatography profile on a Mono Q 5/50 GL column. (c) Assessment of purity and determination of molecular mass on SDS–PAGE. Lane M, molecular-mass markers (labelled in kDa); lane 1, total soluble protein from crude venom; lane 2, BmMP-III (corresponding to peak 1 from anion-exchange chromatography).
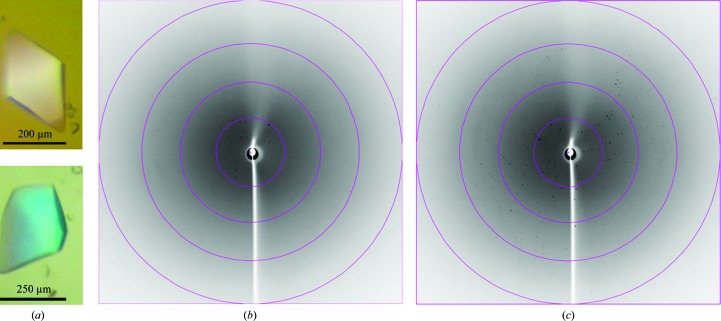
Initially, the BmMP-III crystals (Fig. 2 ▶ a) only diffracted to low resolution (∼10 Å; Fig. 2 ▶ b). However, after crystal annealing diffraction data were observed to a maximum resolution of 3.3 Å (Fig. 2 ▶ c). The crystals belonged to the tetragonal system and analysis of the processed data using POINTLESS (Evans, 2006 ▶) indicated that they belonged to space group I4122. Data-processing and refinement statistics are presented in Table 1 ▶. The Matthews coefficient calculated for the crystal is 2.39 Å3 Da−1, which corresponds to a solvent content of 48.6% (Matthews, 1968 ▶). Considering the molecular weight of the protein (∼60 000 Da), the asymmetric unit contains one molecule.
Figure 2.
(a) Photomicrographs of BmMP-III crystals. (b) X-ray diffraction pattern pre-annealing. (c) X-ray diffraction pattern post-annealing. The circles indicate resolutions of 11.5, 5.8, 3.8 and 2.9 Å.
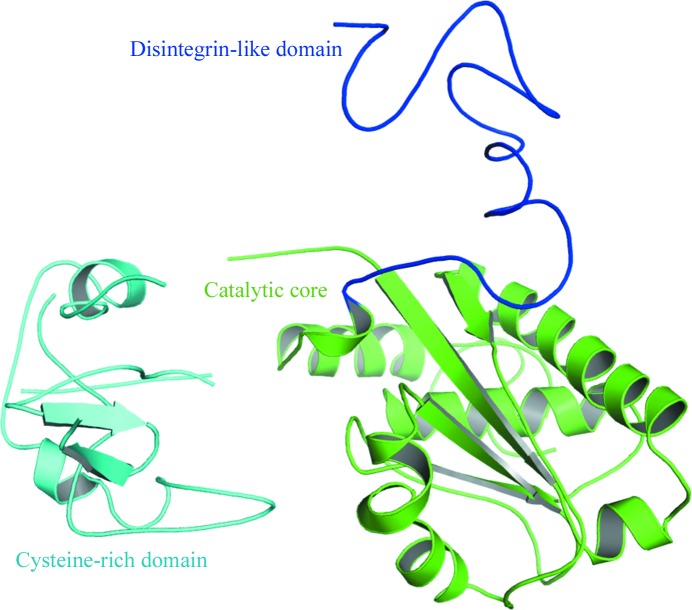
The use of full-length bothropasin as a model for molecular replacement (MR) failed. The structure of a P-I SVMP from B. moojeni (BmooMPα-I) yielded better scores in MR calculations than the truncated catalytic domain of bothropasin. Once the solution for the catalytic core had been fixed (R factor = 49%), solutions for the cysteine-rich (R factor = 43%) and disintegrin-like (R factor = 40%) domains were obtained when these domains were used separately. The final MR model comprised of the three domains was submitted to rigid-body refinement and converged to an R factor of 38% (Fig. 3 ▶).
Figure 3.
Partially refined crystal structure of BmMP-III indicating the domain architecture.
Acknowledgments
This work was supported by grants from CNPq, FAPESP, CAPES and TWAS.
References
- Akao, P. K., Tonoli, C. C., Navarro, M. S., Cintra, A. C., Neto, J. R., Arni, R. K. & Murakami, M. T. (2010). Toxicon, 55, 361–368. [DOI] [PubMed]
- Bjarnason, J. B. & Fox, J. W. (1995). Methods Enzymol. 248, 345–368. [DOI] [PubMed]
- Calvete, J. J., Juárez, P. & Sanz, L. (2007). J. Mass Spectrom. 42, 1405–1414. [DOI] [PubMed]
- Escalante, T., Shannon, J., Moura-da-Silva, A. M., Gutiérrez, J. M. & Fox, J. W. (2006). Arch. Biochem. Biophys. 455, 144–153. [DOI] [PubMed]
- Evans, P. (2006). Acta Cryst. D62, 72–82. [DOI] [PubMed]
- Fox, J. W. & Serrano, S. M. (2005). Toxicon, 45, 969–985. [DOI] [PubMed]
- Fox, J. W. & Serrano, S. M. (2008). Proteomics, 8, 909–920. [DOI] [PubMed]
- Jancarik, J. & Kim, S.-H. (1991). J. Appl. Cryst. 24, 409–411.
- Jeon, O.-H. & Kim, D.-S. (1999). Eur. J. Biochem. 263, 526–533. [DOI] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Kang, T. S. et al. (2011). FEBS J. 278, 4544–4576. [DOI] [PubMed]
- Laemmli, U. K. (1970). Nature (London), 227, 680–685. [DOI] [PubMed]
- Leslie, A. G. W. & Powell, H. R. (2007). Evolving Methods for Macromolecular Crystallography, edited by R. J. Read & J. L. Sussman, pp. 41–51. Dordrecht: Springer.
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Muniz, J. R., Ambrosio, A. L., Selistre-de-Araujo, H. S., Cominetti, M. R., Moura-da-Silva, A. M., Oliva, G., Garratt, R. C. & Souza, D. H. (2008). Toxicon, 52, 807–816. [DOI] [PubMed]
- Serrano, S. M. & Maroun, R. C. (2005). Toxicon, 45, 1115–1132. [DOI] [PubMed]
- Vagin, A. & Teplyakov, A. (2010). Acta Cryst. D66, 22–25. [DOI] [PubMed]