To the Editor:
Hypertension continues to be an important public health problem, with a substantial proportion of patients failing to achieve optimal blood pressure (BP) control.
Recent data suggest that hypertension is characterized by a relative deficiency of the natriuretic peptide system, which has cardiorenal and vascular protective properties.1,2 Furthermore, we previously demonstrated that B-type natriuretic peptide (BNP) supplementation has BP-lowering actions in models of acute and chronic experimental hypertension.3,4 With all of this in mind, we designed a pilot study to investigate the effects of low-dose subcutaneous (SQ) BNP (nesiritide; Scios, Inc, Mountain View, CA) in patients with uncontrolled hypertension despite the use of conventional antihypertensive therapy. Herein we report the results from the first patient enrolled for the safety and dose-finding study (Trial Registration clinicaltrials.gov Identifier: NCT00953472; “B-Type Natriuretic Peptide [BNP] in Human Hypertension”), for which funding is currently being pursued.
A 59-year-old white man (weight, 79 kg; body mass index, 27 kg/m2) with office BP of 150/90 mm Hg despite treatment with an angiotensin receptor blocker (losartan, 50 mg once a day orally) and a diuretic (hydrochlorothiazide, 25 mg once a day orally) consented to participate in this study. Ambulatory BP monitoring indicated hypertension (while awake: 148/87 mm Hg; heart rate, 67 beats/min; during sleep: 132/76 mm Hg; heart rate, 51 beats/min). Results of physical examination and laboratory analyses were normal, with no signs of secondary hypertension or renal insufficiency (serum creatinine, 0.8 mg/dL; to convert to μmol/L, multiply by 88.4).
The patient was on a no-added-salt diet (120 mEq of sodium per day) for 7 days before admission to the Clinical Research Unit at Mayo Clinic. The Figure illustrates BP levels before and after repeated administration of 10 μg/kg of SQ BNP. (The dose was based on experience in patients with heart failure.5) On the morning of the study the patient withheld his standard medications to receive the first SQ BNP dose. Systolic and diastolic BP decreased (from 148/86 to 105/72 mm Hg) and remained reduced for the following 12 hours. Twelve hours after the first SQ BNP injection, the second dose was administered, and a reduction of both systolic and diastolic BP (from 123/75 to 101/56 mm Hg) followed, with no other antihypertensive therapy being given. On the following morning, 12 hours after the second dose, BP was 135/81 mm Hg. Again, the patient's standard medications were withheld. and the last dose of SQ BNP was given, which induced a BP reduction (from 135/81 to 113/74 mm Hg). The patient was discharged with a BP of 121/78 mm Hg and instructed to restart his standard therapy the following day. Plasma BNP1-32 increased from 53 pg/mL before treatment to 72 pg/mL 4 hours after the last injection; in contrast, corresponding plasma levels of endogenous N-terminal proBNP1-76 decreased from 48 pg/mL to 22 pg/mL. (to convert to pmol/L, multiply BNP1-32 by 0.289 and NT-proBNP1-76 by 0.118, respectively.)
FIGURE.
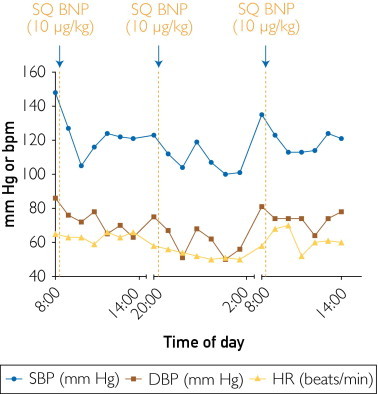
Hyoptensive effects of 10 μg/kg of SQ BNP in a patient with uncontrolled hypertension. DBP = diastolic blood pressure; HR = heart rate; SBP = systolic blood pressure; SQ BNP = subcutaneous B-type natriuretic peptide.
Thus, this patient demonstrated effective BP reduction after BNP administration. This study was designed to assess the safety of low-dose (10 μg/kg) BNP, which normalized BP for the duration of the study without additional therapy. These encouraging results support further studies with SQ BNP as a potential antihypertensive drug, perhaps in combination with standard therapy, in patients who have resistant hypertension or poorly controlled BP.
Footnotes
This work was supported by grant CSDA 2006064 (A.C.) from the Doris Duke Charitable Foundation.
References
- 1.Belluardo P., Cataliotti A., Bonaiuto L. Lack of activation of molecular forms of the BNP system in human grade 1 hypertension and relationship to cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2006;291(4):H1529–H1535. doi: 10.1152/ajpheart.00107.2006. [DOI] [PubMed] [Google Scholar]
- 2.Cataliotti A., Macheret F., McKie P. Deficiency of the cardiorenal protective hormone BNP in early stages of hypertension [abstract] J Hypertens. 2010;28:e21. [Google Scholar]
- 3.Cataliotti A., Schirger J.A., Martin F.L. Oral human brain natriuretic peptide activates cyclic guanosine 3',5'-monophosphate and decreases mean arterial pressure. Circulation. 2005;112(6):836–840. doi: 10.1161/CIRCULATIONAHA.105.538520. [DOI] [PubMed] [Google Scholar]
- 4.Cataliotti A., Tonne J.M., Bellavia D. Long-term cardiac pro-B-type natriuretic peptide gene delivery prevents the development of hypertensive heart disease in spontaneously hypertensive rats. Circulation. 2011;123(12):1297–1305. doi: 10.1161/CIRCULATIONAHA.110.981720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H.H., Redfield M.M., Nordstrom L.J., Horton D.P., Burnett J.C., Jr. Subcutaneous administration of the cardiac hormone BNP in symptomatic human heart failure. J Card Fail. 2004;10(2):115–119. doi: 10.1016/j.cardfail.2003.08.011. [DOI] [PubMed] [Google Scholar]


