Abstract
Far more extensive than the epicardial coronary vasculature that can be visualized angiographically is the coronary microcirculation, which foregoes routine imaging. Probably due to the lack of techniques able to provide tangible evidence of its crucial role, the clinical importance of coronary microvascular dysfunction is not fully appreciated. However, evidence gathered over the last several decades indicates that both functional and structural abnormalities of the coronary microvasculature can lead to myocardial ischaemia, often comparable with that caused by obstructive coronary artery disease. Indeed, a marked increase in coronary microvascular resistance can impair coronary blood flow and trigger angina pectoris, ischaemic ECG shifts, and myocardial perfusion defects, and lead to left ventricular dysfunction in patients who otherwise have patent epicardial coronary arteries. This condition—often referred to as ‘chest pain with normal coronary arteries’ or ‘cardiac syndrome X’—encompasses several pathogenic mechanisms involving the coronary microcirculation. Of importance, coronary microvascular dysfunction can occur in conjunction with several other cardiac disease processes. In this article, we review the pathogenic mechanisms leading to coronary microvascular dysfunction and its diagnostic assessment, as well as the different clinical presentations and prognostic implications of microvascular angina. As such, this review aims to remove at least some of the mystery surrounding the notion of coronary microvascular dysfunction and to show why it represents a true clinical entity.
Keywords: Cardiac syndrome X, Coronary flow reserve, Microvascular angina, Prognosis
Introduction
Contrary to the epicardial coronary vasculature, the coronary microcirculation has remained elusive to conventional imaging techniques (Figure 1). For this reason, possibly, the clinical significance of coronary microvascular dysfunction (CMVD) has not been given as much attention as epicardial coronary artery disease (CAD). In particular, a condition often referred to as ‘chest pain with normal coronary arteries’ or ‘cardiac syndrome X’ (CSX) has puzzled physicians over the years and continues to represent an unsolved ‘mystery’ rather than a reality for many in clinical practice.1,2 However, various lines of evidence in recent years have identified an important role for the coronary microcirculation in the clinical presentation and prognosis of patients who have typical chest pain despite a normal coronary angiogram and also in patients with other cardiac conditions. This article intends to bring this subject closer to the practising cardiologist. We will review the functional aspects of the coronary microcirculation, the diagnostic tests used for the assessment of CMVD, its clinical presentation, and prognosis. The therapeutic management of patients with CMVD, however, will not be reviewed in the present article.
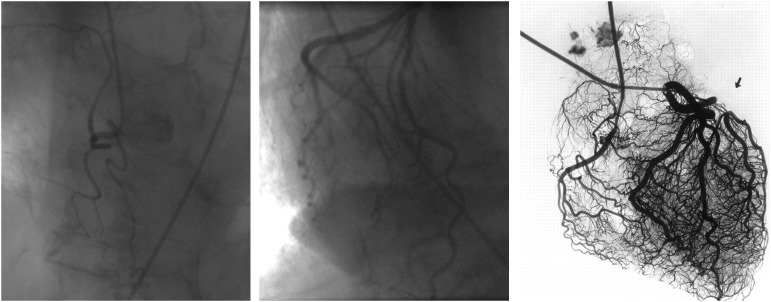
Figure 1.
Angiogram of the right coronary artery (left panel) and a dominant left coronary artery system (middle panel), which do not reveal the rich microvascular network noted on an ex vivo arteriogram.111
Functional aspects of the coronary microcirculation
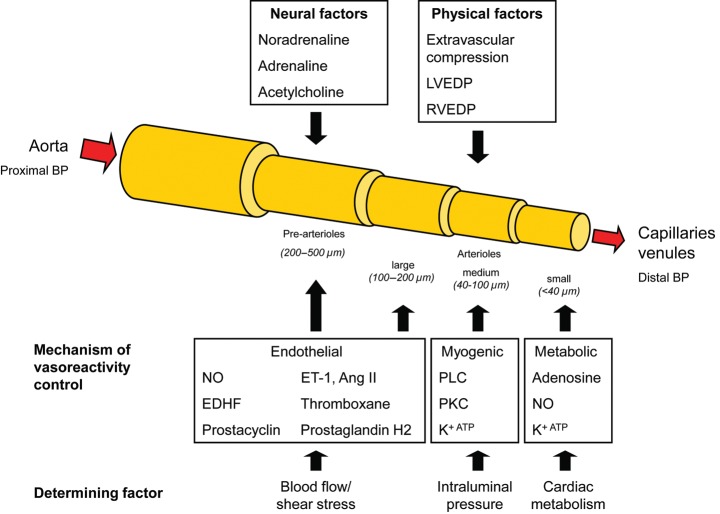
The coronary blood flow (CBF) is driven by the pressure difference between the aortic sinus and the coronary sinus (or the right atrium pressure). In the absence of obstructive stenoses, the epicardial arteries offer very little (∼10%) resistance to CBF and serve mainly as conductance vessels. Capillaries and venules are likewise responsible for only 10% of CBF resistance and mainly function as capacitance vessels, holding 90% of the total myocardial blood volume. Under normal conditions and to a large extent also under pathological conditions, coronary vascular resistance is primarily controlled by the pre-arterioles (vessels <500 µm in diameter) and arterioles (<200 µm). The pre-arterioles are epicardial (extra-myocardial) vessels that react to changes in shear stress and intravascular pressure to preserve adequate perfusion pressure in the distal arteriolar bed. They are responsible for ∼25% of the total coronary vascular resistance.3 The arterioles are the true intramyocardial regulatory component of the coronary circulation and these vessels represent the largest proportion (∼55%) of the total coronary vascular resistance. Arterioles are usually subdivided in two categories, according to their diameter and the mechanism(s) that regulates their tone (Figure 2).3,4 Endothelium-dependent vasoreactivity prevails in the larger arterioles (100–200 µm in diameter) and translates flow-related stimuli into vasomotor responses, i.e. vasodilation with increase in flow and vice versa. Medium-sized microvessels (40–100 µm in diameter) react predominantly to intraluminal pressure changes sensed by stretch receptors located in vascular smooth muscle cells (myogenic control), i.e. they constrict when the intraluminal pressure increases and, conversely, dilate when the pressure decreases.5 Finally, the tone of the smaller arterioles (vessels <40 µm in diameter) is modulated by the metabolic activity of the myocardium. As such, increased metabolic activity leads to vasodilatation of the smaller arterioles, which leads to pressure reduction in the medium-sized microvessels and myogenic dilation, which, in turn, increases flow upstream resulting in endothelium-dependent vasodilation.3 These mechanisms effectively and efficiently allow the microcirculation to regulate myocardial perfusion both at rest and at different levels of myocardial metabolic demand.
Figure 2.
Coronary blood flow is driven by the pressure difference between the aorta and the capillary bed and modulated further by various physical and neural factors, which affect the microcirculation. Moreover, the different compartments of the microcirculation are influenced by one main physiological mechanism to control their vascular tone with cardiac metabolism as the final determining factor.
Assessment of the coronary microcirculation: functional vs. anatomical techniques
A technique that allows an approximate ‘visualization’ of the microcirculation in clinical practice is the injection of dye into the coronary artery resulting in myocardial opacification—also known as myocardial blush (Figure 3).6 Magnetic resonance imaging (MRI) can also outline microvascular obstruction albeit indirectly and with relatively low resolution (Figure 3).7 Of importance, CMVD often results from functional and not necessarily structural abnormalities, or represents a combination of both mechanisms. Hence, even if there was a technique that could clearly visualize the anatomy of the coronary microcirculation in humans in vivo, it would still be an incomplete evaluation. A reliable functional test, on the other hand, provides pragmatic assessment, reflecting CMVD irrespective of whether the cause is structural or functional.
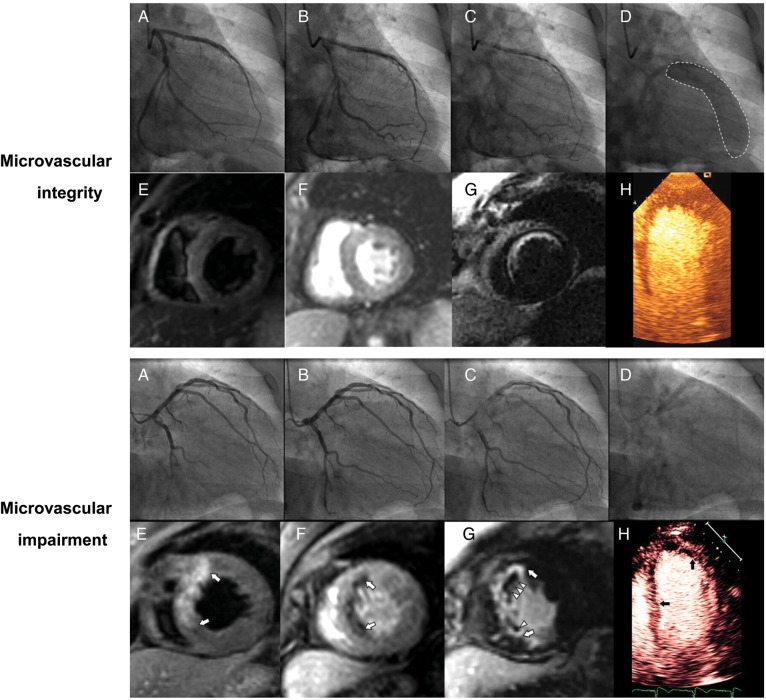
Figure 3.
Illustration of two cases of anterior myocardial infarction with the restoration of blood flow in the left anterior descending artery (A–C, upper and lower panel). In the presence of microvascular integrity, the following can be seen: myocardial blush grade 3 (D, upper panel) a lack of oedema, homogeneous myocardial perfusion, subendocardial anteroseptal enhancement of 25–50% wall thickness on magnetic resonance imaging (MRI, E–G, upper panel) and normal perfusion on myocardial contrast echocardiography (MCE, H, upper panel). On the contrary, in the setting of coronary microvascular impairment, myocardial blush is poor (D, lower panel) along with a large area of oedema, an anteroseptal perfusion defect and extensive delayed enhancement with microvascular obstruction on MRI (E–G, lower panel), and a large perfusion defect on MCE (H, lower panel). Modified from Porto et al.112 Used with the permission of Elsevier.
Consistent with the primary haemodynamic function of the coronary microcirculation, functional techniques for the assessment of the coronary microvasculature rely on the measurement of CBF that changes mainly as a result of alterations in vascular tone (Table 1).8 Positron emission tomography (PET) is the most established non-invasive technique for the assessment of CBF, as it allows the determination of absolute regional myocardial blood flow (MBF) at rest and in response to various stimuli. Importantly, however, non-invasive techniques such as PET may lack sensitivity and specificity for the diagnosis of coronary vasomotor dysfunction and, in general, are unable to differentiate between epicardial and microvascular abnormalities.9 Thus, at present, the most definite evaluation of the coronary microcirculation remains invasive in nature. Simple angiographic techniques such a TIMI frame count can provide an approximate estimation of epicardial vs. microvascular mechanisms.10 Intravascular ultrasound (IVUS) can be useful to identify atherosclerotic areas not necessarily visible on conventional angiography and to provide an accurate estimate of arterial cross-sectional area, which can then be used along with intracoronary Doppler-derived coronary flow velocity to calculate CBF and CBF reserve.11 Alternatively, quantitative angiography (QCA) can be used for the determination of the cross-sectional area at the tip of the Doppler wire.
Table 1.
Modalities to assess coronary microvascular function
| Method | Tracer | Primary parameter | Secondary parameter | Microvascular distinction | Endothelial assessment | Pros | Cons |
|---|---|---|---|---|---|---|---|
| PET101 | Radioisotopes | MBF (0.6–1.3 mL/min/g) | MBF reserve (>2–2.5) | No | No | Validated and reproducibility | Limited availability, radioactivity |
| SPECT | Radioisotopes | Perfusion (no defect) | (Perfusion reserve) | No | No | Availability, low costs | MBF only with dynamic upgrade, radioactivity |
| MDCT102 | Iodine contrast | MBF (0.9–1.3 mL/min/g) | MBF reserve (>2–2.5) | No | No | Availability | Investigational, image quality, radiation |
| MRI103 | Gadolinium | MBF (0.7–1.1 mL/min/g) | MBF reserve (>2–2.5) | No | No | One-stop test, no radiation or radioactivity | Investigational, technical limitations |
| MCE104 | Echo contrast | Perfusion, MBF option (0.5–2.9 mL/min/g) | MBF reserve option (>2–2.5) | No | No | One-stop test, no radiation or radioactivity | Volumetric modelling, image quality |
| Doppler echo 105 | Echo contrast | Flow velocity (24–36 cm/s) | Flow reserve (>2–2.5) | No | No | One-stop test, no radiation or radioactivity | No MBF option, position and image dependent |
| TFC8 | Iodine contrast | Contrast flow velocity (18–24) | TFC reserve (>2–2.5) | Assumed if no epicardial dx | No | Ease of use, low cost | No CBF option, subjectivity |
| MBG4 | Iodine contrast | Contrast staining (Grade 3) | None | Assumed if no epicardial dx | No | Ease of use, low cost | No CBF option, subjectivity |
| ICD106 | None | Flow velocity (10–22 cm/s) | (relative) flow velocity reserve | Assumed if no epicardial dx | Yes | Direct measurement | No CBF option, invasiveness |
| ICD +QCA/IVUS 107 | Iodine contrast | CBF (44–59 mL/min) | CBF reserve (>2–2.5) | Yes | Yes | Complete assessment | Costs, invasiveness |
| TPS108 | Saline | IMF (15–22 U) | None | Yes | Yes | Complete assessment | Costs, invasiveness |
PET, positron emission tomography; SPECT, single photo emission computed tomography; MDCT, multi-detector computed tomography; MRI, magnetic resonance imaging; MCE, myocardial contrast echocardiography; TFC, TIMI frame count; MBG, myocardial blush grade; ICD, intracoronary Doppler; QCA, quantitative coronary angiography; IVUS, intravascular ultrasound; TPS, temperature and pressure sensor; MBF, myocardial blood flow (mL/time/myocardial mass); CBF, coronary blood flow (mL/time unit).
The use of a pressure–temperature sensor-tipped guidewire represents another effective mode of evaluation, allowing simultaneous measurement of the fractional flow reserve (FFR, by coronary pressure) and the coronary flow reserve (CFR, by coronary thermodilution) and calculation of the index of microvascular resistance (IMR).12–14 IMR is defined as the distal coronary pressure divided by the inverse of the hyperaemic mean transit time.13 This index—which is mainly used in the context of CAD—was validated in experimental models but has several limitations. For instance, it is necessary to incorporate the collateral blood flow in the calculations (accomplished by multiplying IMR by the ratio of coronary FFR and myocardial FFR), as otherwise IMR progressively increases with increasing degrees of epicardial coronary artery stenoses (as seen with studies using Doppler-derived FFR).15
The functional status of the coronary microcirculation can be assessed further by testing endothelium-dependent and endothelium-independent vascular responses.16 Adenosine, dipyridamole, and papaverine are often used to trigger arteriolar vasodilation, and hence increase CBF, mainly by a direct relaxing effect on vascular smooth muscle cells. Thus, these agents are not suitable for the assessment of endothelium-dependent coronary microcirculation abnormalities.14 Classically, intracoronary acetylcholine (ACH) has been used as a sensitive and safe test for the assessment of coronary vasomotor function in the catheterization laboratory. Its administration causes vasodilation under normal conditions but, in the absence of a functional endothelium, it leads to vasoconstriction by the unopposed stimulation of muscarinic receptors on vascular smooth muscle cells.16 Bradykinin and substance-P are alternative agents to test the endothelium, and like ACH, also elicit a rapid vascular response.17 Substance P has a good side effect profile and is especially useful in patients in whom the induction of coronary vasoconstriction may be undesirable. For all of these substances the mode of delivery is extremely important. Bolus injections need to be kept to the smallest volume and followed by an adequate catheter flush to allow a distinction between the vascular response to the drug from the mechanical effects of increased flow.17 Also, bradycardia often develops with this type of administration. Graded infusions, on the other hand, allow larger dosages to be safely given over a longer period of time (e.g. 1–1000 nmol/min with infusion vs. 1–100 nmol with bolus injection for ACH). The administration of the agent through an infusion catheter minimizes inconsistencies in drug delivery and the underestimation of the drug response that may occur with the use of guiding catheters.17 Infusion rates, however, have to be kept low at 1–2 mL/min not to affect the CBF. In part related to these considerations, atrial pacing, arm exercise, cold pressure, and mental stress testing are also used to assess endothelium-dependent, flow-related responses associated with increased myocardial oxygen demand.16
With regard to grading of the response, this can be based on symptoms, signs (such as ECG changes), and vascular responses.18 Medication holiday, anxiety, and sedation can significantly influence symptomatic assessment and, on occasion, CMVD can still be present even if signs or symptoms do not develop with any given mode of challenge at any given time point. For this reason, parameters objectively reflecting the occurrence of myocardial ischaemia (i.e. biochemical or imaging variables) and functional abnormalities of the coronary microcirculation are preferred (i.e. CBF responses).
Clinical presentation of coronary microvascular dysfunction
Coronary microvascular dysfunction can present clinically primarily associated with the syndrome of chest pain despite normal coronary arteriograms (i.e. microvascular angina) or in the context of cardiac disease processes. This has been captured in the CMVD classification proposed by Camici and Crea4 (types 1–4, Table 2). In agreement with this approach, one may further add CMVD after cardiac transplantation as an additional subtype (i.e. type 5, Table 2), which is mediated by alterations in autonomic tone, inflammation and immune mechanisms, and, possibly, defective endothelial progenitor cell recruitment.19–23 A listing of underlying mechanisms of CMVD in disease conditions is provided in Table 3.
Table 2.
Modified clinical classification of coronary microvascular dysfunction
| CMVD | Definition |
|---|---|
| Type 1 | Primary, i.e. in the absence of structural heart disease |
| Type 2 | In the presence of cardiomyopathies (incl. LVH, HCM, DCM, amyloidosis) |
| Type 3 | In the presence of obstructive CAD (incl. ACS) |
| Type 4 | After coronary interventions |
| Type 5 | After cardiac transplantation |
| Modifiers | |
| Duration | Acute or chronic |
| Symptoms | Asymptomatic or symptomatic |
| Therapy | None, minimal, moderate, or maximal level |
ACS, acute coronary syndrome; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; LVH, left ventricular hypertrophy.
Table 3.
Mechanisms of coronary blood flow alteration
| Effect on baseline CBF | Clinical example | Effect on hyperaemic CBF | Clinical example | |
|---|---|---|---|---|
| Extravascular | ||||
| Cardiac metabolism ↑ | ↑ | Pathological hypertrophy | ↑ | Physiological hypertrophy |
| Compressive forces ↑ | ↓↑ | ↓ | Various cardiomyopathies, LVH | |
| Diastolic perfusion time ↓ | ↓↑ | ↓ | Aortic stenosis | |
| Vascular dysfunction | ||||
| Endothelial cells | (↓) | CSY | ↓ | CV risk factors, CSX, heart transplantation, post-PCI |
| Smooth muscle cells | (↓) | CSY | ↓ | Hypertension, HCM, CSX |
| Autonomic nervous system | (↓) | CSY | ↓ | CSX, cardiomyopathies, heart transplantation, post-PCI |
| Vaso-structural changes | ||||
| Vascular plugging/obstruction | ↓ | Acute coronary syndromes | ↓ | AMI, post-PCI |
| Vascular infiltration | ↓↑ | ↓ | Amyloidosis, Fabry | |
| Vascular remodelling | ↓↑ | ↓ | Systemic hypertension, HCM | |
| Vascular rarefaction | ↓↑ | ↓ | Aortic stenosis, LVH, DCM | |
| Perivascular fibrosis | ↓↑ | ↓ | Aortic stenosis, LVH, HCM | |
AMI, acute myocardial infarction; CBF, coronary blood flow; CSX, cardiac syndrome X; CSY, cardiac syndrome Y (coronary slow flow syndrome); DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; LVH, left ventricular hypertrophy; PCI, percutaneous coronary intervention.
Obviously, it is quite challenging to define the clinical contribution of CMVD to any coronary or cardiac disease process. Lanza and Crea24 advocated an additional clinical distinction for primary CMVD according to the mode of presentation, i.e. either as an acute (unstable) or chronic (stable) angina. This may help in distinguishing pathogenic mechanisms and perhaps identifying patients with different clinical outcomes.25 The challenge, however, remains to identify aetiologic factors and specific triggers (Table 4).
Table 4.
Coronary microvascular dysfunction—key points
| The coronary microvasculature is the primary gatekeeper for myocardial blood flow beyond the easily visible epicardial coronary arteries |
| Dysfunction of the coronary microvasculature can be noted under a number of clinical circumstances |
| Coronary microvascular dysfunction can lead to acute and chronic signs and symptoms of myocardial ischaemia and can affect ventricular remodelling and function long term |
| Assessment of the coronary microvasculature in clinical practice relies on its functional aspects |
| Currently, invasive catheterization techniques are superior to non-invasive modalities for the functional assessment of the coronary microvasculature |
| The functional assessment may provide important prognostic information under various clinical circumstances |
Microvascular angina
It is conceivable that the main clinical consequence of the inability of the microvessels to match CBF to increased myocardial demand is the development of myocardial ischaemia, similar to that seen with flow-limiting epicardial stenoses. As such, patients with CMVD often present with chronic stable angina and/or dyspnoea. The term ‘microvacular angina’ was coined in an effort to confine and define the underlying functional abnormality in patients with chest pain and normal coronary arteries.26 Obviously, documentation of abnormal coronary microvascular responses to functional testing with the reproduction of symptoms is of central significance for this diagnosis.27 As CMVD is not confined to one coronary artery territory, it often leads to a patchy distribution pattern of perfusion abnormalities rather than to a condensed area of ischaemia, as typically seen in CAD patients.28 Of interest, it has been reported that despite the occurrence of angina, dyspnoea, ECG changes, and perfusion abnormalities, a reduction in LV contractility, as assessed by echocardiography, represents a less consistent finding in patients with CMVD compared with those with obstructive CAD.2,29
As confirmed in recent clinical studies, ∼50% of patients undergoing coronary angiography with signs and/or symptoms of myocardial ischaemia are found to have normal or ‘near normal (non-obstructed)’ coronary arteries.30–32 Of note, as shown by the ACOVA study, intracoronary ACH elicits profound diffuse epicardial vasoconstriction (≥75% diameter reduction) with the reproduction of symptoms (so-called ‘epicardial coronary artery spasm’) in one-third of these patients.32 In another third of patients, intracoronary ACH causes ‘microvascular spasm’, i.e. it reproduces angina symptoms and ischaemic ECG changes without eliciting changes in epicardial coronary artery diameter. Intriguingly, nearly half of the patients with microvascular spasm in the study also showed epicardial vasoconstriction of at least moderate degree.32 Compared with patients with primary epicardial spasm, patients with microvascular spasm presented more frequently with ischaemic ECG changes during non-invasive testing, exertional dyspnoea, and intermittent rest angina rather than isolated exertional chest pain (which is most commonly and typically seen in obstructive CAD).32 This is an important observation and, in fact, the occurrence of exertional angina (exclusively) is not a diagnostic requirement for ‘microvascular angina’ (which can presents with both exertional and rest angina).32,33 Distinct from patients with Prinzmetal's variant angina, ST-segment elevation is extremely rare in patients with microvascular angina.2,27 Furthermore, it has been reported that nitroglycerin may not provide quick and/or sufficient chest pain control in microvascular angina compared with Prinzmetal's variant angina, as the small arterioles can forgo the vasodilatory effect of nitroglycerin.34 If associated with a significant drop in aortic perfusion pressure, nitroglycerin may even worsen myocardial ischaemia, as seen especially in patients with coronary slow flow syndrome, also known as ‘cardiac syndrome Y’. The term cardiac syndrome Y has been chosen mainly because of the possible causal role of neuropeptide Y in this entity, which is characterized by an abnormally high microvascular resistance at rest but a normal vasodilatory response to direct vasodilators and pacing.35–40 Patients with this condition present with rest angina rather than effort angina and abnormal stress testing results and, in fact, often have a history of multiple admissions for unstable angina. In distinction, an abnormal (ECG) stress test response is an integral criterion for CSX, which typically presents with exercise-induced angina but can also sustain episodes of angina at rest in the presence of angiographically normal coronary arteries.1,27
Patients with epicardial coronary atherosclerosis also have, on average, a higher microvascular resistance (even though with a noteworthy overlap in values with patients with and without epicardial CAD).41 In particular, the impairment in endothelium-dependent dilation of the coronary microvasculature in the early stages of epicardial atherosclerosis has been viewed as evidence that the pathophysiological consequences of atherosclerosis may extend into the human coronary microcirculation.42 However, one may also argue that the abnormalities can be first encountered in the microcirculation, i.e. before any epicardial disease. This view is supported by the fact that an impairment in the CBF reserve can be found already in patients with risk factors but without obstructive CAD.4 Furthermore, there is evidence for progressive impairment of microvascular dysfunction involving endothelium-dependent and endothelium-independent function as the underlying disease process progresses, such as in pre-diabetes and diabetes before macrovascular disease.43 The presence of microvascular dysfunction may also explain why at least 20% of patients with CAD continue to experience angina even after successful elimination of all haemodynamically significant lesions by revascularization procedures.44 This holds true even in the acute post-PCI period when the signs and symptoms of myocardial ischaemia can become quite notable despite an ‘otherwise successful intervention’.45,46
Finally, the presence of CMVD can contribute to the signs and symptoms of myocardial ischaemia in patients with other forms of structural heart disease. For instance, the CFR is markedly impaired in patients with aortic stenosis due to an increase in the baseline CBF which is to meet the increased metabolic demand.47,48 In this patient population, reduced CFR, increased transvalvular gradient, and reduced transcoronary perfusion pressure have all been considered to mediate angina despite normal coronary arteries. More recently, decoupling of the normal regulatory mechanisms of CBF at the microvascular level has been suggested to play an important role as well.49
Acute coronary syndrome
Angina at rest, increasing angina, and new-onset angina are the three principal presentations of unstable angina/non-ST-segment myocardial infarction.50 Microvascular angina may also present as an acute coronary syndrome (ACS) and should be considered a differential diagnosis in the 10% male and 25% female patients admitted to hospital with the diagnosis of ACS and found to have ‘normal’ coronary angiograms.51,52 Intriguingly, ACS entails a broad spectrum of clinical presentations, and evidence of CMVD by the TIMI frame count extends beyond the presumed culprit artery in many cases.53 These facts prompted us to postulate that the coronary microcirculation may take a more important role in ACS than traditionally thought.54 Indeed, microvascular resistance can increase during ischaemia in patients with unstable angina, contrary to the classical concept of maximal compensatory vasodilation.55 Moreover, CMVD may reduce the reserve of the myocardium to tolerate ischaemia. This has been highlighted in the setting of PCI, in which patients with evidence of myocardial injury and microvascular impairment have a reduced CFR pre-procedurally.56,57 Along these lines, it is noteworthy that diabetes and the metabolic syndrome, often considered to represent the epitome of microvascular disease, are both associated with poor myocardial perfusion and larger infarcts in the setting of ACS.58 An important (and more accepted) aspect is that CMVD can influence the clinical course following reperfusion therapies. The restoration of the CBF can (paradoxically) harm endothelial cells and myocytes further and lead to so-called reperfusion injury.54,59 Even prior to this primarily oxidative-inflammatory (reperfusion) insult the function of the coronary microcirculation can be considerably compromised in the context of an ACS due to embolization of plaque debris and thrombus as well as the release of numerous vasoconstrictor molecules and reduced nitric oxide production and bioavailability. As a consequence, chest pain and ST-segment elevation can persist or recur despite the successful resolution of epicardial artery occlusions. Acute in-hospital complications such as heart failure, cardiac rupture, and cardiac death are also more frequent under these circumstances.60
In addition to these considerations, it is conceivable that acute and extensive CMVD (in terms of intensity, duration, and localization) can induce severe ischaemia that involves a larger area of the myocardium, yet not confined to a territory defined by the large epicardial coronary arteries. As a clinical example, CMVD may be responsible for cases of apical ballooning syndrome (APS), also known as stress(-induced) or takotsubo cardiomyopathy. By definition, APS patients do not have obstructive CAD; yet abnormal myocardial perfusion can be documented in 70% of patients.61 In studies using serial echocardiography, CFR responses to dipyridamole and adenosine were found to be impaired in the acute phase of presentation and to improve thereafter, correlating with improvements in contractile function.62,63 PET imaging studies confirmed these CFR dynamics and showed an inverse perfusion/metabolism mismatch, usually characteristic of stunning but observed here in the presence of impaired perfusion.64,65 A yet stronger case for causality in this setting was made by the observation that myocardial perfusion, contractility, and LV function improve markedly with the administration of i.v. adenosine in patients with APS but not in those with acute myocardial infarction.66 Moreover, in patients with a history of APS, cold pressure testing induced new regional wall motion abnormalities that were similar to those seen in the acute phase of the syndrome, in association with prominent blunting of the MBF response from a normal baseline level. 67 In addition, a study from our group pointed out increased vascular reactivity and decreased endothelial function in response to acute mental stress in patients with a history of APS.68 Taken together, these findings suggest that the reduction in LV contractility is the consequence of a reduction in myocardial perfusion not due to epicardial disease but rather abnormal vasoreactivity at the level of the coronary microcirculation.
Chronic heart failure
Coronary microvascular dysfunction has also been considered to underlie the reduction in LV function in association with stress-induced myocardial ischaemia in women with angina and normal coronary arteries (post-stress stunning).69,70 Repetition of such episodes could lead to hibernation and more persistent LV dysfunction. However, patients with presumed primary chronic CMVD are not at an increased risk of developing heart failure symptoms, in keeping with a functional disease process.25 On the contrary, structural, non-reversible abnormalities of the coronary microcirculation may be associated with a greater pre-disposition to a persistent reduction in LV function. Indeed, several studies have demonstrated that CMVD after myocardial infarction increases the risk of congestive heart failure and heart failure hospitalizations up to six to eight times.71,72 Similarly in patients with hypertrophic cardiomyopathy, severe microvascular dysfunction was predictive of adverse left ventricular remodelling and systolic dysfunction.73 Furthermore, impairment of the coronary flow velocity reserve was the only independent predictor of LV systolic function dynamics in patients with hypertensive dilated cardiomyopathy over time.74 Finally, the coronary microcirculation is considered to play an important role in the development of diabetic cardiomyopathy.75,76 Indeed, in experimental models of diabetes, the earliest abnormality occurred in the growth factor milieu that maintains the coronary microcirculation, followed by a decrease in capillary density and impaired myocardial perfusion with subsequent cardiomyocyte apoptosis and necrosis, replacement fibrosis, and progressive diastolic and systolic dysfunction.77 Also, experimental studies have demonstrated a reduction in systolic and diastolic function with progressive obliteration of the coronary microcirculation by repetitive injection of microemboli.78 Collectively, these data indicate that persistent impairment of the integrity of the coronary microcirculation can lead to impairment of cardiac function and heart failure.
Prognosis of coronary microvascular dysfunction
The prognostic impact of CMVD is inherently intertwined with any concomitant coronary or cardiac disease process.
Chronic presentation with angina with or without coronary artery disease
Historically, studies in CSX indicated an overall good prognosis,79,80 particularly in patients with chest pain and completely normal coronary arteriograms, even in the presence of abnormal exercise stress test findings. Indeed, as shown by Kaski et al.81 in CSX patients with completely normal coronary angiograms, normal ventricular function, and evidence of mild ischaemia, prognosis is good, but the debate continues regarding the prognosis in CSX, particularly in relation to some patient subgroups.
More recently, and perhaps as a result of the incorporation of larger numbers of patients—therefore increasing the heterogeneity of the population—longer follow-up studies in larger sample sizes suggested a more adverse prognosis in at least some CSX subgroups.82,83 Also, better characterization of the patients, specifically those with documented ischaemia, mild CAD, and microvascular dysfunction has resulted in the identification of at-risk subgroups. Studies have shown that coronary microvascular dysfunction and a reduced CFR predict an adverse prognosis, albeit the issue is confounded by the inclusion of patients with mild or moderate CAD in these studies.84,85
Of interest, patients with a reduction in the CBF to intracoronary ACH (abnormal microvascular response) appear to be at a higher risk of developing cardiovascular events during follow-up, regardless of the presence or absence of obstructive epicardial coronary artery stenoses.86,87 With the caveats outlined above, an abnormal CFR seems to be a marker of a worse long-term outcome including a >6-fold higher adjusted mortality risk in patients with a CFR <3.0.84,88 In agreement, other non-invasive studies pointed CFR out as the strongest independent risk factor for non-STEMI and death in patients with coronary luminal irregularities.89
Among women with persistent signs and symptoms of ischaemia, a relatively higher proportion of adverse events, i.e. heart failure rather than myocardial infarction or increased mortality, has been reported in association with microvascular dysfunction. Data from the NIH-NHLBI-sponsored Women's Ischemia Syndrome Evaluation (WISE) and related studies implicate adverse outcomes (albeit not necessarily regarding mortality or other hard endpoints) in relation to CMVD.85,90 Intriguingly, the event-free survival (index events including death, stroke, and hospitalization for heart failure, rather than MI) diverged more strongly after 4 years. In an unselected population of patients undergoing PET perfusion imaging, an adenosine CFR <2 was found to provide additional prognostic information, in particular for cardiac death, for which it remains the most potent independent predictor.91 While it may be argued that the extent of CAD was not taken into consideration in these studies, an abnormal MBF response to cold pressure testing predicted a 6–8 times higher incidence of ACS and revascularization events during long-term follow-up even in patients without luminal irregularities on angiography.92 Hence, regardless of the epicardial disease status and even in those with normal coronary arteries, the presence of CMVD indicated a significantly elevated risk for epicardial events. Of interest, this risk does not appear immediately in the follow-up but emerges during the long-term (>2 years) follow-up.
It is important to stress that most of these studies, in general, have included heterogeneous patient groups, i.e. ACS cases and patients with different degrees of CAD and LV dysfunction. Lumping together CSX patients with effort-induced angina and completely normal coronary angiograms with patients presenting with acute chest pain, coronary artery stenoses ranging from 20 to 50%, impaired LV function, conduction disturbances, and comorbidities affecting the coronary microcirculation (and overall prognosis) is likely to confuse the issue. Future prospective studies will be required to define very specifically the different patient subgroups that are considered for analysis.
Acute myocardial infarction
The absence of the restoration of the MBF despite an open epicardial artery has been attributed to CMVD. In this setting, myocardial microvascular resistance remains high, myocardial blush and perfusion remain poor, and ST-segments remain elevated (Figure 3).93,94 Clinically important is the fact that the presence of any of these parameters of inadequate tissue level perfusion (and even more so if associated with persistent abnormalities on MRI) portrays a worse prognosis (Figure 4).7,71,72,95–97 Thus, there is a pathophysiological link between microvascular dysfunction and progressive LV dilation, the development of heart failure, and cardiac death after AMI and primary PCI.72 Microvascular dysfunction is unlikely to be simply the consequence of the extent of AMI as it remains an outcome predictor even after adjustment for the infarct size. While some patients sustain microvascular injury before reperfusion and others develop it afterwards, a vital question is how much the coronary microcirculation contributes to the recovery of infarcted myocardium.60 Most likely, the functional and structural integrity of the coronary microcirculation contributes to the recovery of stunned myocardium and limits permanent damage. This has been suggested by the observation that lesser degrees of microvascular impairment are associated with better functional recovery after an AMI.93,94,96,98
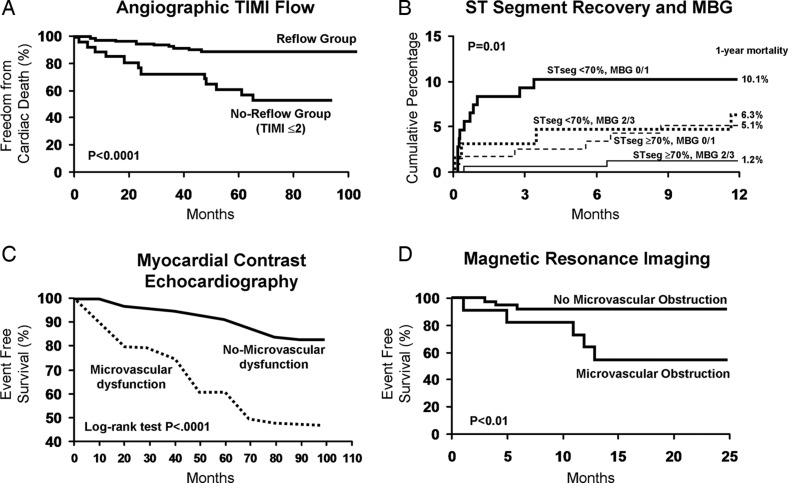
Figure 4.
Illustration of the prognostic indicator function of the coronary microcirculation in acute myocardial infarction. Whether assessed by TIMI flow,71 myocardial blush grade or ST-segment resolution,95 myocardial contrast echocardiography,72 or magnetic resonance imaging,7 prognosis is significantly worse if myocardial perfusion is not restored despite an open epicardial artery. Images used with the permission of the American Heart Association.
Percutaneous coronary intervention
One might argue that under no other circumstance can the onset and hence the impact of CMVD be more defined clinically than in the setting of elective revascularization procedures, particularly PCI. Numerous studies have provided tangible evidence for the occurrence of embolization of particulate matter and the release of vasoactive molecules into the microcirculation at the time of PCI.56,99–101 Furthermore, the considerable reduction in no-reflow events and periprocedural myocardial infarction (PMI) with distal embolization protection devices (especially in saphenous vein graft interventions) substantiates the view that PCI-related embolic events impair the integrity of the microcirculation and the viability of myocytes.102 Unfortunately, no long-term follow-up data are available that could provide further insight into the long-term clinical implications of microvascular dysfunction in this setting. Obviously, the extent of underlying CAD plays an important prognostic role. This holds true also for the much-debated entity of PMI, and no study so far has evaluated the differential prognostic impact of PMI due to side-branch occlusion (type I or proximal type) or microcirculatory impairment (type II or distal type).45,46 For this reason, the prognostic implications of CMVD in the setting of PCI remain uncertain.
Cardiomyopathy
In patients with dilated cardiomyopathy, a severely (>60%) reduced hyperaemic MBF response to dipyridamole increases the relative risk of death and heart failure development or progression 3.5 times, independent of other factors such as the degree of LV dysfunction and the presence of overt heart failure.103 Likewise, an abnormal CFR (<2) and lack of inotropic reserve in response to dipyridamole were independent predictors of survival in patients with idiopathic DCM (adjusted harzard ratios 2.8 and 2.3, respectively).104 Importantly, the prognostic merit of severe CFR impairment in heart failure is independent of CAD and the ischaemic burden and is evident in both ischaemic and non-ischaemic cardiomyopathy.105
In hypertrophic obstructive cardiomyopathy, the MBF response to dipyridamole potently predicts symptomatic progression to NYHA class III and IV and life-threatening ventricular arrhythmias requiring ICD placement and is an independent mortality predictor.106 Especially those patients with the lowest MBF response are seemingly at the highest risk (adjusted hazard ratio 10 for cardiovascular mortality and 20 for all cardiovascular events), which again becomes apparent not immediately but during long-term follow-up (i.e. 6 years). Interestingly, these patients also had a higher risk of progressive LV remodelling and systolic dysfunction.73 Intriguingly, microvascular dysfunction colocalized with areas of late gadolinium enhancement on MRI and hence may lead to recurrent myocardial ischaemia and myocyte death, and eventually replacement fibrosis.107
Finally, in a study of cardiac transplant recipients, a CFR <2.5 in response to dipyridamole was found to be associated with a decline in the LVEF during exercise at the 2-year follow-up.108 Moreover, an abnormal CBF response to ACH was found to be associated with ischaemic events and death >1 year after heart transplantation; however, only in association with angiographically significant epicardial disease (luminal diameter reduction ≥50%).109 The central question of an independent prognostic role of CMVD was eventually answered by the finding that a CFR <2.7 in response to adenosine predicts long-term survival independent of other echocardiographic and angiographic variables, donor age, predisposition to ischaemic heart disease, and treatment.110 Hence, the status of the coronary microcirculation is an important outcome predictor in patients with cardiomyopathy and heart failure, underscoring once again, its vital role in different clinical settings (Table 4).
Conclusions
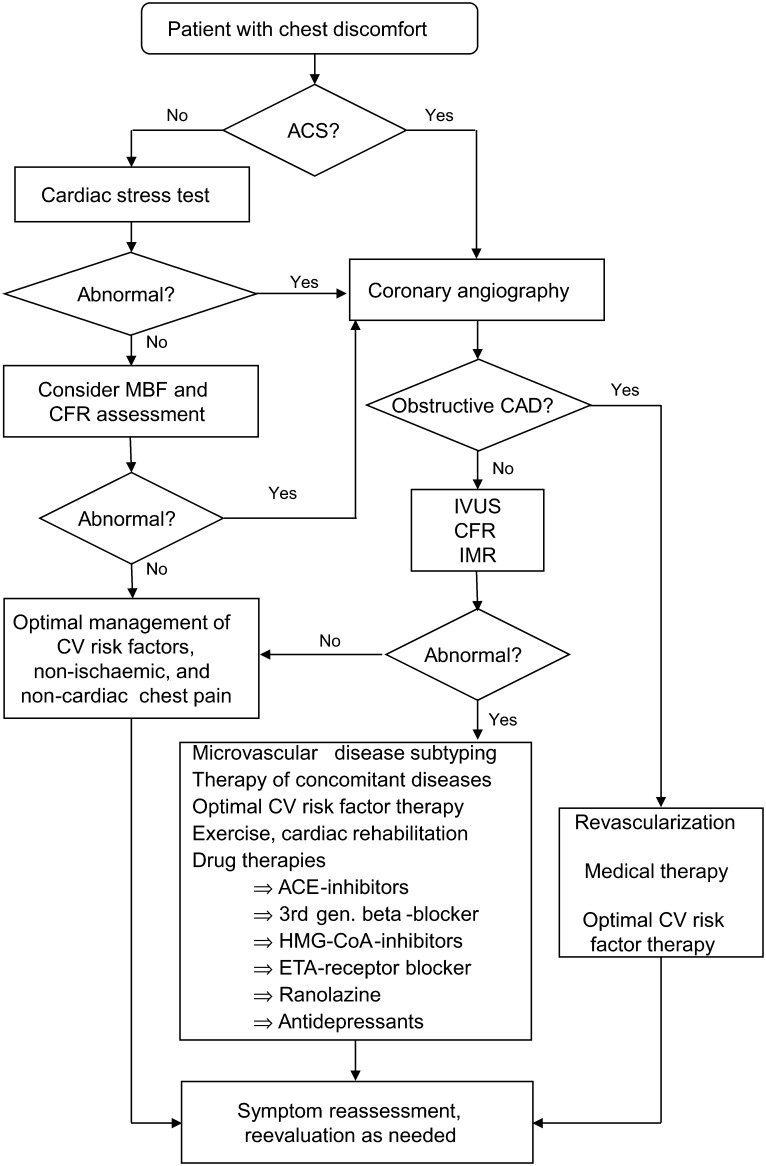
Over the past decades many studies have highlighted the functional significance of the coronary microcirculation in a diversity of clinical settings. Functional and/or structural coronary microvascular abnormalities often explain the signs and symptoms of myocardial ischaemia in individuals with normal coronary angiograms and can possibly contribute to the clinical presentation of patients with CAD and other cardiac conditions. As such, an assessment of CMVD should be considered in the evaluation of angina patients, particularly those with normal coronary arteries or non-obstructive CAD (Figure 5). Identifying the mechanisms underlying the patient's symptoms is important to provide a rational treatment that aims at both improving the quality of life and long-term prognosis when feasible. Taken together, evidence gathered in recent years has shown that CMVD is a true clinical entity rather than a mystery or an academic curiosity.
Figure 5.
Although the discussion of management strategies is beyond the scope of the present manuscript, this figure shows a flow chart outlining a suggested algorithm for the management of patients with chest pain despite angiographically normal coronary arteries, in whom the underlying mechanism is coronary microvascular dysfunction. ACS, acute coronary syndrome; CAD, coronary artery disease; CFR, coronary flow reserve; CV, cardiovascular; ETA, endothelin-type A receptor; IMR, index of microvascular resistance; IVUS, intravascular ultrasound; MBF, myocardial blood flow.
Funding
The study was supported by grants (HL92954 and AG31750 to A.L.) from the National Institutes of Health and St George's, University of London.
Conflict of interest: none declared.
References
- 1.Kemp HG, Jr, Vokonas PS, Cohn PF, Gorlin R. The anginal syndrome associated with normal coronary arteriograms. Report of a six year experience. Am J Med. 1973;54:735–742. doi: 10.1016/0002-9343(73)90060-0. doi:10.1016/0002-9343(73)90060-0. [DOI] [PubMed] [Google Scholar]
- 2.Kaski JC. Pathophysiology and management of patients with chest pain and normal coronary arteriograms (cardiac syndrome X) Circulation. 2004;109:568–572. doi: 10.1161/01.CIR.0000116601.58103.62. doi:10.1161/01.CIR.0000116601.58103.62. [DOI] [PubMed] [Google Scholar]
- 3.Patel B, Fisher M. Therapeutic advances in myocardial microvascular resistance: unravelling the enigma. Pharmacol Ther. 2010;127:131–147. doi: 10.1016/j.pharmthera.2010.04.014. doi:10.1016/j.pharmthera.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. doi: 10.1056/NEJMra061889. doi:10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 5.Kuo L, Chilian WM, Davis MJ. Coronary arteriolar myogenic response is independent of endothelium. Circ Res. 1990;66:860–866. doi: 10.1161/01.res.66.3.860. doi:10.1161/01.RES.66.3.860. [DOI] [PubMed] [Google Scholar]
- 6.van 't Hof AW, Liem A, Suryapranata H, Hoorntje JC, de Boer MJ, Zijlstra F. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation. 1998;97:2302–2306. doi: 10.1161/01.cir.97.23.2302. doi:10.1161/01.CIR.97.23.2302. [DOI] [PubMed] [Google Scholar]
- 7.Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, Blumenthal RS, Lima JA. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765–772. doi: 10.1161/01.cir.97.8.765. doi:10.1161/01.CIR.97.8.765. [DOI] [PubMed] [Google Scholar]
- 8.Leung DY, Leung M. Non-invasive/invasive imaging: significance and assessment of coronary microvascular dysfunction. Heart. 2011;97:587–595. doi: 10.1136/hrt.2009.183327. doi:10.1136/hrt.2009.183327. [DOI] [PubMed] [Google Scholar]
- 9.Cassar A, Chareonthaitawee P, Rihal CS, Prasad A, Lennon RJ, Lerman LO, Lerman A. Lack of correlation between noninvasive stress tests and invasive coronary vasomotor dysfunction in patients with nonobstructive coronary artery disease. Circ Cardiovasc Interv. 2009;2:237–244. doi: 10.1161/CIRCINTERVENTIONS.108.841056. doi:10.1161/CIRCINTERVENTIONS.108.841056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson CM, Cannon CP, Daley WL, Dodge JT, Jr, Alexander B, Jr, Marble SJ, McCabe CH, Raymond L, Fortin T, Poole WK, Braunwald E. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93:879–888. doi: 10.1161/01.cir.93.5.879. doi:10.1161/01.CIR.93.5.879. [DOI] [PubMed] [Google Scholar]
- 11.Kern MJ. Coronary physiology revisited: practical insights from the cardiac catheterization laboratory. Circulation. 2000;101:1344–1351. doi: 10.1161/01.cir.101.11.1344. doi:10.1161/01.CIR.101.11.1344. [DOI] [PubMed] [Google Scholar]
- 12.Pijls NH, De Bruyne B, Smith L, Aarnoudse W, Barbato E, Bartunek J, Bech GJ, Van De Vosse F. Coronary thermodilution to assess flow reserve: validation in humans. Circulation. 2002;105:2482–2486. doi: 10.1161/01.cir.0000017199.09457.3d. doi:10.1161/01.CIR.0000017199.09457.3D. [DOI] [PubMed] [Google Scholar]
- 13.Fearon WF, Balsam LB, Farouque HM, Caffarelli AD, Robbins RC, Fitzgerald PJ, Yock PG, Yeung AC. Novel index for invasively assessing the coronary microcirculation. Circulation. 2003;107:3129–3132. doi: 10.1161/01.CIR.0000080700.98607.D1. doi:10.1161/01.CIR.0000080700.98607.D1. [DOI] [PubMed] [Google Scholar]
- 14.Melikian N, Kearney MT, Thomas MR, De Bruyne B, Shah AM, MacCarthy PA. A simple thermodilution technique to assess coronary endothelium-dependent microvascular function in humans: validation and comparison with coronary flow reserve. Eur Heart J. 2007;28:2188–2194. doi: 10.1093/eurheartj/ehm269. doi:10.1093/eurheartj/ehm269. [DOI] [PubMed] [Google Scholar]
- 15.Yong AS, Ho M, Shah MG, Ng MK, Fearon WF. Coronary microcirculatory resistance is independent of epicardial stenosis. Circ Cardiovasc Interv. 2012;5:103–108. doi: 10.1161/CIRCINTERVENTIONS.111.966556. S1–2 doi:10.1161/CIRCINTERVENTIONS.111.966556. [DOI] [PubMed] [Google Scholar]
- 16.Herrmann J, Lerman A. The endothelium: dysfunction and beyond. J Nucl Cardiol. 2001;8:197–206. doi: 10.1067/mnc.2001.114148. doi:10.1067/mnc.2001.114148. [DOI] [PubMed] [Google Scholar]
- 17.Newby DE. Intracoronary infusions and the assessment of coronary blood flow in clinical studies. Heart. 2000;84:118–120. doi: 10.1136/heart.84.2.118. doi:10.1136/heart.84.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Summers MR, Lerman A, Lennon RJ, Rihal CS, Prasad A. Myocardial ischaemia in patients with coronary endothelial dysfunction: insights from body surface ECG mapping and implications for invasive evaluation of chronic chest pain. Eur Heart J. 2011;32:2758–2765. doi: 10.1093/eurheartj/ehr221. doi:10.1093/eurheartj/ehr221. [DOI] [PubMed] [Google Scholar]
- 19.Hirohata A, Nakamura M, Waseda K, Honda Y, Lee DP, Vagelos RH, Hunt SA, Valantine HA, Yock PG, Fitzgerald PJ, Yeung AC, Fearon WF. Changes in coronary anatomy and physiology after heart transplantation. Am J Cardiol. 2007;99:1603–1607. doi: 10.1016/j.amjcard.2007.01.039. doi:10.1016/j.amjcard.2007.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Carli MF, Tobes MC, Mangner T, Levine AB, Muzik O, Chakroborty P, Levine TB. Effects of cardiac sympathetic innervation on coronary blood flow. N Engl J Med. 1997;336:1208–1215. doi: 10.1056/NEJM199704243361703. doi:10.1056/NEJM199704243361703. [DOI] [PubMed] [Google Scholar]
- 21.Wildhirt SM, Weis M, Schulze C, Conrad N, Rieder G, Enders G, Hoepp C, von Scheidt W, Reichart B. An association between microvascular endothelial dysfunction, transcardiac nitric oxide production and pro-inflammatory cytokines after heart transplantation in humans. Transpl Int. 2000;13(Suppl. 1):S228–S234. doi: 10.1007/s001470050330. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y, Chester AH, Hariri B, McCormack A, Sarathchandra P, Rose ML. The indirect alloimmune response causes microvascular endothelial dysfunction-a possible role for alloantibody. Transplantation. 2010;90:1157–1164. doi: 10.1097/TP.0b013e3181fa9480. doi:10.1097/TP.0b013e3181fa9480. [DOI] [PubMed] [Google Scholar]
- 23.Osto E, Castellani C, Fadini GP, Baesso I, Gambino A, Agostini C, Avogaro A, Gerosa G, Thiene G, Iliceto S, Angelini A, Tona F. Impaired endothelial progenitor cell recruitment may contribute to heart transplant microvasculopathy. J Heart Lung Transplant. 2011;30:70–76. doi: 10.1016/j.healun.2010.07.004. doi:10.1016/j.healun.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Lanza GA, Crea F. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation. 2010;121:2317–2325. doi: 10.1161/CIRCULATIONAHA.109.900191. doi:10.1161/CIRCULATIONAHA.109.900191. [DOI] [PubMed] [Google Scholar]
- 25.Takeuchi M, Kuroiwa A. Alteration of coronary flow velocity during spontaneous angina in a patient with microvascular angina. Catheter Cardiovasc Interv. 2000;50:63–67. doi: 10.1002/(sici)1522-726x(200005)50:1<63::aid-ccd13>3.0.co;2-1. doi:10.1002/(SICI)1522-726X(200005)50:1<63::AID-CCD13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 26.Cannon RO, III, Epstein SE. ‘Microvascular angina’ as a cause of chest pain with angiographically normal coronary arteries. Am J Cardiol. 1988;61:1338–1343. doi: 10.1016/0002-9149(88)91180-0. doi:10.1016/0002-9149(88)91180-0. [DOI] [PubMed] [Google Scholar]
- 27.Kaski JC, Aldama G, Cosin-Sales J. Cardiac syndrome X. Diagnosis, pathogenesis and management. Am J Cardiovasc Drugs. 2004;4:179–194. doi: 10.2165/00129784-200404030-00005. doi:10.2165/00129784-200404030-00005. [DOI] [PubMed] [Google Scholar]
- 28.Maseri A, Crea F, Kaski JC, Davies G. Mechanisms and significance of cardiac ischemic pain. Prog Cardiovasc Dis. 1992;35:1–18. doi: 10.1016/0033-0620(92)90031-t. doi:10.1016/0033-0620(92)90031-T. [DOI] [PubMed] [Google Scholar]
- 29.Nihoyannopoulos P, Kaski JC, Crake T, Maseri A. Absence of myocardial dysfunction during stress in patients with syndrome X. J Am Coll Cardiol. 1991;18:1463–1470. doi: 10.1016/0735-1097(91)90676-z. doi:10.1016/0735-1097(91)90676-Z. [DOI] [PubMed] [Google Scholar]
- 30.Douglas PS, Patel MR, Bailey SR, Dai D, Kaltenbach L, Brindis RG, Messenger J, Peterson ED. Hospital variability in the rate of finding obstructive coronary artery disease at elective, diagnostic coronary angiography. J Am Coll Cardiol. 2011;58:801–809. doi: 10.1016/j.jacc.2011.05.019. doi:10.1016/j.jacc.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, Jorgensen E, Kelbaek H, Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–744. doi: 10.1093/eurheartj/ehr331. doi:10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- 32.Ong P, Athanasiadis A, Borgulya G, Mahrholdt H, Kaski JC, Sechtem U. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries. The ACOVA Study (Abnormal COronary VAsomotion in patients with stable angina and unobstructed coronary arteries) J Am Coll Cardiol. 2012;59:655–662. doi: 10.1016/j.jacc.2011.11.015. doi:10.1016/j.jacc.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Cosin-Sales J, Pizzi C, Brown S, Kaski JC. C-reactive protein, clinical presentation, and ischemic activity in patients with chest pain and normal coronary angiograms. J Am Coll Cardiol. 2003;41:1468–1474. doi: 10.1016/s0735-1097(03)00243-2. doi:10.1016/S0735-1097(03)00243-2. [DOI] [PubMed] [Google Scholar]
- 34.Kanatsuka H, Eastham CL, Marcus ML, Lamping KG. Effects of nitroglycerin on the coronary microcirculation in normal and ischemic myocardium. J Cardiovasc Pharmacol. 1992;19:755–763. [PubMed] [Google Scholar]
- 35.Clarke JG, Davies GJ, Kerwin R, Hackett D, Larkin S, Dawbarn D, Lee Y, Bloom SR, Yacoub M, Maseri A. Coronary artery infusion of neuropeptide Y in patients with angina pectoris. Lancet. 1987;1:1057–1059. doi: 10.1016/s0140-6736(87)90483-1. doi:10.1016/S0140-6736(87)90483-1. [DOI] [PubMed] [Google Scholar]
- 36.Beltrame JF, Limaye SB, Horowitz JD. The coronary slow flow phenomenon–a new coronary microvascular disorder. Cardiology. 2002;97:197–202. doi: 10.1159/000063121. doi:10.1159/000063121. [DOI] [PubMed] [Google Scholar]
- 37.Fineschi M, Gori T. Coronary slow flow: description of a new ‘cardiac Y’ syndrome. Int J Cardiol. 2009;137:308–310. doi: 10.1016/j.ijcard.2008.05.076. doi:10.1016/j.ijcard.2008.05.076. [DOI] [PubMed] [Google Scholar]
- 38.Fragasso G, Chierchia SL, Arioli F, Carandente O, Gerosa S, Carlino M, Palloshi A, Gianolli L, Calori G, Fazio F, Margonato A. Coronary slow-flow causing transient myocardial hypoperfusion in patients with cardiac syndrome X: long-term clinical and functional prognosis. Int J Cardiol. 2009;137:137–144. doi: 10.1016/j.ijcard.2008.06.070. doi:10.1016/j.ijcard.2008.06.070. [DOI] [PubMed] [Google Scholar]
- 39.Beltrame JF, Limaye SB, Wuttke RD, Horowitz JD. Coronary hemodynamic and metabolic studies of the coronary slow flow phenomenon. Am Heart J. 2003;146:84–90. doi: 10.1016/S0002-8703(03)00124-8. doi:10.1016/S0002-8703(03)00124-8. [DOI] [PubMed] [Google Scholar]
- 40.Fineschi M, Bravi A, Gori T. The ‘slow coronary flow’ phenomenon: evidence of preserved coronary flow reserve despite increased resting microvascular resistances. Int J Cardiol. 2008;127:358–361. doi: 10.1016/j.ijcard.2007.06.010. doi:10.1016/j.ijcard.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Melikian N, Vercauteren S, Fearon WF, Cuisset T, MacCarthy PA, Davidavicius G, Aarnoudse W, Bartunek J, Vanderheyden M, Wyffels E, Wijns W, Heyndrickx GR, Pijls NH, de Bruyne B. Quantitative assessment of coronary microvascular function in patients with and without epicardial atherosclerosis. EuroIntervention. 2010;5:939–945. doi:10.4244/EIJV5I8A158. [PubMed] [Google Scholar]
- 42.Zeiher AM, Drexler H, Wollschlager H, Just H. Endothelial dysfunction of the coronary microvasculature is associated with coronary blood flow regulation in patients with early atherosclerosis. Circulation. 1991;84:1984–1992. doi: 10.1161/01.cir.84.5.1984. doi:10.1161/01.CIR.84.5.1984. [DOI] [PubMed] [Google Scholar]
- 43.Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging. 2010;3:623–640. doi: 10.1016/j.jcmg.2010.04.007. doi:10.1016/j.jcmg.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 44.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van't Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. doi:10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 45.Herrmann J. Peri-procedural myocardial injury: 2005 update. Eur Heart J. 2005;26:2493–2519. doi: 10.1093/eurheartj/ehi455. doi:10.1093/eurheartj/ehi455. [DOI] [PubMed] [Google Scholar]
- 46.Prasad A, Herrmann J. Myocardial infarction due to percutaneous coronary intervention. N Engl J Med. 2011;364:453–464. doi: 10.1056/NEJMra0912134. doi:10.1056/NEJMra0912134. [DOI] [PubMed] [Google Scholar]
- 47.Marcus ML, Doty DB, Hiratzka LF, Wright CB, Eastham CL. Decreased coronary reserve: a mechanism for angina pectoris in patients with aortic stenosis and normal coronary arteries. N Engl J Med. 1982;307:1362–1366. doi: 10.1056/NEJM198211253072202. doi:10.1056/NEJM198211253072202. [DOI] [PubMed] [Google Scholar]
- 48.Eberli FR, Ritter M, Schwitter J, Bortone A, Schneider J, Hess OM, Krayenbuehl HP. Coronary reserve in patients with aortic valve disease before and after successful aortic valve replacement. Eur Heart J. 1991;12:127–138. doi: 10.1093/oxfordjournals.eurheartj.a059858. [DOI] [PubMed] [Google Scholar]
- 49.Davies JE, Sen S, Broyd C, Hadjiloizou N, Baksi J, Francis DP, Foale RA, Parker KH, Hughes AD, Chukwuemeka A, Casula R, Malik IS, Mikhail GW, Mayet J. Arterial pulse wave dynamics after percutaneous aortic valve replacement: fall in coronary diastolic suction with increasing heart rate as a basis for angina symptoms in aortic stenosis. Circulation. 2011;124:1565–1572. doi: 10.1161/CIRCULATIONAHA.110.011916. doi:10.1161/CIRCULATIONAHA.110.011916. [DOI] [PubMed] [Google Scholar]
- 50.Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D, Bax JJ, Auricchio A, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Knuuti J, Kolh P, McDonagh T, Moulin C, Poldermans D, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Torbicki A, Vahanian A, Windecker S, Achenbach S, Badimon L, Bertrand M, Botker HE, Collet JP, Crea F, Danchin N, Falk E, Goudevenos J, Gulba D, Hambrecht R, Herrmann J, Kastrati A, Kjeldsen K, Kristensen SD, Lancellotti P, Mehilli J, Merkely B, Montalescot G, Neumann FJ, Neyses L, Perk J, Roffi M, Romeo F, Ruda M, Swahn E, Valgimigli M, Vrints CJ, Widimsky P. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. doi:10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 51.Lagerqvist B, Safstrom K, Stahle E, Wallentin L, Swahn E. Is early invasive treatment of unstable coronary artery disease equally effective for both women and men? FRISC II Study Group Investigators. J Am Coll Cardiol. 2001;38:41–48. doi: 10.1016/s0735-1097(01)01308-0. doi:10.1016/S0735-1097(01)01308-0. [DOI] [PubMed] [Google Scholar]
- 52.Germing A, Lindstaedt M, Ulrich S, Grewe P, Bojara W, Lawo T, von Dryander S, Jager D, Machraoui A, Mugge A, Lemke B. Normal angiogram in acute coronary syndrome-preangiographic risk stratification, angiographic findings and follow-up. Int J Cardiol. 2005;99:19–23. doi: 10.1016/j.ijcard.2004.07.003. doi:10.1016/j.ijcard.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 53.Gibson CM, Ryan KA, Murphy SA, Mesley R, Marble SJ, Giugliano RP, Cannon CP, Antman EM, Braunwald E. Impaired coronary blood flow in nonculprit arteries in the setting of acute myocardial infarction. The TIMI Study Group. Thrombolysis in myocardial infarction. J Am Coll Cardiol. 1999;34:974–982. doi: 10.1016/s0735-1097(99)00335-6. doi:10.1016/S0735-1097(99)00335-6. [DOI] [PubMed] [Google Scholar]
- 54.Lerman A, Holmes DR, Herrmann J, Gersh BJ. Microcirculatory dysfunction in ST-elevation myocardial infarction: cause, consequence, or both? Eur Heart J. 2007;28:788–797. doi: 10.1093/eurheartj/ehl501. doi:10.1093/eurheartj/ehl501. [DOI] [PubMed] [Google Scholar]
- 55.Marzilli M, Sambuceti G, Fedele S, L'Abbate A. Coronary microcirculatory vasoconstriction during ischemia in patients with unstable angina. J Am Coll Cardiol. 2000;35:327–334. doi: 10.1016/s0735-1097(99)00554-9. doi:10.1016/S0735-1097(99)00554-9. [DOI] [PubMed] [Google Scholar]
- 56.Herrmann J, Haude M, Lerman A, Schulz R, Volbracht L, Ge J, Schmermund A, Wieneke H, von Birgelen C, Eggebrecht H, Baumgart D, Heusch G, Erbel R. Abnormal coronary flow velocity reserve after coronary intervention is associated with cardiac marker elevation. Circulation. 2001;103:2339–2345. doi: 10.1161/01.cir.103.19.2339. doi:10.1161/01.CIR.103.19.2339. [DOI] [PubMed] [Google Scholar]
- 57.Hoole SP, White PA, Heck PM, Khan SN, Densem CG, Clarke SC, Shapiro LM, Schofield PM, O'Sullivan M, Dutka DP. Primary coronary microvascular dysfunction and poor coronary collaterals predict post-percutaneous coronary intervention cardiac necrosis. Coron Artery Dis. 2009;20:253–259. doi: 10.1097/MCA.0b013e32832ac5ac. doi:10.1097/MCA.0b013e32832ac5ac. [DOI] [PubMed] [Google Scholar]
- 58.Prasad A, Stone GW, Stuckey TD, Costantini CO, Zimetbaum PJ, McLaughlin M, Mehran R, Garcia E, Tcheng JE, Cox DA, Grines CL, Lansky AJ, Gersh BJ. Impact of diabetes mellitus on myocardial perfusion after primary angioplasty in patients with acute myocardial infarction. J Am Coll Cardiol. 2005;45:508–514. doi: 10.1016/j.jacc.2004.10.054. doi:10.1016/j.jacc.2004.10.054. [DOI] [PubMed] [Google Scholar]
- 59.Roe MT, Ohman EM, Maas AC, Christenson RH, Mahaffey KW, Granger CB, Harrington RA, Califf RM, Krucoff MW. Shifting the open-artery hypothesis downstream: the quest for optimal reperfusion. J Am Coll Cardiol. 2001;37:9–18. doi: 10.1016/s0735-1097(00)01101-3. doi:10.1016/S0735-1097(00)01101-3. [DOI] [PubMed] [Google Scholar]
- 60.Yamamuro A, Akasaka T, Tamita K, Yamabe K, Katayama M, Takagi T, Morioka S. Coronary flow velocity pattern immediately after percutaneous coronary intervention as a predictor of complications and in-hospital survival after acute myocardial infarction. Circulation. 2002;106:3051–3056. doi: 10.1161/01.cir.0000043022.44032.77. doi:10.1161/01.CIR.0000043022.44032.77. [DOI] [PubMed] [Google Scholar]
- 61.Elesber A, Lerman A, Bybee KA, Murphy JG, Barsness G, Singh M, Rihal CS, Prasad A. Myocardial perfusion in apical ballooning syndrome correlate of myocardial injury. Am Heart J. 2006;152:469. doi: 10.1016/j.ahj.2006.06.007. e9–13. [DOI] [PubMed] [Google Scholar]
- 62.Meimoun P, Malaquin D, Sayah S, Benali T, Luycx-Bore A, Levy F, Zemir H, Tribouilloy C. The coronary flow reserve is transiently impaired in tako-tsubo cardiomyopathy: a prospective study using serial Doppler transthoracic echocardiography. J Am Soc Echocardiogr. 2008;21:72–77. doi: 10.1016/j.echo.2007.05.024. doi:10.1016/j.echo.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 63.Rigo F, Sicari R, Citro R, Ossena G, Buja P, Picano E. Diffuse, marked, reversible impairment in coronary microcirculation in stress cardiomyopathy: a Doppler transthoracic echo study. Ann Med. 2009;41:462–470. doi: 10.1080/07853890903022793. doi:10.1080/07853890903022793. [DOI] [PubMed] [Google Scholar]
- 64.Kurowski V, Kaiser A, von Hof K, Killermann DP, Mayer B, Hartmann F, Schunkert H, Radke PW. Apical and midventricular transient left ventricular dysfunction syndrome (tako-tsubo cardiomyopathy): frequency, mechanisms, and prognosis. Chest. 2007;132:809–816. doi: 10.1378/chest.07-0608. doi:10.1378/chest.07-0608. [DOI] [PubMed] [Google Scholar]
- 65.Feola M, Rosso GL, Casasso F, Morena L, Biggi A, Chauvie S, Ribichini F, Uslenghi E. Reversible inverse mismatch in transient left ventricular apical ballooning: perfusion/metabolism positron emission tomography imaging. J Nuclear Cardiol. 2006;13:587–590. doi: 10.1016/j.nuclcard.2006.05.004. doi:10.1016/j.nuclcard.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 66.Galiuto L, De Caterina AR, Porfidia A, Paraggio L, Barchetta S, Locorotondo G, Rebuzzi AG, Crea F. Reversible coronary microvascular dysfunction: a common pathogenetic mechanism in apical ballooning or Tako-Tsubo Syndrome. Eur Heart J. 2010;31:1319–1327. doi: 10.1093/eurheartj/ehq039. doi:10.1093/eurheartj/ehq039. [DOI] [PubMed] [Google Scholar]
- 67.Barletta G, Del Pace S, Boddi M, Del Bene R, Salvadori C, Bellandi B, Coppo M, Saletti E, Gensini GF. Abnormal coronary reserve and left ventricular wall motion during cold pressor test in patients with previous left ventricular ballooning syndrome. Eur Heart J. 2009;30:3007–3014. doi: 10.1093/eurheartj/ehp325. doi:10.1093/eurheartj/ehp325. [DOI] [PubMed] [Google Scholar]
- 68.Martin EA, Prasad A, Rihal CS, Lerman LO, Lerman A. Endothelial function and vascular response to mental stress are impaired in patients with apical ballooning syndrome. J Am Coll Cardiol. 2010;56:1840–1846. doi: 10.1016/j.jacc.2010.03.107. doi:10.1016/j.jacc.2010.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Demir H, Kahraman G, Isgoren S, Tan YZ, Kilic T, Berk F. Evaluation of post-stress left ventricular dysfunction and its relationship with perfusion abnormalities using gated SPECT in patients with cardiac syndrome X. Nuc Med Commun. 2008;29:208–214. doi: 10.1097/MNM.0b013e3282f52c49. doi:10.1097/MNM.0b013e3282f52c49. [DOI] [PubMed] [Google Scholar]
- 70.Peix A, Gonzalez A, Garcia EJ, Valiente J, Cabrera LO, Sixto S, Filgueiras CE, Cabale B, Hechavarria S, Gonzalez I, Carrillo R, Garcia-Barreto D. Left ventricular dysfunction secondary to ischemia in women with angina and normal coronary angiograms. J Womens Health. 2009;18:155–161. doi: 10.1089/jwh.2008.0844. doi:10.1089/jwh.2008.0844. [DOI] [PubMed] [Google Scholar]
- 71.Morishima I, Sone T, Okumura K, Tsuboi H, Kondo J, Mukawa H, Matsui H, Toki Y, Ito T, Hayakawa T. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J Am Coll Cardiol. 2000;36:1202–1209. doi: 10.1016/s0735-1097(00)00865-2. doi:10.1016/S0735-1097(00)00865-2. [DOI] [PubMed] [Google Scholar]
- 72.Bolognese L, Carrabba N, Parodi G, Santoro GM, Buonamici P, Cerisano G, Antoniucci D. Impact of microvascular dysfunction on left ventricular remodeling and long-term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation. 2004;109:1121–1126. doi: 10.1161/01.CIR.0000118496.44135.A7. doi:10.1161/01.CIR.0000118496.44135.A7. [DOI] [PubMed] [Google Scholar]
- 73.Olivotto I, Cecchi F, Gistri R, Lorenzoni R, Chiriatti G, Girolami F, Torricelli F, Camici PG. Relevance of coronary microvascular flow impairment to long-term remodeling and systolic dysfunction in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2006;47:1043–1048. doi: 10.1016/j.jacc.2005.10.050. doi:10.1016/j.jacc.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 74.Pereira VF, de Carvalho Frimm C, Rodrigues AC, Curi M. Coronary reserve impairment prevents the improvement of left ventricular dysfunction and adversely affects the long-term outcome of patients with hypertensive dilated cardiomyopathy. JASH. 2010;4:14–21. doi: 10.1016/j.jash.2009.12.002. doi:10.1016/j.jash.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 75.Strauer BE, Motz W, Vogt M, Schwartzkopff B. Evidence for reduced coronary flow reserve in patients with insulin-dependent diabetes. A possible cause for diabetic heart disease in man. Exp Clin Endocrinol Diabetes. 1997;105:15–20. doi: 10.1055/s-0029-1211722. [DOI] [PubMed] [Google Scholar]
- 76.Galderisi M. Diastolic dysfunction and diabetic cardiomyopathy: evaluation by Doppler echocardiography. J Am Coll Cardiol. 2006;48:1548–1551. doi: 10.1016/j.jacc.2006.07.033. doi:10.1016/j.jacc.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 77.Yoon YS, Uchida S, Masuo O, Cejna M, Park JS, Gwon HC, Kirchmair R, Bahlman F, Walter D, Curry C, Hanley A, Isner JM, Losordo DW. Progressive attenuation of myocardial vascular endothelial growth factor expression is a seminal event in diabetic cardiomyopathy: restoration of microvascular homeostasis and recovery of cardiac function in diabetic cardiomyopathy after replenishment of local vascular endothelial growth factor. Circulation. 2005;111:2073–2085. doi: 10.1161/01.CIR.0000162472.52990.36. doi:10.1161/01.CIR.0000162472.52990.36. [DOI] [PubMed] [Google Scholar]
- 78.Gill RM, Jones BD, Corbly AK, Wang J, Braz JC, Sandusky GE, Shen W. Cardiac diastolic dysfunction in conscious dogs with heart failure induced by chronic coronary microembolization. Am J Physiol Heart Circ Physiol. 2006;291:H3154–H3158. doi: 10.1152/ajpheart.00052.2006. doi:10.1152/ajpheart.00052.2006. [DOI] [PubMed] [Google Scholar]
- 79.Bemiller CR, Pepine CJ, Rogers AK. Long-term observations in patients with angina and normal coronary arteriograms. Circulation. 1973;47:36–43. doi: 10.1161/01.cir.47.1.36. doi:10.1161/01.CIR.47.1.36. [DOI] [PubMed] [Google Scholar]
- 80.Day LJ, Sowton E. Clinical features and follow-up of patients with angina and normal coronary arteries. Lancet. 1976;2:334–337. doi: 10.1016/s0140-6736(76)92591-5. doi:10.1016/S0140-6736(76)92591-5. [DOI] [PubMed] [Google Scholar]
- 81.Kaski JC, Rosano GM, Collins P, Nihoyannopoulos P, Maseri A, Poole-Wilson PA. Cardiac syndrome X: clinical characteristics and left ventricular function. Long-term follow-up study. J Am Coll Cardiol. 1995;25:807–814. doi: 10.1016/0735-1097(94)00507-M. doi:10.1016/0735-1097(94)00507-M. [DOI] [PubMed] [Google Scholar]
- 82.Romeo F, Rosano GM, Martuscelli E, Lombardo L, Valente A. Long-term follow-up of patients initially diagnosed with syndrome X. Am J Cardiol. 1993;71:669–673. doi: 10.1016/0002-9149(93)91008-6. doi:10.1016/0002-9149(93)91008-6. [DOI] [PubMed] [Google Scholar]
- 83.Sicari R, Palinkas A, Pasanisi EG, Venneri L, Picano E. Long-term survival of patients with chest pain syndrome and angiographically normal or near-normal coronary arteries: the additional prognostic value of dipyridamole echocardiography test (DET) Eur Heart J. 2005;26:2136–2141. doi: 10.1093/eurheartj/ehi408. doi:10.1093/eurheartj/ehi408. [DOI] [PubMed] [Google Scholar]
- 84.Britten MB, Zeiher AM, Schachinger V. Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long-term outcome. Coron Artery Dis. 2004;15:259–264. doi: 10.1097/01.mca.0000134590.99841.81. doi:10.1097/01.mca.0000134590.99841.81. [DOI] [PubMed] [Google Scholar]
- 85.Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–2832. doi: 10.1016/j.jacc.2010.01.054. doi:10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. doi:10.1161/01.CIR.101.9.948. [DOI] [PubMed] [Google Scholar]
- 87.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. doi: 10.1161/01.cir.0000025404.78001.d8. doi:10.1161/01.CIR.0000025404.78001.D8. [DOI] [PubMed] [Google Scholar]
- 88.Marks DS, Gudapati S, Prisant LM, Weir B, diDonato-Gonzalez C, Waller JL, Houghton JL. Mortality in patients with microvascular disease. J Clin Hypertens. 2004;6:304–309. doi: 10.1111/j.1524-6175.2004.03254.x. doi:10.1111/j.1524-6175.2004.03254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sicari R, Rigo F, Cortigiani L, Gherardi S, Galderisi M, Picano E. Additive prognostic value of coronary flow reserve in patients with chest pain syndrome and normal or near-normal coronary arteries. Am J Cardiol. 2009;103:626–631. doi: 10.1016/j.amjcard.2008.10.033. doi:10.1016/j.amjcard.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 90.von Mering GO, Arant CB, Wessel TR, McGorray SP, Bairey Merz CN, Sharaf BL, Smith KM, Olson MB, Johnson BD, Sopko G, Handberg E, Pepine CJ, Kerensky RA. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:722–725. doi: 10.1161/01.CIR.0000115525.92645.16. doi:10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 91.Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, Burkhard N, Wyss CA, Kaufmann PA. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol. 2009;54:150–156. doi: 10.1016/j.jacc.2009.02.069. doi:10.1016/j.jacc.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 92.Schindler TH, Nitzsche EU, Schelbert HR, Olschewski M, Sayre J, Mix M, Brink I, Zhang XL, Kreissl M, Magosaki N, Just H, Solzbach U. Positron emission tomography-measured abnormal responses of myocardial blood flow to sympathetic stimulation are associated with the risk of developing cardiovascular events. J Am Coll Cardiol. 2005;45:1505–1512. doi: 10.1016/j.jacc.2005.01.040. doi:10.1016/j.jacc.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 93.Fearon WF, Shah M, Ng M, Brinton T, Wilson A, Tremmel JA, Schnittger I, Lee DP, Vagelos RH, Fitzgerald PJ, Yock PG, Yeung AC. Predictive value of the index of microcirculatory resistance in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2008;51:560–565. doi: 10.1016/j.jacc.2007.08.062. doi:10.1016/j.jacc.2007.08.062. [DOI] [PubMed] [Google Scholar]
- 94.Lim HS, Yoon MH, Tahk SJ, Yang HM, Choi BJ, Choi SY, Sheen SS, Hwang GS, Kang SJ, Shin JH. Usefulness of the index of microcirculatory resistance for invasively assessing myocardial viability immediately after primary angioplasty for anterior myocardial infarction. Eur Heart J. 2009;30:2854–2860. doi: 10.1093/eurheartj/ehp313. doi:10.1093/eurheartj/ehp313. [DOI] [PubMed] [Google Scholar]
- 95.Sorajja P, Gersh BJ, Costantini C, McLaughlin MG, Zimetbaum P, Cox DA, Garcia E, Tcheng JE, Mehran R, Lansky AJ, Kandzari DE, Grines CL, Stone GW. Combined prognostic utility of ST-segment recovery and myocardial blush after primary percutaneous coronary intervention in acute myocardial infarction. Eur Heart J. 2005;26:667–674. doi: 10.1093/eurheartj/ehi167. doi:10.1093/eurheartj/ehi167. [DOI] [PubMed] [Google Scholar]
- 96.Poli A, Fetiveau R, Vandoni P, del Rosso G, D'Urbano M, Seveso G, Cafiero F, De Servi S. Integrated analysis of myocardial blush and ST-segment elevation recovery after successful primary angioplasty: Real-time grading of microvascular reperfusion and prediction of early and late recovery of left ventricular function. Circulation. 2002;106:313–318. doi: 10.1161/01.cir.0000022691.71708.94. doi:10.1161/01.CIR.0000022691.71708.94. [DOI] [PubMed] [Google Scholar]
- 97.de Waha S, Desch S, Eitel I, Fuernau G, Zachrau J, Leuschner A, Gutberlet M, Schuler G, Thiele H. Impact of early vs. late microvascular obstruction assessed by magnetic resonance imaging on long-term outcome after ST-elevation myocardial infarction: a comparison with traditional prognostic markers. Eur Heart J. 2010;31:2660–2668. doi: 10.1093/eurheartj/ehq247. doi:10.1093/eurheartj/ehq247. [DOI] [PubMed] [Google Scholar]
- 98.Nijveldt R, Beek AM, Hofman MB, Umans VA, Algra PR, Spreeuwenberg MD, Visser CA, van Rossum AC. Late gadolinium-enhanced cardiovascular magnetic resonance evaluation of infarct size and microvascular obstruction in optimally treated patients after acute myocardial infarction. J Cardiovasc Magn Reson. 2007;9:765–770. doi: 10.1080/10976640701545008. doi:10.1080/10976640701545008. [DOI] [PubMed] [Google Scholar]
- 99.Grube E, Gerckens U, Yeung AC, Rowold S, Kirchhof N, Sedgewick J, Yadav JS, Stertzer S. Prevention of distal embolization during coronary angioplasty in saphenous vein grafts and native vessels using porous filter protection. Circulation. 2001;104:2436–2441. doi: 10.1161/hc4501.099317. doi:10.1161/hc4501.099317. [DOI] [PubMed] [Google Scholar]
- 100.Bahrmann P, Werner GS, Heusch G, Ferrari M, Poerner TC, Voss A, Figulla HR. Detection of coronary microembolization by Doppler ultrasound in patients with stable angina pectoris undergoing elective percutaneous coronary interventions. Circulation. 2007;115:600–608. doi: 10.1161/CIRCULATIONAHA.106.660779. doi:10.1161/CIRCULATIONAHA.106.660779. [DOI] [PubMed] [Google Scholar]
- 101.Kleinbongard P, Bose D, Baars T, Mohlenkamp S, Konorza T, Schoner S, Elter-Schulz M, Eggebrecht H, Degen H, Haude M, Levkau B, Schulz R, Erbel R, Heusch G. Vasoconstrictor potential of coronary aspirate from patients undergoing stenting of saphenous vein aortocoronary bypass grafts and its pharmacological attenuation. Circ Res. 2011;108:344–352. doi: 10.1161/CIRCRESAHA.110.235713. doi:10.1161/CIRCRESAHA.110.235713. [DOI] [PubMed] [Google Scholar]
- 102.Baim DS, Wahr D, George B, Leon MB, Greenberg J, Cutlip DE, Kaya U, Popma JJ, Ho KK, Kuntz RE. Randomized trial of a distal embolic protection device during percutaneous intervention of saphenous vein aorto-coronary bypass grafts. Circulation. 2002;105:1285–1290. [PubMed] [Google Scholar]
- 103.Neglia D, Michelassi C, Trivieri MG, Sambuceti G, Giorgetti A, Pratali L, Gallopin M, Salvadori P, Sorace O, Carpeggiani C, Poddighe R, L'Abbate A, Parodi O. Prognostic role of myocardial blood flow impairment in idiopathic left ventricular dysfunction. Circulation. 2002;105:186–193. doi: 10.1161/hc0202.102119. doi:10.1161/hc0202.102119. [DOI] [PubMed] [Google Scholar]
- 104.Rigo F, Gherardi S, Galderisi M, Sicari R, Picano E. The independent prognostic value of contractile and coronary flow reserve determined by dipyridamole stress echocardiography in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2007;99:1154–1158. doi: 10.1016/j.amjcard.2006.11.049. doi:10.1016/j.amjcard.2006.11.049. [DOI] [PubMed] [Google Scholar]
- 105.Anantharam B, Janardhanan R, Hayat S, Hickman M, Chahal N, Bassett P, Senior R. Coronary flow reserve assessed by myocardial contrast echocardiography predicts mortality in patients with heart failure. Eur J Echocardiogr. 2011;12:69–75. doi: 10.1093/ejechocard/jeq109. [DOI] [PubMed] [Google Scholar]
- 106.Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, Camici PG. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med. 2003;349:1027–1035. doi: 10.1056/NEJMoa025050. doi:10.1056/NEJMoa025050. [DOI] [PubMed] [Google Scholar]
- 107.Maron MS, Olivotto I, Maron BJ, Prasad SK, Cecchi F, Udelson JE, Camici PG. The case for myocardial ischemia in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009;54:866–875. doi: 10.1016/j.jacc.2009.04.072. doi:10.1016/j.jacc.2009.04.072. [DOI] [PubMed] [Google Scholar]
- 108.Weis M, Hartmann A, Olbrich HG, Hor G, Zeiher AM. Prognostic significance of coronary flow reserve on left ventricular ejection fraction in cardiac transplant recipients. Transplantation. 1998;65:103–108. doi: 10.1097/00007890-199801150-00020. doi:10.1097/00007890-199801150-00020. [DOI] [PubMed] [Google Scholar]
- 109.Hollenberg SM, Klein LW, Parrillo JE, Scherer M, Burns D, Tamburro P, Oberoi M, Johnson MR, Costanzo MR. Coronary endothelial dysfunction after heart transplantation predicts allograft vasculopathy and cardiac death. Circulation. 2001;104:3091–3096. doi: 10.1161/hc5001.100796. doi:10.1161/hc5001.100796. [DOI] [PubMed] [Google Scholar]
- 110.Tona F, Caforio AL, Montisci R, Gambino A, Angelini A, Ruscazio M, Toscano G, Feltrin G, Ramondo A, Gerosa G, Iliceto S. Coronary flow velocity pattern and coronary flow reserve by contrast-enhanced transthoracic echocardiography predict long-term outcome in heart transplantation. Circulation. 2006;114(1 Suppl):I49–I55. doi: 10.1161/CIRCULATIONAHA.105.001321. [DOI] [PubMed] [Google Scholar]
- 111.Berry C, Balachandran KP, L'Allier PL, Lesperance J, Bonan R, Oldroyd KG. Importance of collateral circulation in coronary heart disease. Eur Heart J. 2007;28:278–291. doi: 10.1093/eurheartj/ehl446. doi:10.1093/eurheartj/ehl446. [DOI] [PubMed] [Google Scholar]
- 112.Porto I, Hamilton-Craig C, Brancati M, Burzotta F, Galiuto L, Crea F. Angiographic assessment of microvascular perfusion–myocardial blush in clinical practice. Am Heart J. 2010;160:1015–1022. doi: 10.1016/j.ahj.2010.08.009. doi:10.1016/j.ahj.2010.08.009. [DOI] [PubMed] [Google Scholar]