Abstract
Aims
Men and women differ in terms of presentation and management in coronary artery disease (CAD). Whether these differences translate into different clinical outcomes in stable CAD is unclear. We analysed data from the international prospective CLARIFY registry to compare cardiovascular clinical outcomes in men and women with stable CAD.
Methods and results
We analysed 1-year outcomes in 30 977 outpatients with stable CAD [23 975 (77.4%) men; 7002 (22.6%) women]. Women were older than men, more likely to have hypertension and diabetes, and less likely to exercise or smoke. They had more frequent angina, but were less likely to have undergone diagnostic non-invasive testing or coronary angiography. Women received less optimized treatment for stable CAD. One-year outcomes were similar for men and women for the composite of cardiovascular death, non-fatal myocardial infarction, or stroke [adjusted rates 1.7 vs. 1.8%, respectively, odds ratio (OR) 0.93, 95% confidence interval (CI) 0.75–1.15]; all-cause death (adjusted 1.5 vs. 1.6%, OR: 0.91, 95% CI: 0.72–1.13); fatal or non-fatal myocardial infarction (adjusted 1.0 vs. 0.9%, OR: 0.81, 95 CI: 0.60–1.08); and cardiovascular death or non-fatal myocardial infarction (adjusted 1.4 vs. 1.4%, OR: 0.89, 95% CI: 0.70–1.12). Fewer women underwent revascularization (2.6 vs. 2.2%, OR: 0.77, 95% CI: 0.64–0.93), although appropriateness was not analysed.
Conclusion
The risk profiles of women and men with stable CAD differ substantially. However, 1-year outcomes were similar. Fewer women underwent revascularization. Further research is needed to better understand gender determinants of outcome and devise strategies to minimize bias in the management and treatment of women.
Keywords: CAD, CLARIFY, Gender, Prognosis, Registry, Women
See page 2769 for the editorial comment on this article (doi:10.1093/eurheartj/ehs276)
Introduction
Within cardiovascular diseases, coronary artery disease (CAD) is the single most frequent cause of death in both sexes and is responsible for more than half of all cardiovascular events.1,2 There are differences between men and women in terms of presentation and management in all forms of CAD: stable angina or acute coronary syndromes (ACS).3,4 Women with CAD are on average older than men, with higher rates of cardiovascular risk factors; they are also less likely to receive optimized medical treatment or revascularization for their condition.5–9 Whether these gender differences translate into a different prognosis is unclear. While some reports found gender differences in outcomes for stable angina or ACS,6,9,10 others did not.11–14 Furthermore, most contemporary studies of CAD patients are limited to a single country or specific geographical region, or a particular manifestation of disease [e.g. angina symptoms or acute myocardial infarction (MI)].6,15–20
CLARIFY (ProspeCtive observational LongitudinAl RegIstry oF patients with stable coronary arterY disease) is an ongoing international prospective observational longitudinal registry in >33 000 patients with stable CAD in 45 countries.21,22 Women make up nearly a quarter of the CLARIFY population.21 We set out to use the CLARIFY database to explore differences in outcomes between men and women treated for stable CAD, in particular in patients post-MI or post-revascularization.
Methods
Study design and patients
CLARIFY is an ongoing prospective, international, observational, and longitudinal registry in 33 285 outpatients with stable CAD receiving standard management. The rationale, design, and baseline characteristics of CLARIFY have been described elsewhere21,22 (further information can also be found online at www.clarify-registry.com). Patients were enrolled in 45 countries in Africa, Asia, Australia, Europe, the Middle East, and North, Central, and South America. A detailed list of countries, sites, and investigators is available from Steg et al.21 Patients are being treated according to usual clinical practice at each institution, with no specific tests or therapies defined in the study protocol. The 2898 participating physicians were selected on the basis of geographic distribution; each was requested to recruit 10–15 consecutive stable CAD outpatients to meet a predefined country target of 25 patients per million inhabitants (range 12.5–50) and obtain an epidemiologically representative population in each country. Eligible patients had stable CAD defined as at least one of the following: documented MI >3 months before enrolment; angiographic demonstration of coronary stenosis >50%; chest pain with evidence of myocardial ischaemia (stress electrocardiogram); or coronary artery bypass graft (CABG) or percutaneous coronary intervention (PCI) >3 months before enrolment. These criteria were not mutually exclusive. Exclusion criteria were hospital admission for cardiovascular reasons (including revascularization) in the past 3 months, planned revascularization, or conditions hampering the participation or the 5-year follow-up (such as limited cooperation, limited legal capacity, serious non-cardiovascular disease or conditions interfering with life expectancy (e.g. cancer, drug abuse) or severe other cardiovascular disease (e.g. advanced heart failure, severe valve disease, history of valve repair/replacement). To ensure that the study population was representative of stable CAD outpatients, recruitment of sites and subjects was based on predefined selection of physicians (cardiologists, as well as office-based primary care physicians and physicians based in hospitals with outpatient clinics) by national coordinators, using the best available epidemiological data in each country reflecting the burden of CAD, to provide a distribution of physicians across regions and locations (i.e. urban, suburban, or rural areas) mimicking the epidemiological patterns in each country. In each practice, patient enrolment was restricted over a brief period to achieve near consecutive patient enrolment. The first patient was included on 26 November 2009 and recruitment was completed on 30 June 2010.
The study is in accordance with the principles in the Declaration of Helsinki and local ethical approval was obtained in all countries prior to recruitment. All patients gave written informed consent. The study is registered (ISRCTN43070564).
Data collection
The investigators completed standardized electronic case report forms (eCRFs) at baseline and at an actual patient visit 1-year ± 3 months after enrolment. For patients missing the 1-year visit, telephone contact with the patient, a designated relative or contact, or his/her physician was attempted. Where applicable, registries could be used to retrieve the vital status. A number of measures were implemented to ensure data quality, including onsite monitoring visits of 100% of the data in 5% of centres selected at random; regular telephone contact with investigators to limit missing data and loss to follow-up; and centralized verification of the eCRF for completeness, consistency, and accuracy. At baseline and follow-up, data were collected on demographics, risk factors and lifestyle, medical history, physical condition and vital signs, current symptoms, and current treatments. Available results of invasive and non-invasive tests were collected, but no test was mandated by the study and there was no standardized measurement of the left ventricular ejection fraction. At the 1-year visit, clinical outcomes occurring over the year were recorded. We analysed the CLARIFY population by gender and compared the rates of the following outcomes in men and women at 1 year: all-cause death; fatal or non-fatal MI; cardiovascular death; or non-fatal MI; a combined endpoint of cardiovascular death, non-fatal MI, or non-fatal stroke; all coronary events [fatal or non-fatal MI, revascularization (PCI or CABG), or unstable angina]; and revascularization (PCI or CABG). For the purpose of this analysis, we defined the main outcome as the composite of cardiovascular death, MI, and stroke, as these events are objectively defined and are less likely to be affected by gender differences in ascertainment or management than coronary events, which include myocardial revascularization. As sensitivity analyses, we also examined these clinical outcomes in two patient subsets of interest, expected to represent more severe disease: patients with angiographic evidence of CAD (defined as >50% stenosis in at least one vessel) and patients with a history of MI or revascularization. For all composite outcomes, we analysed the number of patients with at least one event from the combination outcomes. Patients experiencing more than one contributing event were counted only once. There was no time to event analysis. Events were accepted as reported by physicians and were not adjudicated. However, all events were source-verified during the audits, which were performed at 5% of randomly selected sites.
Statistical analysis
Baseline characteristics for the whole population and by gender are presented using descriptive statistics with mean (SD) or median [Q1, Q3] for continuous variables, depending on the distribution of the data, and numbers (%) for categorical variables. Baseline values were compared between men and women using Student's t-test or the Kruskal–Wallis test for continuous variables, again depending on the distribution of the data, and χ2 tests for categorical variables. The risk of outcomes was compared between men and women using a logistic regression analysis to produce odds ratios (ORs) and corresponding 95% confidence intervals (CIs) adjusted for baseline differences (age, presence and severity of angina, diabetes, hypertension, MI history, peripheral artery disease, heart rate, and systolic blood pressure). Statistical analysis was performed at the Robertson Centre for Biostatistics at the University of Glasgow, UK, using the SAS (version 9.2) statistical program.
Results
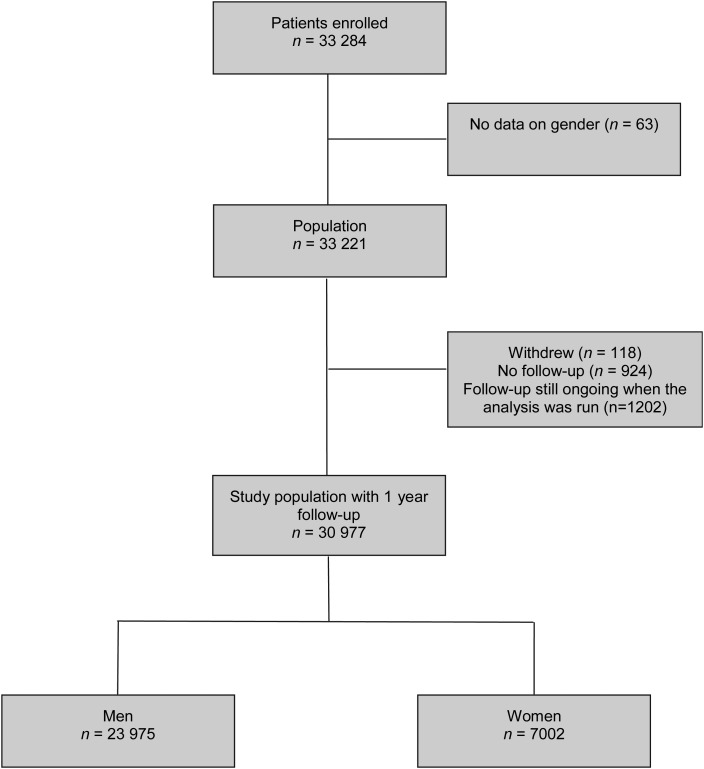
A total of 33 284 patients were included at baseline,21 and gender was recorded on the eCRF for 33 221 (99.8%). The flow of patient from enrolment to 1-year analysis is depicted in Figure 1. Of these, 118 patients (0.4%) withdrew consent before 1 year and 924 patients (2.8%) were lost to follow-up. Finally, at the time of this analysis, follow-up was ongoing and data were incomplete in 1202 (3.6%) patients. These patients were not included in the present analyses. Complete outcomes were collected and available for 30 977 patients (93.2%) who completed a 1-year follow-up or died before the annual visit. There were 23 975 (77.4%) men and 7002 (22.6%) women. Major baseline characteristics were compared between patients with and without follow-up in Supplementary material online, Table S1 and show important differences in baseline characteristics, which are associated with similarly important differences in geographic distribution.
Figure 1.
Patient flow from enrolment to 1-year analysis.
At baseline, the CLARIFY women were older than their male counterparts [66.5 (9.9) vs. 63.4 (10.5) years] and were more likely to have diabetes (32.7 vs. 28.0%) and treated hypertension (78.5 vs. 68.9%); they were more likely to have no physical activity (22.6 vs. 13.9%) and were less likely to smoke (7.2 vs. 14.1%) (Table 1). With regard to disease characteristics and medical history, women had a slightly higher heart rate (palpation) [69.6 (10.5) vs. 67.9 (10.6) b.p.m.] and systolic blood pressure [133.3 (17.5) vs. 130.4 (16.3) mmHg]; they also had slightly higher left ventricular ejection fraction than men [58.0 (10.6) vs. 55.6% (11.1%)].
Table 1.
Baseline characteristics
| Parameter (no. of patients with data available) | Total (n = 30 977) | Men (n = 23 975) | Women (n = 7002) | P-valuea |
|---|---|---|---|---|
| Demographic characteristic | ||||
| Age, years (n = 30 977) | 64.1 (10.5) | 63.4 (10.5) | 66.5 (9.9) | <0.0001 |
| Body mass index, kg/m2 (n = 30 949) | 27.3 (24.8, 30.4) | 27.3 (24.9, 30.1) | 27.3 (24.2, 31.1) | 0.87 |
| Ethnicity (n = 30 977) | ||||
| Caucasian | 20 458 (66.0) | 15 825 (66.0) | 4633 (66.2) | <0.0001 |
| South Asian | 2462 (7.9) | 1945 (8.1) | 517 (7.4) | |
| Chinese | 2618 (8.5) | 1999 (8.3) | 619 (8.8) | |
| Japanese/Korean | 1024 (3.3) | 758 (3.2) | 266 (3.8) | |
| Hispanic | 1352 (4.4) | 993 (4.1) | 359 (5.1) | |
| Black/African | 301 (1.0) | 208 (0.9) | 93 (1.3) | |
| Unknown | 2762 (8.9) | 2247 (9.4) | 515 (7.4) | |
| Cardiovascular risk factors | ||||
| Smoking status (n = 30 972) | ||||
| Current | 3870 (12.5) | 3368 (14.1) | 502 (7.2) | <0.0001 |
| Former | 14 139 (45.7) | 12 713 (53.0) | 1426 (20.4) | |
| Never | 12 963 (41.9) | 7890 (32.9) | 5073 (72.5) | |
| Dyslipidaemiab (n = 30 974) | 23 179 (74.8) | 17 940 (74.8) | 5239 (74.8) | 0.98 |
| Treated hypertension (n = 30 974) | 22 023 (71.1) | 16 525 (68.9) | 5498 (78.5) | <0.0001 |
| Family history of premature CADc (n = 30 970) | 8833 (28.5) | 6661 (27.8) | 2172 (31.0) | <0.0001 |
| Diabetesd (n= 30 973) | 8995 (29.0) | 6705 (28.0) | 2290 (32.7) | <0.0001 |
| Physical activity (n = 30 972) | ||||
| None | 4916 (15.9) | 3331 (13.9) | 1585 (22.6) | <0.0001 |
| Light physical activity most weeks | 16 056 (51.8) | 12 161 (50.7) | 3895 (55.7) | |
| >20 min physical activity once or twice weekly | 5167 (16.7) | 4329 (18.1) | 838 (12.0) | |
| >20min physical activity >3 times weekly | 4833 (15.6) | 4152 (17.3) | 681 (9.7) | |
| Medical history | ||||
| Time since diagnosis of CAD, years (n = 30 975) | 5 (2, 9) | 5 (2, 10) | 4 (2, 8) | <0.0001 |
| Myocardial infarction (n = 30 976) | 18 464 (59.6) | 14 883 (62.1) | 3581 (51.1) | <0.0001 |
| Coronary angiography performed (n = 30 977) | 26 320 (85.0) | 20 747 (86.5) | 5573 (79.6) | <0.0001 |
| Non-invasive test for myocardial ischaemia (n = 30 969) | 19 066 (61.6) | 15 003 (62.6) | 4063 (58.1) | <0.0001 |
| Evidence for myocardial ischaemia (n = 30 970) | 5082 (16.4) | 3851 (16.1) | 1231 (17.6) | 0.0025 |
| Percutaneous coronary intervention (n = 30 975) | 18 101 (58.4) | 14 261 (59.5) | 3840 (54.8) | <0.0001 |
| Coronary artery bypass graft (n = 30 974) | 7246 (23.4) | 6023 (25.1) | 1223 (17.5) | <0.0001 |
| Peripheral arterial disease (n = 30 972) | 3012 (9.7) | 2418 (10.1) | 594 (8.5) | <0.0001 |
| Asthma/COPD (n = 30 975) | 2276 (7.3) | 1677 (7.0) | 599 (8.6) | <0.0001 |
| Hospital admission for heart failure (n = 30 976) | 1434 (4.6) | 1072 (4.5) | 362 (5.2) | 0.014 |
| Stroke (n = 30 976) | 1231 (4.0) | 936 (3.9) | 295 (4.2) | 0.24 |
| Transient ischaemic attack (n = 30 975) | 962 (3.1) | 707 (2.9) | 255 (3.6) | 0.0033 |
| Symptomatic status | ||||
| Angina (n = 30 977) | 7007 (22.6) | 5002 (20.9) | 2005 (28.6) | <0.0001 |
| CCS class (if angina) (n = 7003) | ||||
| I | 1992 (28.4) | 1483 (29.7) | 509 (25.4) | 0.0018 |
| II | 3727 (53.2) | 2634 (52.7) | 1093 (54.5) | |
| III | 1209 (17.3) | 833 (16.7) | 376 (18.8) | |
| IV | 75 (1.1) | 49 (1.0) | 26 (1.3) | |
| Heart failure symptoms (NYHA class) (n = 30 977) | ||||
| No heart failure | 26 242 (84.7) | 20 497 (85.5) | 5745 (82.0) | <0.0001 |
| Class II | 3964 (12.8) | 2922 (12.2) | 1042 (14.9) | |
| Class III | 771 (2.5) | 556 (2.3) | 215 (3.1) | |
| Angiographic findings (n = 26 282) | ||||
| No diseased vessel | 968 (3.7) | 610 (2.9) | 358 (6.4) | <0.0001 |
| One-vessel disease | 10 815 (41.1) | 8239 (39.8) | 2576 (46.3) | |
| Two or more vessel disease | 14 499 (55.2) | 11 868 (57.3) | 2631 (47.3) | |
| Cardiac parameters | ||||
| Heart rate (palpation), b.p.m. (n = 30 963) | 68.3 (10.6) | 67.9 (10.6) | 69.6 (10.5) | <0.0001 |
| Heart rate (electrocardiography), b.p.m. (n = 23 034) | 67.2 (11.4) | 66.7 (11.4) | 69.0 (11.5) | <0.0001 |
| Systolic blood pressure, mmHg (n = 30 969) | 131.0 (16.7) | 130.4 (16.3) | 133.3 (17.5) | <0.0001 |
| Diastolic blood pressure, mmHg (n = 30 969) | 77.3 (10.0) | 77.3 (9.9) | 77.0 (10.4) | 0.0042 |
| Left ventricular ejection fraction, % (n = 21 283) | 56.1 (11.0) | 55.6 (11.1) | 58.0 (10.6) | <0.0001 |
| Electrocardiography rhythm (n = 23 020) | ||||
| Sinus rhythm | 21 888 (95.1) | 16 974 (94.9) | 4914 (95.6) | 0.10 |
| Atrial fibrillation/flutter | 772 (3.4) | 614 (3.4) | 158 (3.1) | |
| Paced rhythm | 360 (1.6) | 293 (1.6) | 67 (1.3) | |
CCS, Canadian Cardiovascular Society; COPD, chronic obstructive pulmonary disease; NYHA, New York Heart Association.
Values are numbers (percentages), means (SD), or medians [Q1, Q3].
aMen vs. women.
bDefined as a history of documented total cholesterol >2 g/L (5.18 mmol/L) or high-density lipoprotein cholesterol <0.4 g/L or <1 mmol/L.
cDefined as myocardial infarction, sudden death, or stable angina at age <55 years (men) and <65 years (women) in a first-degree relative.
dRefers to a history of diabetes or current diabetes (diagnosed by two fasting blood glucose measures >7 mmol/L or 126 mg/L, or abnormal oral glucose tolerance test) treated or not.
Women had lower rates than men of previous MI (51.1 vs. 62.1%), previous PCI (54.8 vs. 59.5%), and previous CABG (17.5 vs. 25.1%). More women than men had anginal symptoms (28.6 vs. 20.9% in men), and the symptoms were more severe, as determined by the Canadian Cardiovascular Society Class. Women also had more frequent and severe symptoms of heart failure, as measured by New York Heart Association functional class.
At baseline, women were less likely than men to have undergone coronary angiography (79.6 vs. 86.5%, P < 0.0001) or non-invasive testing for myocardial ischaemia (58.1 vs. 62.6%, P < 0.0001) at any time. Among patients with angiographic data, the extent of CAD was less in women with more men having multivessel disease than women and, conversely, more women with single vessel disease than men. Likewise, more women had no evidence of angiographic stenosis >50% in at least one vessel than men. The presence of myocardial ischaemia on non-invasive testing was higher in women.
At baseline, the use of evidence-based drugs for prevention in CAD was high in the CLARIFY population. A large majority of patients from both sexes were receiving lipid-lowering agents (92.2%) and aspirin (87.7%), and three-quarters were receiving angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (76.0%) and beta-blockers (75.2%) (Table 2). There were few differences between the treatments received by men and women at baseline, although women in CLARIFY were slightly less likely to be receiving lipid-lowering agents (90.1 vs. 92.9%), aspirin (87.0 vs. 87.9%), and thienopyridines (25.3 vs. 26.8%), and more likely to be treated with all categories of antianginal agents (except beta-blockers), than men, and were more likely to be receiving diuretics (37.7 vs. 26.9%).
Table 2.
Treatments at baseline
| Treatment (no. of patients with data available) | Whole population (n= 30 977) | Men (n= 23 975) | Women (n= 7002) | P-valuea |
|---|---|---|---|---|
| Lipid-lowering drug (n = 30 972) | 28 566 (92.2) | 22 262 (92.9) | 6304 (90.1) | <0.0001 |
| Statins (n = 30 972) | 25 638 (82.8) | 20 027 (83.5) | 5611 (80.2) | <0.0001 |
| Aspirin (n = 30 971) | 27 165 (87.7) | 21 073 (87.9) | 6092 (87.0) | 0.048 |
| Thienopyridine (n = 30 957) | 8201 (26.5) | 6431 (26.8) | 1770 (25.3) | 0.01 |
| Other antiplatelet agent (n = 30 957) | 2868 (9.3) | 2208 (9.2) | 660 (9.4) | 0.59 |
| Oral anticoagulant agent (n = 30 970) | 2548 (8.2) | 2055 (8.6) | 493 (7.0) | <0.0001 |
| ACE inhibitor and/or ARB (n = 30 969) | 23 543 (76.0) | 18 203 (75.9) | 5340 (76.3) | 0.54 |
| Beta-blocker (n = 30 972) | 23 299 (75.2) | 18 047 (75.3) | 5252 (75.0) | 0.66 |
| Diuretic (n = 30 969) | 9091 (29.4) | 6449 (26.9) | 2642 (37.7) | <0.0001 |
| Calcium channel blocker (n = 30 967) | 8404 (27.1) | 6137 (25.6) | 2267 (32.4) | <0.0001 |
| Long-acting nitrate (n = 30 969) | 6837 (22.1) | 5028 (21.0) | 1809 (25.8) | <0.0001 |
| Ivabradine (n = 30 970) | 3120 (10.1) | 2313 (9.6) | 807 (11.5) | <0.0001 |
| Verapamil or diltiazem (n = 30 967) | 1775 (5.7) | 1276 (5.3) | 499 (7.1) | <0.0001 |
| Other antianginal agent (n = 30 966) | 4403 (14.2) | 3192 (13.3) | 1211 (17.3) | <0.0001 |
ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker.
Values are numbers (percentages).
aMen vs. women.
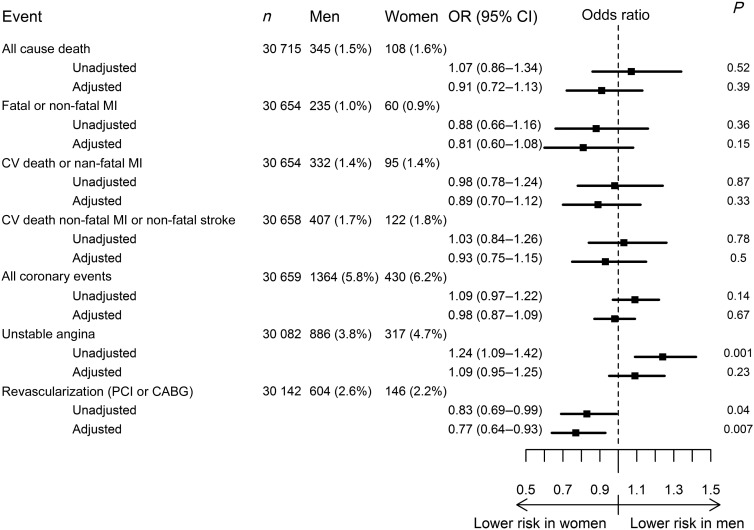
Overall, at the 1-year follow up, there were 1794 coronary events and 529 patients experienced either cardiovascular death, non-fatal MI, or non-fatal stroke. Unadjusted outcome event rates were very similar between men and women (Figure 2). After adjustment for baseline differences, event rates were similar for men and women for the composite outcome of cardiovascular death, non-fatal MI, or non-fatal stroke [407 (1.7%) vs. 122 (1.8%), OR: 0.93, 95% CI: 0.75–1.15, P= 0.50]. Likewise, there was no difference in clinical event rates for all-cause death (P= 0.39), fatal or non-fatal MI (P= 0.15), cardiovascular death or non-fatal MI (P = 0.33), unstable angina (P = 0.23), and the composite of all coronary events (P = 0.67) (Figure 2). These results were homogeneous across geographic regions (see Supplementary material online, Table S2). Conversely, fewer women than men underwent myocardial revascularization (PCI or CABG) (P = 0.007), driven largely by patients from Western and Eastern Europe.
Figure 2.
Events by first annual visit and odds ratios. Odds ratios and 95% confidence intervals, crude and adjusted for risk factors, age, and baseline differences. All coronary events include: fatal myocardial infarction, non-fatal myocardial infarction, coronary revascularization [PCI (percutaneous coronary intervention) or coronary artery bypass graft (CABG)], or unstable angina.
Sensitivity analyses were performed to assess outcomes in selected patient subsets (Table 3). First, we analysed patients with angiographic evidence of CAD (with stenosis >50% in at least one vessel) (n = 25 314) and found no interaction between outcomes by gender and presence and the extent of angiographic evidence of CAD (data not shown). The results were therefore consistent with those from the overall analysis (Table 3). Then, we studied patients with either a history of MI or of previous revascularization (n = 28 026). In that subset, results were also consistent with the overall analysis. However, there was a statistically significant interaction between outcomes by gender and a history of MI or previous revascularization: women without prior MI/revascularization being less likely than men with a similar medical history to experience a coronary event and were less likely to undergo revascularization (see Supplementary material online, Table S3). Finally, we examined whether there was an interaction between gender and age, and analysed outcomes by gender and tertiles of age (see Supplementary material online, Table S4). There was a significant interaction for the outcomes of fatal/non-fatal MI, the composite of cardiovascular death and non-fatal MI and the triple composite of cardiovascular death, non-fatal MI, and non-fatal stroke. For all of these outcomes, women in the younger tertile had better outcomes than men of similar age, whereas such differences were not apparent and were even directionally opposite in older women.
Table 3.
Outcomes in selected patient subsets
| Event | No. of events | ORa (95% CI) | P-value |
|---|---|---|---|
| Patients with MI or revascularization at baseline (n = 28 026) | |||
| All-cause death | 414 | 0.97 (0.77–1.23) | 0.80 |
| Fatal or non-fatal MI | 268 | 0.82 (0.60–1.12) | 0.20 |
| CV death or non-fatal MI | 389 | 0.93 (0.72–1.19) | 0.54 |
| CV death/non-fatal MI/non-fatal stroke | 482 | 0.98 (0.78–1.22) | 0.83 |
| All coronary eventsb | 1596 | 1.04 (0.92–1.18) | 0.52 |
| Unstable angina | 1067 | 1.14 (0.99, 1.32) | 0.08 |
| Revascularization (PCI or CABG) | 677 | 0.86 (0.71–1.05) | 0.13 |
| Patients with angiographic evidence of CAD (≥1 vessel disease) (n = 25 314) | |||
| All-cause death | 327 | 0.93 (0.71–1.22) | 0.61 |
| Fatal or non-fatal MI | 229 | 0.90 (0.64–1.24) | 0.49 |
| CV death or non-fatal MI | 315 | 0.95 (0.72–1.25) | 0.74 |
| CV death/non-fatal MI/non-fatal stroke | 387 | 0.98 (0.76–1.26) | 0.87 |
| All coronary eventsb | 1367 | 1.06 (0.92–1.21) | 0.42 |
| Unstable angina | 887 | 1.14 (0.97–1.34) | 0.11 |
| Revascularization (PCI or CABG) | 635 | 0.84 (0.68–1.03) | 0.10 |
CAD, coronary artery disease; CV, cardiovascular.
aOdds ratio for women vs. men and 95% confidence interval adjusted for risk factors, age, and baseline differences.
bFatal myocardial infarction, non-fatal myocardial infarction, coronary revascularization [PCI (percutaneous coronary intervention) or coronary artery bypass graft (CABG)], or unstable angina.
Discussion
The main finding from this analysis of a large, international, contemporary population of outpatients with stable CAD (most of whom had a previous MI or revascularization) is that despite substantial differences in baseline clinical characteristics and management, the rates for cardiovascular clinical outcomes were similar between men and women, both with and without adjustment, and fewer women had a history of myocardial revascularization at baseline. These results hold when sensitivity analyses restrict the population studied to that with angiographic evidence of CAD or with prior clinical events. However, it is noteworthy that younger women (in the lower age tertile) and women without a history of MI or revascularization actually fare better than men with a similar profile.
These results suggest that there is no discernible excess of cardiovascular events in women with stable CAD compared with men and that, if anything, among younger patients or lower risk patient groups, women actually fare better than men.
There were important baseline differences between men and women at baseline. The women in CLARIFY were older, and were more likely to have hypertension, diabetes, and symptomatic angina than men, and to be receiving all types of anti-anginals, except beta-blockers. On the other hand, they were less likely to have had coronary angiography, revascularization, or non-invasive testing or to be receiving statins or beta-blockers.
The data from clinical trials related to women should be interpreted carefully since they are often performed in highly selected populations with under-representation of women.23 Registry studies, particularly when performed nationwide,24,25 provide more representative data regarding gender differences. CLARIFY, as a large contemporary cohort of outpatients with stable CAD, with broad geographic representation, and as such, provides information to improve our understanding of gender issues in patients with established but stable CAD.
The observation of gender differences in baseline characteristics and management in this stable CAD population is in line with previous registries and cohort studies. Gender differences in risk profile have been reported in patients with angina and ACS,5,6,26 and women appear to be more likely than men to have a higher risk factor burden. Coronary artery disease tends to manifest itself at a later age in women. This may be related to a cardioprotective effect of oestrogen,26 although this has been debated by others.27 Even though international guidelines do not dictate gender-specific management strategies, women with stable CAD tend to have less access to non-invasive and invasive investigations and receive less intensive medical therapy as well as revascularization.5–7,9,14,15,28
The underlying reasons for these differences remain unclear, though the underlying pathophysiological substrate for CAD may be different in men and women.8,26,29,30 Women tend to have smaller lumens and brachial artery diameters, which may influence endothelium-dependent vasodilatation.31,32 Women are less likely than men to undergo angiography (as was seen in CLARIFY during the 1-year follow-up) and, if they do, have lower rates of obstructive CAD,6,30,33 which account in part for the lower rates of revascularization in women in CLARIFY, and they also have less severe disease, with lower rates of multivessel disease.34 There are higher rates of microvascular disease in women.26,35 Older women have lower plaque burden than men, smaller atheroma volume, and are less likely to have plaque calcification and rupture.26,30,32 However, in younger women, the prevalence of coronary lesions appears similar in women compared with men, although plaque burden may be lower.36 On the other hand, the gender differences may also be related to physician and patient behaviour; for example, women are often perceived by physicians to be at lower cardiovascular risk than men and appear to be underdiagnosed—possibly because of more frequent atypical presentation37—undergo far less frequent non-invasive or invasive testing, and are referred less frequently for revascularization than men (as indeed seen in CLARIFY). They may also be less willing to undergo invasive procedures, which may reduce the level of care they receive.6 Clearly, concerted efforts are needed to modify both physician and patient behaviours by increasing awareness of the prevalence of CAD in women (as done through mass media campaigns such as the ‘Women at Heart’ initiative by the European Society of Cardiology or the ‘Go Red for Women’ campaign of the American Heart Association), by educating physicians to the differences in biology, and clinical presentation of women with CAD compared with men, the need for more active testing and referral of women with suspected or proven heart disease and the need for uniform application of guidelines and evidence to the management of women and men with CAD.38
Some6,9 but not all11 studies have reported gender differences in outcomes in stable CAD patients with angina. In ACS, the evidence is somewhat conflicting, with observations of gender differences5,7,8,39,40 or no gender difference.12–14 However, many studies suggest that women have worse outcomes following ACS, even after adjustment for age. This may be partly due to the lower use of revascularization in women.41 In contrast, data in patients with stable CAD and hypertension indicated that despite higher blood pressures and more comorbidities, women had a lower rate of cardiovascular events (death, MI, or stroke).42 Across all types of CAD subsets, the use of revascularization is generally lower in women than in men in the literature, as was seen in CLARIFY.6,39 It remains possible that while women with CAD tend to have less severe coronary obstructive disease than men31—and therefore possibly a better outcome—this may be balanced by the more severe outcomes of non-obstructive CAD among women.43 The known gender bias in diagnosis and access to diagnostic interventions, as found in CLARIFY and elsewhere,9,14,44,45 is likely to modify long-term prognosis. Although the present study did not explore appropriateness for interventions, there are no data to support reduced efficacy of optimal medical therapy or revascularization in women.45
While we found no overall gender difference in outcomes in stable CAD, this may actually reflect offsetting trends by age: there was an interaction between age and gender, with younger women having better outcomes than men in the same age class, whereas this was not the case for the middle or higher age tertiles.
Differences between studies may also be related to the role and prognostic impact of percutaneous interventions: in ACS, where the use of PCI is associated with established clinical benefit, the lower rate of use seen in women may impact outcomes, whereas in patients with stable CAD, where the benefits of routine revascularization are less established,46,47 the major difference between men and women in the use of PCI would not be expected to impact outcomes.
Finally, with improved outcomes related to broader use of effective evidence-based secondary prevention therapies, such as statins, renin–angiotensin system inhibitors, and antiplatelet agents, the potential to demonstrate differences between genders diminishes. Therefore, a more contemporary study such as CLARIFY may not identify differences seen a decade ago in large trials or registries.
Our study is not without limitations.21 First, the purely observational nature of our study prevents any causality inference, because even though we attempted to adjust for multiple measured potential confounders, it is not possible to exclude confounding by unmeasured variables. Secondly, despite the large size of the CLARIFY population, there were no patients enrolled from the USA, though it did include >2500 patients from Canada, Mexico, and Central America. Thirdly, physician and patient participation in the CLARIFY registry was on a voluntary basis, and as such, is not as representative as a population-based sample. However, every effort was made to maximize the representativeness of the physician and patient samples in each country. The 23% rate of representation of women in CLARIFY may still be lower than in routine clinical practice. The cohort enrolled patients with established CAD, but who survived the first episode and this may underestimate gender differences in mortality related to a first episode of CAD. One of the selection criteria was the presence of chest pain with evidence of myocardial ischaemia, yet exercise electrocardiographic changes in women are more often ‘false positives’ than in men, which may have skewed enrolment of women towards lower risk. One cannot exclude that enrolment based in part on prior revascularization or angiographic demonstration of obstructive CAD may have minimized the participation of those women who tend to undergo less frequently revascularization and have more frequently non-obstructive CAD, in whom outcomes may differ from women with the more typical form of CAD.48 It also may explain the relatively low proportion of women in the registry, as women are less likely than men to achieve eligibility based on prior angiography or revascularization. Another important consideration is that this analysis was not a non-inferiority study and thus was not pre-specified to rule out a given difference in event rates between men and women. Despite the very large size of the trial, event rates are somewhat low and the CIs for ORs by gender show that, even though point estimates for event rates are below 1, one cannot rule out an actual increase in risk of cardiovascular event rates of ∼15% (Figure 2). Finally, 2.8% of the patients were lost to follow-up, and 3.6% of the patients had incomplete data at the time of database lock and were not included in the analyses, although this is unlikely to change the results dramatically.
In summary, there were important differences in baseline characteristics and in risk between men and women, and fewer women underwent revascularization. Nevertheless, we found similar crude and adjusted rates of mortality and cardiovascular events in men and women with stable CAD. Longer follow-up of this population should provide further insights into gender issues in stable CAD. Further research is also needed to devise strategies to minimize bias in the management and treatment of women.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by Servier, France. Sophie Rushton-Smith provided editorial assistance and was compensated by the sponsor. Funding to pay the Open Access publication charges for this article was provided by Servier, France.
Disclaimer
The CLARIFY registry enforces a no ghost-writing policy. This manuscript was written and edited by the authors, who take full responsibility for its content.
Conflict of interest: R.F. has received speaker's bureau fees from Servier, Roche and Boehringer Ingelheim, and research grants from Servier, Boehringer Ingelheim and Roche; Advisory Board: Servier, Bayer, Roche, and Boehringer Ingelheim. J.-C.T. has received research grants and honoraria from Servier. M.T. has received fees, honoraria, and research grants from Amgen, Bayer, Menarini, Servier, and TIMI Group. I.F. has received research grants, honoraria for committee membership, and support for conference attendance from Servier. S.K. is supported by the DZHK (Deutsches Zentrum für Herz-Kreislauf-Forschung—German Centre for Cardiovascular Research) and by the BMBF (German Ministry of Education and Research) and has received consultancy fees/honoraria from Servier. K.M.F. has received fees, honoraria, and research grants from Servier. P.G.S. has received research grants from NYU School of Medicine, Sanofi, Servier; consultancy fees/honoraria from Ablynx, Amarin, Amgen, Astellas, AstraZeneca, Bayer, Boehringer-Ingelheim, BMS, Daiichi/sankyo, Eisai, GSK, Lilly, Medtronic, MSD, Novartis, Otsuka, Pfizer, Roche, Sanofi, Servier, The Medicines Company, and Vivus; and has equity ownership in Aterovax.
Supplementary Material
Acknowledgements
K.M.F. is an NIHR senior investigator and supported by the NIHR Cardiovascular Biomedical Research Unit at the Royal Brompton Hospital. All data were collected and analysed by the independent academic statistics centre at the Robertson Centre for Biostatistics at the University of Glasgow, UK. Servier had no role in study design, data collection and analysis, decision to publish, or writing of the manuscript, but did assist with the set-up and management of the study in each country. Sophie Rushton-Smith provided editorial assistance, limited to editing, checking content and language, formatting, referencing, and preparing tables and figures.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. doi:10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. World Health Statistics 2012. http://www.who.int/gho/publications/world_health_statistics/EN_WHS2012_Full.pdf. (22 July 2012)
- 3.Canto JG, Goldberg RJ, Hand MM, Bonow RO, Sopko G, Pepine CJ, Long T. Symptom presentation of women with acute coronary syndromes: myth vs reality. Arch Intern Med. 2007;167:2405–2413. doi: 10.1001/archinte.167.22.2405. doi:10.1001/archinte.167.22.2405. [DOI] [PubMed] [Google Scholar]
- 4.Brieger D, Eagle KA, Goodman SG, Steg PG, Budaj A, White K, Montalescot G. Acute coronary syndromes without chest pain, an underdiagnosed and undertreated high-risk group: insights from the Global Registry of Acute Coronary Events. Chest. 2004;126:461–469. doi: 10.1378/chest.126.2.461. doi:10.1378/chest.126.2.461. [DOI] [PubMed] [Google Scholar]
- 5.Dey S, Flather MD, Devlin G, Brieger D, Gurfinkel EP, Steg PG, Fitzgerald G, Jackson EA, Eagle KA. Sex-related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: the Global Registry of Acute Coronary Events. Heart. 2009;95:20–26. doi: 10.1136/hrt.2007.138537. doi:10.1136/hrt.2007.138537. [DOI] [PubMed] [Google Scholar]
- 6.Daly C, Clemens F, Lopez Sendon JL, Tavazzi L, Boersma E, Danchin N, Delahaye F, Gitt A, Julian D, Mulcahy D, Ruzyllo W, Thygesen K, Verheugt F, Fox KM. Gender differences in the management and clinical outcome of stable angina. Circulation. 2006;113:490–498. doi: 10.1161/CIRCULATIONAHA.105.561647. doi:10.1161/CIRCULATIONAHA.105.561647. [DOI] [PubMed] [Google Scholar]
- 7.Alfredsson J, Stenestrand U, Wallentin L, Swahn E. Gender differences in management and outcome in non-ST-elevation acute coronary syndrome. Heart. 2007;93:1357–1362. doi: 10.1136/hrt.2006.102012. doi:10.1136/hrt.2006.102012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hochman JS, Tamis JE, Thompson TD, Weaver WD, White HD, Van de Werf F, Aylward P, Topol EJ, Califf RM. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes IIb Investigators. N Engl J Med. 1999;341:226–232. doi: 10.1056/NEJM199907223410402. doi:10.1056/NEJM199907223410402. [DOI] [PubMed] [Google Scholar]
- 9.Crilly M, Bundred P, Hu X, Leckey L, Johnstone F. Gender differences in the clinical management of patients with angina pectoris: a cross-sectional survey in primary care. BMC Health Serv Res. 2007;7:142. doi: 10.1186/1472-6963-7-142. doi:10.1186/1472-6963-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deckers JW, Goedhart DM, Boersma E, Briggs A, Bertrand M, Ferrari R, Remme WJ, Fox K, Simoons ML. Treatment benefit by perindopril in patients with stable coronary artery disease at different levels of risk. Eur Heart J. 2006;27:796–801. doi: 10.1093/eurheartj/ehi809. doi:10.1093/eurheartj/ehi809. [DOI] [PubMed] [Google Scholar]
- 11.Hemingway H, McCallum A, Shipley M, Manderbacka K, Martikainen P, Keskimaki I. Incidence and prognostic implications of stable angina pectoris among women and men. JAMA. 2006;295:1404–1411. doi: 10.1001/jama.295.12.1404. doi:10.1001/jama.295.12.1404. [DOI] [PubMed] [Google Scholar]
- 12.Kumbhani DJ, Shishehbor MH, Willis JM, Karim S, Singh D, Bavry AA, Zishiri E, Ellis SG, Menon V. Influence of gender on long-term mortality in patients presenting with non-ST-elevation acute coronary syndromes undergoing percutaneous coronary intervention. Am J Cardiol. 2012;109:1087–1091. doi: 10.1016/j.amjcard.2011.11.044. doi:10.1016/j.amjcard.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 13.Lansky AJ, Mehran R, Cristea E, Parise H, Feit F, Ohman EM, White HD, Alexander KP, Bertrand ME, Desmet W, Hamon M, Stone GW. Impact of gender and antithrombin strategy on early and late clinical outcomes in patients with non-ST-elevation acute coronary syndromes (from the ACUITY trial) Am J Cardiol. 2009;103:1196–1203. doi: 10.1016/j.amjcard.2009.01.030. doi:10.1016/j.amjcard.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 14.Blomkalns AL, Chen AY, Hochman JS, Peterson ED, Trynosky K, Diercks DB, Brogan GX, Jr, Boden WE, Roe MT, Ohman EM, Gibler WB, Newby LK. Gender disparities in the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes: large-scale observations from the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the American College of Cardiology/American Heart Association Guidelines) National Quality Improvement Initiative. J Am Coll Cardiol. 2005;45:832–837. doi: 10.1016/j.jacc.2004.11.055. doi:10.1016/j.jacc.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 15.Lahoz C, Mantilla T, Taboada M, Soler B, Tranche S, Lopez-Rodriguez I, Monteiro B, Martin-Jadraque R, Sanchez-Zamorano MA, Mostaza JM. Gender differences in evidence-based pharmacological therapy for patients with stable coronary heart disease. Int J Cardiol. 2009;133:336–340. doi: 10.1016/j.ijcard.2007.12.115. doi:10.1016/j.ijcard.2007.12.115. [DOI] [PubMed] [Google Scholar]
- 16.Pepine CJ, Abrams J, Marks RG, Morris JJ, Scheidt SS, Handberg E. Characteristics of a contemporary population with angina pectoris. TIDES Investigators. Am J Cardiol. 1994;74:226–231. doi: 10.1016/0002-9149(94)90361-1. doi:10.1016/0002-9149(94)90361-1. [DOI] [PubMed] [Google Scholar]
- 17.Shaw LJ, Shaw RE, Merz CN, Brindis RG, Klein LW, Nallamothu B, Douglas PS, Krone RJ, McKay CR, Block PC, Hewitt K, Weintraub WS, Peterson ED. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation. 2008;117:1787–1801. doi: 10.1161/CIRCULATIONAHA.107.726562. doi:10.1161/CIRCULATIONAHA.107.726562. [DOI] [PubMed] [Google Scholar]
- 18.Reibis RK, Bestehorn K, Pittrow D, Jannowitz C, Wegscheider K, Voller H. Elevated risk profile of women in secondary prevention of coronary artery disease: a 6-year survey of 117,913 patients. J Womens Health (Larchmt) 2009;18:1123–1131. doi: 10.1089/jwh.2008.1082. doi:10.1089/jwh.2008.1082. [DOI] [PubMed] [Google Scholar]
- 19.Coppieters Y, Collart P, Leveque A. Gender differences in acute myocardial infarction, twenty-five years registration. Int J Cardiol. 2011 doi: 10.1016/j.ijcard.2011.04.012. Published online ahead of print 6 May. [DOI] [PubMed] [Google Scholar]
- 20.Mannsverk J, Wilsgaard T, Njolstad I, Hopstock LA, Lochen ML, Mathiesen EB, Thelle DS, Rasmussen K, Bonaa KH. Age and gender differences in incidence and case fatality trends for myocardial infarction: a 30-year follow-up. The Tromso Study. Eur J Cardiovasc Prev Rehabil. doi: 10.1177/1741826711421081. doi:10.1177/1741826711421081. Published online ahead of print 22 August 2011. [DOI] [PubMed] [Google Scholar]
- 21.Steg PG, Ferrari R, Ford I, Greenlaw N, Tardif JC, Tendera M, Abergel H, Fox KM. Heart rate and use of beta-blockers in stable outpatients with coronary artery disease. PLoS One. 2012;7:e36284. doi: 10.1371/journal.pone.0036284. doi:10.1371/journal.pone.0036284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steg PG. Heart rate management in coronary artery disease: the CLARIFY registry. Eur Heart J. 2009;11(Suppl. D):D13–D18. doi:10.1093/eurheartj/sup017. [Google Scholar]
- 23.Lee PY, Alexander KP, Hammill BG, Pasquali SK, Peterson ED. Representation of elderly persons and women in published randomized trials of acute coronary syndromes. JAMA. 2001;286:708–713. doi: 10.1001/jama.286.6.708. doi:10.1001/jama.286.6.708. [DOI] [PubMed] [Google Scholar]
- 24.Lawesson SS, Alfredsson J, Fredrikson M, Swahn E. Time trends in STEMI–improved treatment and outcome but still a gender gap: a prospective observational cohort study from the SWEDEHEART register. BMJ Open. 2012;2:e000726. doi: 10.1136/bmjopen-2011-000726. doi:10.1136/bmjopen-2011-000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alfredsson J, Lindback J, Wallentin L, Swahn E. Similar outcome with an invasive strategy in men and women with non-ST-elevation acute coronary syndromes: from the Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART) Eur Heart J. 2011;32:3128–3136. doi: 10.1093/eurheartj/ehr349. doi:10.1093/eurheartj/ehr349. [DOI] [PubMed] [Google Scholar]
- 26.Andreotti F, Marchese N. Women and coronary disease. Heart. 2008;94:108–116. doi: 10.1136/hrt.2005.072769. doi:10.1136/hrt.2005.072769. [DOI] [PubMed] [Google Scholar]
- 27.Vaidya D, Becker DM, Bittner V, Mathias RA, Ouyang P. Ageing, menopause, and ischaemic heart disease mortality in England, Wales, and the United States: modelling study of national mortality data. BMJ. 2011;343:d5170. doi: 10.1136/bmj.d5170. doi:10.1136/bmj.d5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hippisley-Cox J, Pringle M, Crown N, Meal A, Wynn A. Sex inequalities in ischaemic heart disease in general practice: cross sectional survey. BMJ. 2001;322:832. doi: 10.1136/bmj.322.7290.832. doi:10.1136/bmj.322.7290.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulcahy D, Dakak N, Zalos G, Andrews NP, Proschan M, Waclawiw MA, Schenke WH, Quyyumi AA. Patterns and behavior of transient myocardial ischemia in stable coronary disease are the same in both men and women: a comparative study. J Am Coll Cardiol. 1996;27:1629–1636. doi: 10.1016/0735-1097(96)00061-7. doi:10.1016/0735-1097(96)00061-7. [DOI] [PubMed] [Google Scholar]
- 30.Vaccarino V. Ischemic heart disease in women: many questions, few facts. Circ Cardiovasc Qual Outcomes. 2010;3:111–115. doi: 10.1161/CIRCOUTCOMES.109.925313. doi:10.1161/CIRCOUTCOMES.109.925313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizia-Stec K, Gasior Z, Mizia M, Haberka M, Holecki M, Zwolinska W, Katarzyna K, Skowerski M. Flow-mediated dilation and gender in patients with coronary artery disease: arterial size influences gender differences in flow-mediated dilation. Echocardiography. 2007;24:1051–1057. doi: 10.1111/j.1540-8175.2007.00531.x. doi:10.1111/j.1540-8175.2007.00531.x. [DOI] [PubMed] [Google Scholar]
- 32.Lansky AJ, Ng VG, Maehara A, Weisz G, Lerman A, Mintz GS, De Bruyne B, Farhat N, Niess G, Jankovic I, Lazar D, Xu K, Fahy M, Serruys PW, Stone GW. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. JACC Cardiovasc Imaging. 2012;5(3 Suppl):S62–S72. doi: 10.1016/j.jcmg.2012.02.003. doi:10.1016/j.jcmg.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Merz CN, Shaw LJ. Stable angina in women: lessons from the National Heart, Lung and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation. J Cardiovasc Med (Hagerstown) 2011;12:85–87. doi: 10.2459/JCM.0b013e3283430969. doi:10.2459/JCM.0b013e3283430969. [DOI] [PubMed] [Google Scholar]
- 34.Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54:1561–1575. doi: 10.1016/j.jacc.2009.04.098. doi:10.1016/j.jacc.2009.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sader MA, Celermajer DS. Endothelial function, vascular reactivity and gender differences in the cardiovascular system. Cardiovasc Res. 2002;53:597–604. doi: 10.1016/s0008-6363(01)00473-4. doi:10.1016/S0008-6363(01)00473-4. [DOI] [PubMed] [Google Scholar]
- 36.Lawesson SS, Stenestrand U, Lagerqvist B, Wallentin L, Swahn E. Gender perspective on risk factors, coronary lesions and long-term outcome in young patients with ST-elevation myocardial infarction. Heart. 2010;96:453–459. doi: 10.1136/hrt.2009.175463. doi:10.1136/hrt.2009.175463. [DOI] [PubMed] [Google Scholar]
- 37.Leuzzi C, Modena MG. Coronary artery disease: clinical presentation, diagnosis and prognosis in women. Nutr Metab Cardiovasc Dis. 2010;20:426–435. doi: 10.1016/j.numecd.2010.02.013. doi:10.1016/j.numecd.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Maas AH, van der Schouw YT, Regitz-Zagrosek V, Swahn E, Appelman YE, Pasterkamp G, Ten Cate H, Nilsson PM, Huisman MV, Stam HC, Eizema K, Stramba-Badiale M. Red alert for women's heart: the urgent need for more research and knowledge on cardiovascular disease in women: proceedings of the workshop held in Brussels on gender differences in cardiovascular disease, 29 September 2010. Eur Heart J. 2011;32:1362–1368. doi: 10.1093/eurheartj/ehr048. doi:10.1093/eurheartj/ehr048. [DOI] [PubMed] [Google Scholar]
- 39.Poon S, Goodman SG, Yan RT, Bugiardini R, Bierman AS, Eagle KA, Johnston N, Huynh T, Grondin FR, Schenck-Gustafsson K, Yan AT. Bridging the gender gap: Insights from a contemporary analysis of sex-related differences in the treatment and outcomes of patients with acute coronary syndromes. Am Heart J. 2012;163:66–73. doi: 10.1016/j.ahj.2011.09.025. doi:10.1016/j.ahj.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 40.Berger JS, Elliott L, Gallup D, Roe M, Granger CB, Armstrong PW, Simes RJ, White HD, Van de Werf F, Topol EJ, Hochman JS, Newby LK, Harrington RA, Califf RM, Becker RC, Douglas PS. Sex differences in mortality following acute coronary syndromes. JAMA. 2009;302:874–882. doi: 10.1001/jama.2009.1227. doi:10.1001/jama.2009.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milcent C, Dormont B, Durand-Zaleski I, Steg PG. Gender differences in hospital mortality and use of percutaneous coronary intervention in acute myocardial infarction: microsimulation analysis of the 1999 nationwide French hospitals database. Circulation. 2007;115:833–839. doi: 10.1161/CIRCULATIONAHA.106.664979. doi:10.1161/CIRCULATIONAHA.106.664979. [DOI] [PubMed] [Google Scholar]
- 42.Pepine CJ. Ischemic heart disease in women: facts and wishful thinking. J Am Coll Cardiol. 2004;43:1727–1730. doi: 10.1016/j.jacc.2004.04.012. doi:10.1016/j.jacc.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 43.Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, Zineh I, Kelsey SF, Arnsdorf MF, Black HR, Pepine CJ, Merz CN. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women's Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med. 2009;169:843–850. doi: 10.1001/archinternmed.2009.50. doi:10.1001/archinternmed.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sekhri N, Timmis A, Chen R, Junghans C, Walsh N, Zaman MJ, Eldridge S, Hemingway H, Feder G. Inequity of access to investigation and effect on clinical outcomes: prognostic study of coronary angiography for suspected stable angina pectoris. BMJ. 2008;336:1058–1061. doi: 10.1136/bmj.39534.571042.BE. doi:10.1136/bmj.39534.571042.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ciambrone G, Kaski JC. Gender differences in the treatment of chronic ischemic heart disease: prognostic implications. Fundam Clin Pharmacol. 2010;24:707–710. doi: 10.1111/j.1472-8206.2009.00774.x. doi:10.1111/j.1472-8206.2009.00774.x. [DOI] [PubMed] [Google Scholar]
- 46.Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. doi:10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 47.Simoons ML, Windecker S. Controversies in cardiovascular medicine: Chronic stable coronary artery disease: drugs vs. revascularization. Eur Heart J. 2010;31:530–541. doi: 10.1093/eurheartj/ehp605. [DOI] [PubMed] [Google Scholar]
- 48.Shaw LJ, Bairey Merz CN, Pepine CJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Wessel TR, Arant CB, Pohost GM, Lerman A, Quyyumi AA, Sopko G. Insights from the NHLBI-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study: Part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol. 2006;47(3 Suppl.):S4–S20. doi: 10.1016/j.jacc.2005.01.072. doi:10.1016/j.jacc.2005.01.072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.