Abstract
Although deep brain stimulation (DBS) has been found to be efficacious for some chronic pain syndromes, its usefulness in patients with central poststroke pain (CPSP) has been disappointing. The most common DBS targets for pain are the periventricular gray region (PVG) and the ventralis caudalis of the thalamus. Despite the limited success of DBS for CPSP, few alternative targets have been explored. The nucleus accumbens (NAC), a limbic structure within the ventral striatum that is involved in reward and pain processing, has emerged as an effective target for psychiatric disease. There is also evidence that it may be an effective target for pain. We describe a 72-year-old woman with a large right hemisphere infarct who subsequently experienced refractory left hemibody pain. She underwent placement of 3 electrodes in the right PVG, ventralis caudalis of the thalamus, and NAC. Individual stimulation of the NAC and PVG provided substantial improvement in pain rating. The patient underwent implantation of permanent electrodes in both targets, and combined stimulation has provided sustained pain relief at nearly 1 year after the procedure. These results suggest that the NAC may be an effective DBS target for CPSP.
Abbreviations and Acronyms: CPSP, central poststroke pain; DBS, deep brain stimulation; ECT, electroconvulive therapy; ICL, intercommissural line; MCS, motor cortex stimulation; NAC, nucleus accumbens; PFC, prefrontal cortex; PVG, periventricular gray region; VC, ventralis caudalis of the thalamus
The efficacy of deep brain stimulation (DBS) is well established in Parkinson disease and essential tremor.1,2 However, results of DBS for chronic pain vary depending on the nature of the pain.3 Deep brain stimulation is generally more effective for nociceptive than for deafferentation pain.4 Several targets have been explored for controlling pain, including the septal area, the posterior limb of the internal capsule, the centromedian and parafascicular nuclei, the ventralis caudalis of the thalamus (VC), and the periventricular gray region (PVG).4-7 The preferred targets are the PVG and VC due to ease of targeting and efficacy. Stimulation of the PVG and VC for central poststroke pain (CPSP), however, has failure rates ranging from 33% to 82%.4,8,9 These poor results suggest that alternative targets should be explored.
Deep brain stimulation of the nucleus accumbens (NAC), an extension of the ventral striatum involved in reward processing, has been found to be effective for depression and obsessive-compulsive disorder.10,11 Based on increasing evidence that the NAC also mediates pain processing, we hypothesized it might be an effective target for CPSP.
We describe a patient with refractory CPSP and controlled depression in whom DBS surgery was performed with the NAC, PVG, and VC as targets for stimulation. Nucleus accumbens and PVG stimulation provided substantial relief 1 day postsurgery. The patient subsequently underwent implantation of permanent electrodes in both targets and has experienced continued pain relief at 11 months postsurgery.
Report of a Case
A 72-year-old woman sustained a right middle cerebral artery infarct in July 2009 resulting in left hemiplegia, hemianopia, and hemisensory loss to pinprick and temperature. Within 3 months, constant, severe left arm and leg pain developed. She had no allodynia or hyperalgesia. Pain persisted despite adequate trials of oxycodone, lamotrigine, gabapentin, pregabalin, citalopram, carbamazepine, amitriptyline, duloxetine, alprazolam, baclofen, transcutaneous electrical nerve stimulation, and botulinum toxin injection to relieve right shoulder pain. She was referred to the Mayo Clinic in Rochester, Minnesota, 11 months poststroke for consideration of DBS. Neuropsychiatric assessment performed at the time of referral revealed that the patient had a history of depression that began shortly after the onset of her stroke. Her depressive symptoms had been refractory to several antidepressant medications including citalopram, amitriptyline, and duloxetine. However, she responded well to electroconvulsive therapy (ECT), which was administered on July 26, 2010, 12 months after her stroke. Relief from depression following ECT was maintained with sertraline, a selective serotonin reuptake inhibitor. The Mayo Clinic DBS committee approved surgery in November 2010.
DBS Procedure
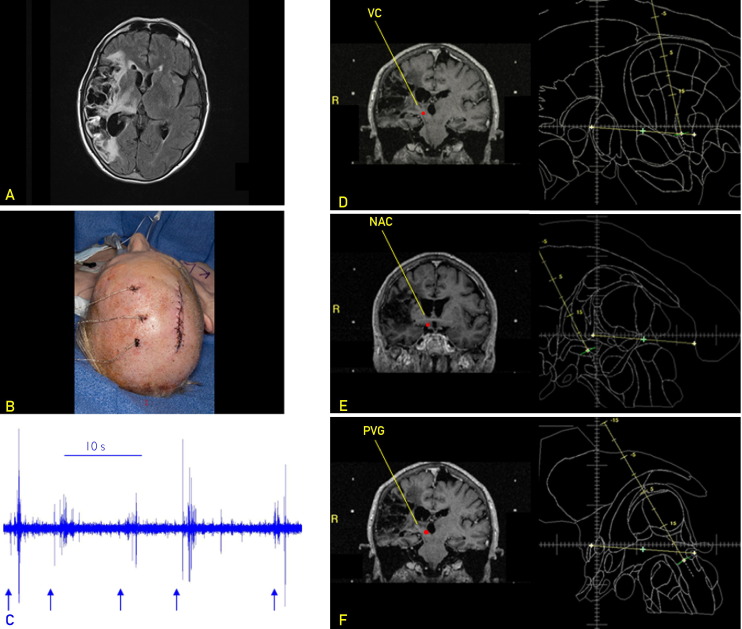
Surgery was performed with local anesthesia. After placing a Leksell head frame (Elekta, Norcross, GA), stereotactic magnetic resonance imaging with gadolinium was performed. Compass software (Compass International Innovations Inc., Rochester, MN) and a Schaltenbrand-Wahren atlas were used to target the PVG and VC.12 The NAC was targeted using coordinates described by Schlaepfer et al.13 Coordinates were as follows: 2.5 mm lateral to the third ventricular wall, 2.5 mm anterior to the posterior commissure, and 2.0 mm inferior to the intercommissural line (ICL) for the PVG; 12 mm lateral to midline, 3.5 mm anterior to the posterior commissure, and 0.0 mm from the ICL for the VC; and 7.0 mm lateral to midline, 1.0 mm anterior to the anterior commissure, and 4 mm inferior to the ICL for the NAC. Through a single incision, 3 separate burr holes were made for the planned electrode trajectories. The PVG was targeted first, followed by the VC and NAC. Microelectrode recordings from the NAC and PVG showed spike activity. The VC recordings demonstrated activation of sensory cells with light touch, despite the fact that the patient exhibited no conscious awareness of the stimuli. Medtronic model 3387 leads (Medtronic, Inc, Minneapolis, MN) were advanced to the intended targets. Macrostimulation yielded no adverse effects, and fluoroscopy ensured no lead migration. The leads were secured and externalized for stimulation, and the wound was closed. Preoperative planning images and VC recordings are shown in Figure 1. Postoperative magnetic resonance imaging showed lead placement at the intended targets (Figure 2).
FIGURE 1.
A, Preoperative axial T2-weighted fluid-attenuated inversion recovery image showing an old right hemispheric infarct with cortical encephalomalacia and Wallerian degeneration in the right thalamus. B, Intraoperative photograph showing externalized leads. Test stimulation was performed on the first postoperative day. C, Microelectrode recordings from the ventralis caudalis of the thalamus (VC) (10-second strip) demonstrating spike activity to light touch (arrows) even though the patient had no conscious perception. D-F, Stereotactic planning for deep brain stimulation of the VC (D), nucleus accumbens (NAC) (E), and periventricular gray region (PVG) (F). The PVG and VC were targeted using the human brain atlas of Schaltenbrand and Wahren,12 and the NAC was targeted using stereotactic coordinates described by Schlaepfer et al.13 Targets are indicated by red dots. R = right.
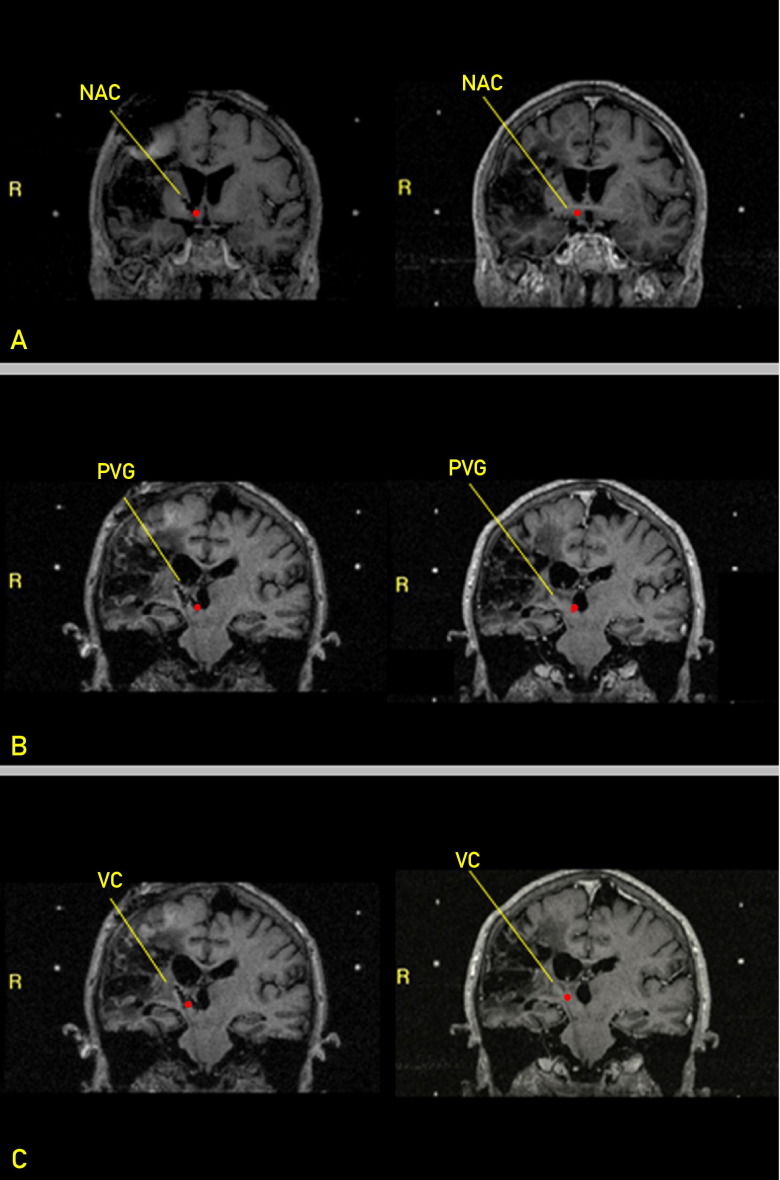
FIGURE 2.
Postoperative (left) and preoperative (right) stereotactic magnetic resonance images for each target. A, Nucleus accumbens (NAC). B, Periventricular gray region (PVG). C, Ventralis caudalis of the thalamus (VC). T1-weighted coronal sequences are shown with the target highlighted with a red dot. Metal artifact for each electrode can be seen, demonstrating that electrodes were placed at intended targets. R = right.
Test Stimulation and Follow-up
Double-blinded test stimulation was initiated on postoperative day 1. Nursing staff recorded hourly pain ratings using a 0 to 10 visual analog scale. The patient rated pain as a 10 before surgery and as an 8 before stimulation. Targets were stimulated individually for 3 hours at a 90-μs pulse width, a frequency of 130 Hz, and an amplitude of 4.0 V. The patient reported no pain relief. Eye deviation occurred with PVG stimulation, and the distal contact was excluded from further stimulation. The pulse width was increased to 300 μs, and the patient reported a pain rating of 3 during NAC and PVG stimulation. She reported no relief with VC stimulation. Combined NAC and PVG stimulation gave further relief without adverse effects. The NAC and PVG electrodes were then internalized for long-term stimulation, and the settings were further adjusted before discharge.
At 3 weeks postoperatively, with the addition of physical therapy, the patient rated her pain level as a 2. At 6 months, her pain ratings ranged from 0 to 2. Long-acting morphine was discontinued, and hydrocodone was reduced from 40 mg/d to 10 mg/d. Carbamazepine, pregabalin, and sertraline were continued. Her depression remained in remission. To further assess individual target efficacy, the PVG electrode was turned off, and NAC stimulation was continued at the original settings. Pain recurred within 1 week. It partially improved with reinitiation of PVG stimulation, although the pulse width for the NAC was eventually increased from 300 to 450 μs because of continued pain. Isolated PVG stimulation was then tried, but pain recurred several weeks later. The PVG voltage was increased from 3.0 to 3.5 V without relief. At that point, NAC stimulation was restarted at 1.0 V. At 11 months postsurgery, the patient reported her pain level as 0 of 10 with combined NAC and PVG stimulation (Table).
TABLE.
Pain Ratings at Different Time Intervals and Corresponding Lead Stimulation
| Time | PVG settings | NAC settings | VAS pain scores |
|---|---|---|---|
| Before surgery | … | … | 10 |
| Postimplantation/prestimulation | … | … | 8 |
| Day 1 stimulation | … | PW: 90 μsF: 130 HzA: 4.0 V | 8 |
| PW: 90 μsF: 130 HzA: 4.0 V | … | 8 | |
| Day 2 stimulation | … | PW: 300 μsF: 130 HzA: 4.0 V | 3 |
| PW: 300 μsF: 130 HzA: 4.0 V | … | 3 | |
| 3 wk postsurgery | PW: 300 μsF: 130 HzA: 3.5 V | PW: 300 μsF: 130 HzA: 4.0 V | 2 |
| 7 mo postsurgery | PW: 300 μsF: 130 HzA: 3.5 V | PW: 300 μsF: 130 HzA: 4.0 V | 0 |
| … | PW: 300 μsF: 130 HzA: 4.0 V | 9 | |
| PW: 300 μsF: 130 HzA: 3.0 V | PW: 300 μsF: 130 HzA: 4.0 V | 7 | |
| 8 mo postsurgery | PW: 300 μsF: 130 HzA: 3.0 V | PW: 450 μsF: 130 HzA: 4.0 V | 5 |
| PW: 300 μsF: 130 HzA: 3.0 V | … | 9 | |
| PW: 300 μsF: 130 HzA: 3.5 V | … | 9 | |
| 11 mo postsurgery | PW: 300 μsF: 130 HzA: 3.5 V | PW: 450 μsF: 130 HzA: 1.0 V cycling | 0 |
A = amplitude; F = frequency; NAC = nucleus accumbens; PVG = periventricular gray region; PW = pulse width; VAS = visual analog scale.
Discussion
Herein, we report sustained analgesia following combined NAC and PVG stimulation in a single patient with treatment-refractory CPSP. There is currently an ongoing randomized trial of NAC stimulation for thalamic pain.14 As many as 8% of stroke patients suffer from CPSP.15 The percentage may be higher in patients with strokes involving the spinothalamic pathway or central pain processing areas.16-18 Antidepressants are considered the first line of treatment, followed by anticonvulsants and opiates.19-21 Deep brain stimulation or motor cortex stimulation (MCS) are considered when medications and less invasive modalities fail. Some investigators advocate that MCS is more effective for CPSP.22 However, MCS requires integrity of the underlying cortex. The large size and location of the infarct in our patient made DBS a better option.
We targeted 3 sites known to play critical roles in pain processing: the VC, NAC, and PVG. The VC receives input from the lateral component of the spinothalamic pathways and projects to the somatosensory cortex, the secondary somatosensory cortex, and eventually the posterior insular and parietal cortices.23,24 The function of this pathway is pain localization. There is evidence that loss of VC afferents results in hyperpolarization of neurons within the thalamic reticular nucleus and transition to bursting, which spreads to lateral and medial thalami via reciprocal projections and could eventually reach conscious perception as aberrant pain signals.24,25 The few studies that have investigated the mechanism of analgesia from VC stimulation suggest that pain relief is provided through activation of both descending and ascending pathways. In animal models, the analgesic effect of VC stimulation is eliminated by lesioning the spinal cord,26 whereas positron emission tomographic studies demonstrate that ascending projections are also activated as relative hypermetabolism of the anterior cingulate gyrus occurs with VC stimulation.27 In our case, analgesia was not obtained by VC simulation but with combined NAC and PVG stimulation.
The NAC is a 10.5 × 14.5 × 7.0-mm ventral extension of the striatum located rostral to the anterior commissure and underneath the anterior limb of the internal capsule.28 The NAC occupies a strategic location. It receives input from the prefrontal cortex (PFC) and limbic structures, such as the hippocampus and amygdala, and sends output to motor control regions. This circuitry allows contextual input from the hippocampus and emotional input from the amygdala to be integrated with cognitive information supplied by the PFC to motor control areas that are important in the selection of goal-directed behaviors.29 This unique neuroanatomic position allows the NAC to modulate pain processing directly through inhibitory projections to the medial thalamus30,31 and from the medial thalamus to the dorsal horn neurons.32,33 The NAC together with the PFC, insula, and anterior cingulate cortex mediates the affective (“unpleasant”) component of pain.34 The NAC also suppresses the aversive and autonomic components of pain through direct inhibitory projections to the amygdala and periaqueductal gray region.35,36
In addition, the NAC is one of few locations where dopamine and glutamate neurotransmissions converge. Recent findings suggest that the opioid receptor agonist TAN-67, a compound that causes antinociception or hyperalgesia (in mice) depending on its enantiomeric form, triggers a release of glutamate that ultimately, via activation of N-methyl-D-aspartate receptors, enhances release of dopamine from dopaminergic nerve terminals in the NAC.37 We have previously reported that high-frequency stimulation of the subthalamic nucleus increases glutamate and dopamine levels in rats and large animal models.38,39 Thus, it is not unreasonable to presume that high-frequency stimulation of the NAC may also result in glutamate and dopamine release, which could result in analgesia through unknown downstream effects.
Although the NAC has never been directly targeted for pain, the analgesic effect of intracranial stimulation was first discovered with septal stimulation, an area in close proximity to the NAC. During behavioral experiments, Heath and Mickle6 discovered that septal stimulation not only provided intense reward but also relieved chronic pain in some patients. Gol7 later reproduced this finding inconsistently in cancer patients. Based on its proximity to the septal area and its function in reward processing, it is tempting to speculate that these investigators were actually stimulating the NAC.
Interestingly, PVG stimulation in our patient was also associated with marked analgesic effect. The PVG has ascending projections to the intralaminar and medial dorsal nuclei in addition to descending projections to raphespinal and reticulospinal nuclei.22 It has been suggested that PVG stimulation may release endogenous opioids and activate descending pain inhibitory pathways.40,41
Further research is needed to elucidate the mechanisms underlying the ameliorative effects of NAC stimulation on pain. For example, there is some evidence that NAC stimulation may have an antidepressant effect.10,11 It could be argued that because our patient had symptoms of depression, NAC stimulation had a mood-modulating effect that had a positive impact on the patient's experience of pain. It is important to note, however, that the patient had relief from her depressive symptoms prior to surgery through ECT, that she was responding well to selective serotonin reuptake inhibitor medication at the time of DBS implantation, and that her subjective experience of depression was unchanged following DBS. Further research is also needed to evaluate the efficacy of NAC stimulation. Currently, Machado14 is conducting a prospective, randomized study of DBS of the ventral striatum and anterior internal capsule for patients with thalamic pain syndrome.
Conclusion
We report sustained analgesia from combined stimulation of the NAC and PVG in a refractory case of CPSP. The durable response suggests that the NAC may be a feasible target for CPSP and other pain disorders. Given the role of the NAC and PVG in processing and inhibiting pain and the fact that neither target was effective in isolation, it may be that only combined stimulation is effective. Further research is needed to confirm our findings, to evaluate the efficacy of targeting the NAC for other disorders, and to investigate the mechanisms of DBS-invoked analgesia.
Acknowledgments
We thank Dr Penelope Duffy for editorial assistance and Stephan Goerss, Laura Haugen, and Bruce Kall for their technical expertise in helping produce figures for this article and utilizing Compass software for stereotactic planning.
Footnotes
Grant Support: This work was supported by a National Institutes of Health grant (K08 NS 52232, K.H.L.) and a Mayo Foundation Research Early Career Development Award for Clinician Scientists (K.H.L.).
Potential Conflicting Interests: Dr Stead serves on the Medtronic Medical Advisory Board. Dr Watson consults on the safety monitoring committee for Nevro Corp spinal cord stimulators.
References
- 1.Deuschl G., Schade-Brittinger C., Krack P., German Parkinson Study Group, Neurostimulation Section A randomized trial of deep-brain stimulation for Parkinson's disease [published correction appears in N Engl J Med. 2006;355(12):1289] N Engl J Med. 2006;355(9):896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 2.Flora E.D., Perera C.L., Cameron A.L., Maddern G.J. Deep brain stimulation for essential tremor: a systematic review. Mov Disord. 2010;25(11):1550–1559. doi: 10.1002/mds.23195. [DOI] [PubMed] [Google Scholar]
- 3.Levy R., Deer T.R., Henderson J. Intracranial neurostimulation for pain control: a review. Pain Physician. 2010;13(2):157–165. [PubMed] [Google Scholar]
- 4.Rasche D., Rinaldi P.C., Young R.F., Tronnier V.M. Deep brain stimulation for the treatment of various chronic pain syndromes. Neurosurg Focus. 2006;21(6):E8. doi: 10.3171/foc.2006.21.6.10. [DOI] [PubMed] [Google Scholar]
- 5.Young R.F., Kroening R., Fulton W., Feldman R.A., Chambi I. Electrical stimulation of the brain in treatment of chronic pain: experience over 5 years. J Neurosurg. 1985;62(3):389–396. doi: 10.3171/jns.1985.62.3.0389. [DOI] [PubMed] [Google Scholar]
- 6.Heath R.G., Mickle W.A. Evaluation of seven years' experience with depth electrode studies in human patients. In: Ramey E.R., O'Doherty D.S., editors. Electrical Studies on the Unanesthesized Brain. PB Hoeber; New York, NY: 1960. pp. 214–247. [Google Scholar]
- 7.Gol A. Relief of pain by electrical stimulation of the septal area. J Neurol Sci. 1967;5(1):115–120. doi: 10.1016/0022-510x(67)90012-3. [DOI] [PubMed] [Google Scholar]
- 8.Owen S.L., Green A.L., Stein J.F., Aziz T.Z. Deep brain stimulation for the alleviation of post-stroke neuropathic pain. Pain. 2006;120(1-2):202–206. doi: 10.1016/j.pain.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 9.Owen S.L., Green A.L., Nandi D.D., Bittar R.G., Wang S., Aziz T.Z. Deep brain stimulation for neuropathic pain. Acta Neurochir Suppl. 2007;97(pt 2):111–116. doi: 10.1007/978-3-211-33081-4_13. [DOI] [PubMed] [Google Scholar]
- 10.Hauptman J.S., DeSalles A.A., Espinoza R., Sedrak M., Ishida W. Potential surgical targets for deep brain stimulation in treatment-resistant depression. Neurosurg Focus. 2008;25(1):E9. doi: 10.3171/FOC/2008/25/7/E3. [DOI] [PubMed] [Google Scholar]
- 11.Franzini A., Messina G., Gambini O. Deep-brain stimulation of the nucleus accumbens in obsessive compulsive disorder: clinical, surgical and electrophysiological considerations in two consecutive patients. Neurol Sci. 2010;31(3):353–359. doi: 10.1007/s10072-009-0214-8. [DOI] [PubMed] [Google Scholar]
- 12.Schaltenbrand G., Wahren W. Georg Thieme Verlag; Stuttgart, Germany: 1977. Atlas for Stereotaxy of the Human Brain. [Google Scholar]
- 13.Schlaepfer T.E., Cohen M.X., Frick C. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33(2):368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 14.Machado A. Safety Study of Deep Brain Stimulation to Manage Thalamic Pain Syndrome (DBS) http://clinicaltrials.gov/ct2/show/NCT01072656 Accessed August 21, 2012.
- 15.Andersen G., Vestergaard K., Ingeman-Nielsen M., Jensen T.S. Incidence of central post-stroke pain. Pain. 1995;61(2):187–193. doi: 10.1016/0304-3959(94)00144-4. [DOI] [PubMed] [Google Scholar]
- 16.Bowsher D. Allodynia in relation to lesion site in central post-stroke pain. J Pain. 2005;6(11):736–740. doi: 10.1016/j.jpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Kim J.S., Choi-Kwon S. Sensory sequelae of medullary infarction: differences between lateral and medial medullary syndrome. Stroke. 1999;30(12):2697–2703. doi: 10.1161/01.str.30.12.2697. [DOI] [PubMed] [Google Scholar]
- 18.Kumar G., Soni C.R. Central post-stroke pain: current evidence. J Neurol Sci. 2009;284(1-2):10–17. doi: 10.1016/j.jns.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 19.Leijon G., Boivie J. Central post-stroke pain—a controlled trial of amitriptyline and carbamazepine. Pain. 1989;36(1):27–36. doi: 10.1016/0304-3959(89)90108-5. [DOI] [PubMed] [Google Scholar]
- 20.Vestergaard K., Nielsen J., Andersen G., Ingeman-Nielsen M., Arendt-Nielsen L., Jensen T.S. Sensory abnormalities in consecutive, unselected patients with central post-stroke pain. Pain. 1995;61(2):177–186. doi: 10.1016/0304-3959(94)00140-A. [DOI] [PubMed] [Google Scholar]
- 21.Frese A., Husstedt I.W., Ringelstein E.B., Evers S. Pharmacologic treatment of central post-stroke pain. Clin J Pain. 2006;22(3):252–260. doi: 10.1097/01.ajp.0000173020.10483.13. [DOI] [PubMed] [Google Scholar]
- 22.Nandi D., Aziz T., Carter H., Stein J. Thalamic field potentials in chronic central pain treated by periventricular gray stimulation—a series of eight cases. Pain. 2003;101(1-2):97–107. doi: 10.1016/s0304-3959(02)00277-4. [DOI] [PubMed] [Google Scholar]
- 23.Taber K.H., Rashid A., Hurley R.A. Functional anatomy of central pain. J Neuropsychiatry Clin Neurosci. 2001;13(4):437–440. doi: 10.1176/jnp.13.4.437. [DOI] [PubMed] [Google Scholar]
- 24.Jeanmonod D., Magnin M., Morel A. Low-threshold calcium spike bursts in the human thalamus: common physiopathology for sensory, motor and limbic positive symptoms. Brain. 1996;119(pt 2):363–375. doi: 10.1093/brain/119.2.363. [DOI] [PubMed] [Google Scholar]
- 25.Rinaldi P.C., Young R.F., Albe-Fessard D., Chodakiewitz J. Spontaneous neuronal hyperactivity in the medial and intralaminar thalamic nuclei of patients with deafferentation pain. J Neurosurg. 1991;74(3):415–421. doi: 10.3171/jns.1991.74.3.0415. [DOI] [PubMed] [Google Scholar]
- 26.Bittar R.G., Kar-Purkayastha I., Owen S.L. Deep brain stimulation for pain relief: a meta-analysis. J Clin Neurosci. 2005;12(5):515–519. doi: 10.1016/j.jocn.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Davis K.D., Taub E., Duffner F. Activation of the anterior cingulate cortex by thalamic stimulation in patients with chronic pain: a positron emission tomography study. J Neurosurg. 2000;92(1):64–69. doi: 10.3171/jns.2000.92.1.0064. [DOI] [PubMed] [Google Scholar]
- 28.Neto L.L., Oliveira E., Correia F., Ferreira A.G. The human nucleus accumbens: where is it?: A stereotactic, anatomical and magnetic resonance imaging study. Neuromodulation. 2008;11(1):13–22. doi: 10.1111/j.1525-1403.2007.00138.x. [DOI] [PubMed] [Google Scholar]
- 29.Heinze H.J., Heldmann M., Voges J. Counteracting incentive sensitization in severe alcohol dependence using deep brain stimulation of the nucleus accumbens: clinical and basic science aspects. Front Hum Neurosci. 2009;3:22. doi: 10.3389/neuro.09.022.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams D.J., Crossman A.R., Slater P. The efferent projections of the nucleus accumbens in the rat. Brain Res. 1977;130(2):217–227. doi: 10.1016/0006-8993(77)90271-2. [DOI] [PubMed] [Google Scholar]
- 31.Smeets W.J., Medina L. The efferent connections of the nucleus accumbens in the lizard Gekko gecko: a combined tract-tracing/transmitter-immunohistochemical study. Anat Embryol (Berl) 1995;191(1):73–81. doi: 10.1007/BF00215299. [DOI] [PubMed] [Google Scholar]
- 32.Albe-Fessard D., Berkley K.J., Kruger L., Ralston H.J., III, Willis W.D., Jr Diencephalic mechanisms of pain sensation. Brain Res. 1985;356(3):217–296. doi: 10.1016/0165-0173(85)90013-x. [DOI] [PubMed] [Google Scholar]
- 33.Kerr F.W., Casey K.L. Pain. Neurosci Res Program Bull. 1978;16(1):1–207. [PubMed] [Google Scholar]
- 34.Lorenz J., Minoshima S., Casey K.L. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126(pt 5):1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- 35.Ma Q.P., Han J.S. Neurochemical studies on the mesolimbic circuitry of antinociception. Brain Res. 1991;566(1-2):95–102. doi: 10.1016/0006-8993(91)91685-t. [DOI] [PubMed] [Google Scholar]
- 36.Ma Q.P., Shi Y.S., Han J.S. Further studies on interactions between periaqueductal gray, nucleus accumbens and habenula in antinociception. Brain Res. 1992;583(1-2):292–295. doi: 10.1016/s0006-8993(10)80036-8. [DOI] [PubMed] [Google Scholar]
- 37.Fusa K., Takahashi I., Watanabe S. The non-peptidic delta opioid receptor agonist TAN-67 enhances dopamine efflux in the nucleus accumbens of freely moving rats via a mechanism that involves both glutamate and free radicals. Neuroscience. 2005;130(3):745–755. doi: 10.1016/j.neuroscience.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Lee K.H., Kristic K., van Hoff R. High-frequency stimulation of the subthalamic nucleus increases glutamate in the subthalamic nucleus of rats as demonstrated by in vivo enzyme-linked glutamate sensor. Brain Res. 2007;1162:121–129. doi: 10.1016/j.brainres.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 39.Shon Y.M., Lee K.H., Goerss S.J. High frequency stimulation of the subthalamic nucleus evokes striatal dopamine release in a large animal model of human DBS neurosurgery. Neurosci Lett. 2010;475(3):136–140. doi: 10.1016/j.neulet.2010.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hosobuchi Y., Adams J.E., Linchitz R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science. 1977;197(4299):183–186. doi: 10.1126/science.301658. [DOI] [PubMed] [Google Scholar]
- 41.Young R.F., Bach F.W., Van Norman A.S., Yaksh T.L. Release of beta-endorphin and methionine-enkephalin into cerebrospinal fluid during deep brain stimulation for chronic pain: effects of stimulation locus and site of sampling. J Neurosurg. 1993;79(6):816–825. doi: 10.3171/jns.1993.79.6.0816. [DOI] [PubMed] [Google Scholar]