Abstract
The protein cargo transported by specific types of vesicles largely defines the different secretory trafficking pathways operating within cells. However, mole per mole the most abundant cargo contained within transport vesicles is not protein, but lipid. Taking a “lipid-centric” point-of-view, we examine the importance of lipid signaling, membrane lipid organization and lipid metabolism for vesicle transport during exocytosis in budding yeast. In fact, the essential requirement for some exocytosis regulatory proteins can be bypassed by making simple manipulations of the lipids involved. During polarized exocytosis the sequential steps required to generate post-Golgi vesicles and target them to the plasma membrane (PM) involves the interplay of several types of lipids that are coordinately linked through PI4P metabolism and signaling. In turn, PI4P levels are regulated by PI4P kinases, the Sac1p PI4P phosphatase and the yeast Osh proteins, which are homologs of mammalian oxysterol-binding protein (OSBP). Together these regulators integrate the transitional steps required for vesicle maturation directly through changes in lipid composition and organization.
Keywords: polarized exocytosis, vesicle transport, lipid metabolism, sterols, PI4P, phosphoinositides, SAC1, oxysterol-binding proteins, Osh proteins, small GTPases
The final delivery of secretory proteins and lipids to the cell cortex begins at the Golgi apparatus when membrane components are sorted into nascent exocytic vesicles and ends at the PM where vesicles dock and bilayers fuse. In budding yeast, the major route of membrane trafficking to the cell cortex involves targeted vesicle transport via polarized exocytosis into the growing bud.1 In this secretory pathway, lipid biosynthesis, lipid signaling by phosphoinositides (PIPs) and other signaling lipids, and transbilayer asymmetry generated by lipid flippases all conspire to modulate membranes for exocytosis. Changes in membrane lipid composition and organization contribute to exocytosis by: (1) generating local deformations in the Golgi membrane bilayer for the extrusion of vesicles; (2) assembling membrane domains that promote the recruitment and sorting of specific proteins into forming vesicles; and (3) initiating signaling cascades that trigger transitional changes in protein complexes that drive subsequent steps for transport. For the latter, lipids orchestrate the activities of Ras-, Rab- and Rho-family small GTPases to promote the assembly of protein complexes involved in actomyosin-based vesicle transfer, vesicle docking and membrane fusion at targeted sites.
In yeast, vesicle traffic from the Golgi to the PM moves along actin filaments propelled forward by Myo2p, a type V myosin motor. Along the way, the vesicle-associated subunits of the exocyst tethering complex are configured for vesicle docking with the PM. Vesicle docking occurs when the vesicle- and PM-associated exocyst complex subunits join, and SNARE-dependent fusion of the vesicle and PM bilayers ensues. At each step during vesicle transport, the protein complexes involved are commanded by specific lipids and the regulation of those lipids ultimately controls exocytosis (Table 1).
Table 1. Lipid-signaling and -metabolism proteins required for yeast polarized exocytosis.
| Protein | Activity | Localization |
|---|---|---|
|
Actinomyosin-based vesicle motility |
|
|
| Myo2 |
Type V myosin motor |
Actin filaments |
|
Exocyst complex/vesicle tethering |
|
|
| Sec3, Exo70 |
PM-associated exocyst complex subunits |
PM/sites of polarized growth |
| Exo84, Sec6, Sec8, Sec10, Sec15 |
Vesicle-associated exocyst complex subunits |
Exocytic vesicles/sites of polarized growth |
|
Lipid flippases and regulators |
|
|
| Cdc50 |
Drs2p chaperone |
Golgi |
| Dnf1, Dnf2 |
Aminophospholipid translocases |
PM |
| Dnf3, Drs2 |
Aminophospholipid translocases |
Golgi |
| Lem3 |
Dnf1p interacting protein |
PM |
|
Markers for exocytic transport |
|
|
| Can1 |
Plasma membrane arginine permease |
PM |
| Fur4 |
Uracil permease |
PM |
| Gas1 |
Beta-1,3-glucanosyltransferase |
PM, ER, Golgi |
| Pma1 |
Plasma membrane H+-ATPase |
PM |
| Tat2 |
High-affinity tryptophan and tyrosine permease |
PM |
|
Phospholipid metabolism and regulators |
|
|
| Cho1 |
PS synthase |
Microsomes |
| Frq1 |
Pik1p regulator |
Golgi |
| Osh4/Kes1 |
Oxysterol-binding protein homolog; Sac1p regulator |
Golgi, exocytic vesicles, cytoplasm, endosomes |
| Mss4 |
PI-4P 5-kinase |
PM |
| Pct1 |
Cholinephosphate cytidylyltransferase |
Golgi |
| Pik1 |
PI 4-kinase |
ER, Golgi |
| Pis1 |
PI synthase |
Golgi |
| Sac1 |
PI 4-P phosphatase |
ER, Golgi |
| Sec14 |
PI/PC transfer protein |
Golgi |
| Sph5 |
PI transfer protein |
Cytoplasm, ER, PM |
| Spo14 |
Phospholipase D |
Endosomes |
| Stt4 |
PI 4-kinase |
PM |
|
Rab GTPases and regulators |
|
|
| Gdi1 |
Rab GDI (GTPase dissociation inhibitor) |
Cytoplasm |
| Mrs6 |
Rab GEP (GTPase escort protein) |
Cytoplasm |
| Sec2 |
Sec4p GEF (guanine nucleotide exchange factor) |
Golgi, exocytic vesicles, sites of polarized growth |
| Sec4 |
Rab-family GTPase |
Golgi, exocytic vesicles, sites of polarized growth |
| Yip1 |
Rab GTPase- and GDI-interacting protein |
ER, Golgi and COPII vesicle membranes |
| Ypt31, Ypt32 |
Rab-family GTPases |
Endosome, Golgi |
|
Ras GTPases and regulators |
|
|
| Age1, Age2 |
ARF GAPs |
Golgi |
| Arf1 |
Ras-family GTPase |
Golgi-associated vesicles |
| Gcs1 |
ARF GAP |
Golgi |
| Sec7 |
ARF GEF |
Cytoplasm, Golgi, Golgi-associated vesicles, |
|
Rho GTPases and regulators |
|
|
| Cdc24 |
Cdc42p GEF |
PM/sites of polarized growth, nucleus |
| Cdc42 |
Rho-family GTPase |
PM/sites of polarized growth |
| Gic2 |
Cdc42p effector |
PM |
| Rdi1 |
Rho GDI |
Cytoplasm, PM/sites of polarized growth |
| Rga1, Rga2 |
Cdc42p GAPs |
PM/Bud neck |
| Rho1 |
Rho-family GTPase |
Golgi, PM/sites of polarized growth |
| Rho3 |
Rho-family GTPase |
PM/sites of polarized growth |
| Rom2 |
Rho1p and Rho2p GEF |
PM/sites of polarized growth |
|
SNARE membrane fusion complex |
|
|
| Sec9 |
t-SNARE, SNAP-25 homolog |
PM |
| Snc1, Snc2 |
v-SNARE, synaptobrevin homolog |
Exocytic vesicles, Golgi |
| Sso1, Sso2 | t-SNARE, syntaxin homolog | PM |
Lipid-Dependent Vesicle Biogenesis at the Golgi Apparatus
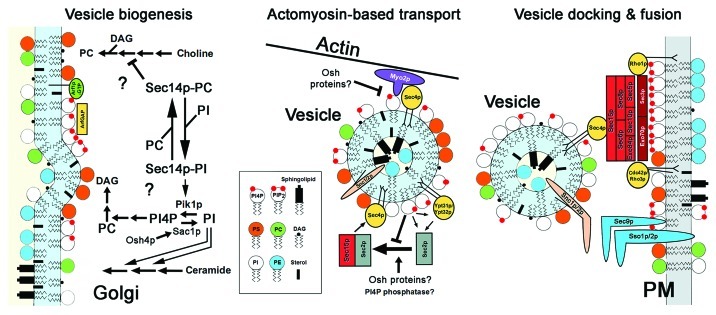
The ebb and flow of lipid precursors through competing biosynthetic pathways largely controls the generation of secretory vesicles emanating from the Golgi (Fig. 1). The structural changes in the Golgi bilayer that initiate membrane curvature for vesicle budding are dictated in part by the dynamic balance between diacylglycerol (DAG) production and DAG consumption in the production of phosphatidylcholine (PC). Inclusion of membrane proteins into nascent vesicles is partly dependent on the balance of phosphatidylinositol (PI) incorporation into complex sphingolipids vs. the generation of PI4P and other PIPs. The PI/PC transfer protein Sec14p plays a pivotal role acting as a traffic cop directing lipid flux through these metabolic pathways.2-6 At the Golgi, Sec14p integrates PI, PC, DAG and complex sphingolipid metabolism, and as a result Sec14p inactivation blocks exocytosis during post-Golgi vesicle biogenesis (Fig. 1).
Figure 1. Lipid-dependent events in yeast polarized exocytosis. During vesicle biogenesis (left) Sec14p-dependent regulation of lipid metabolism both stimulates DAG synthesis and inhibits DAG consumption as a precursor in PC production. As a precursor for the synthesis of PI-containing complex sphingolipids, PI is used in complex sphingolipid production at the expense of DAG production. Concentrated with sterols, de novo synthesized sphingolipids form membrane microdomains that recruit membrane proteins for exocytosis. In transit between the Golgi and PM (center), vesicles move along actin filaments propelled by a type V myosin (Myo2p). Myo2p interactions with vesicles is dependent in part on PI4P as is the reconfiguration of small GTPases (yellow) required for the assembly of vesicle-associated exocyst complex subunits (light red). At the interface between the PM and vesicle membrane (right), Rho GTPases and exocyst complex subunits associated with the PM (dark red) via PI(4,5)P2 assemble with the vesicle-bound exocyst complex subunits to facilitate vesicle docking at sites of polarized growth. Membrane fusion follows after v-SNAREs (tan) and t-SNAREs (blue) interactions.
Sec14p, which is essential for exocytosis and yeast growth, is dispensable if DAG is exogenously supplemented to cells.2,3 This result suggests that Sec14p increases levels of DAG, but this simple outcome belies the complexity of Sec14p-dependent regulation. Sec14p is proposed to have a dual function in DAG production depending on whether it is bound to PI or PC (Fig. 1). When bound to PC, Sec14p appears to inhibit choline-phosphate cytidyl transferase (Pct1p), the rate-limiting enzyme in PC biosynthetic pathway that consumes DAG as a precursor for PC synthesis.4 In this way, Sec14p might increase DAG, thought to be a “pro-secretory” lipid, while at the same time decreasing PC, an “anti-secretory” lipid.5,6 Due to its small head-group and long hydrophobic acyl chains, the cone-shape of DAG is predicted to affect the local curvature of the Golgi bilayer in proximity to DAG-enriched domains.7-9 The structural deformation of the membrane at these sites might promote vesicle membrane budding. In contrast, the cylindrical shape of PC is hypothesized to resist membrane deformation and inhibit vesicle formation. When bound to PI, Sec14p appears to stimulate the PI 4-kinase Pik1p to generate more PI4P for conversion into PI(4,5)P2. PI(4,5)P2 activates Spo14p, phospholipase D, which represents another mechanism for decreasing PC and its potentially anti-secretory effects.10 Spo14p hydrolyzes PC to form choline and phosphatidic acid (PA), which can be metabolized into DAG. Thus, it is possible that Sec14p-PC and Sec14p-PI both tilt the dynamic balance of lipids toward DAG at the expense of PC.11
An alternative mechanism by which DAG might affect vesicle formation is through the recruitment and activation of Gcs1p, an ARF-GAP (ADP-ribosylation factor GTPase-activating protein) implicated in vesicle scission from the Golgi. Gcs1p acts together with other ARF-GAPs, Age1p and Age2p, to regulate the small GTPase Arf1p,12,13 which in turn “primes” vesicle formation by recruiting cargo and other regulators of vesicle transport.14 DAG stimulates the GAP activity of Age1p and Gcs1p in vitro,13,15 and exocytosis defects in GCS1 and AGE2 mutant cells are rescued by the exogenous addition of DAG.16 Based on these findings, it is proposed that DAG-induced membrane curvature might recruit and activate ARF-GAP activity at the Golgi.16
Although it might appear that DAG is a key lipid regulator of Sec14p-dependent vesicle formation,2 other reports suggest that the essential requirement for SEC14 can be bypassed without increasing cellular DAG levels.17 In addition, PI4P levels are reduced by ~45% when sec14ts cells are cultured at elevated temperature.18 This result suggests that PI4P is an important lipid species for Sec14p-dependent vesicle biogenesis.17 Consistent with this suggestion, the deletion of SAC1, which encodes an ER/Golgi PI4P phosphatase, rescues the lethality of SEC14-inactivating mutations.2,19 In SEC14-defective cells, the elimination of SAC1 results in elevated PI4P levels but, unexpectedly, cellular DAG levels are unchanged suggesting rescue of SEC14 mutants is DAG-independent.17 (Although this finding does not preclude the possibility that localized increases in DAG levels within the Golgi membrane induce vesicle biogenesis). PI4P levels might also affect the ARF-GAPs Gcs1p and Age1p, which contain general lipid-binding domains that bind PIPs including PI4P;15,16 a homologous domain can also be found within Age2p. These results suggest that ARF-GAP activities might be affected by PIPs. In addition to PI4P, SEC14-defective cells accumulate ~3-fold more complex sphingolipid precursors, in which levels of very long chain ceramides are particularly elevated.20 Given the broad changes in lipid composition in cells lacking SEC14 function, it is probable the observed defects in vesicle formation is the collective effect of changes to the distribution and synthesis of several lipids.
In addition to regulated changes in lipid metabolism and membrane composition within specific membrane domains at the Golgi, vesicle formation also depends on the maintenance of transbilayer lipid asymmetry. Phosphatidylserine (PS) is synthesized within one leaflet of the ER bilayer but it equilibrates between leaflets.21 When PS arrives at the Golgi membrane, it is restricted to the cytoplasmic membrane leaflet by the P-type ATPase phospholipid flippase Drs2p and its Cdc50p chaperone. Drs2p thereby maintains Golgi bilayer asymmetry, which is functionally linked to Arf1p-dependent vesicle budding; mutations in either CDC50 or DRS2 are synthetically lethal with ARF1 mutations.22 Disruption of Drs2p or Cdc50p results in exocytosis and polarization defects and the accumulation of aberrant membrane structures.23,24 A possible mechanism for how PS asymmetry affects vesicle biogenesis involves the induction of localized membrane curvature, as predicted by the bilayer couple hypothesis.25 Membrane curvature might also promote ARF-GAP recruitment. Consistent with this model, the deletion of DRS2 is synthetically lethal with gcs1Δ,26 perhaps indicating a functional interaction required for post-Golgi vesicle formation. The involvement of a lipid flippase in the initial stages of exocytosis suggests that vesicle formation requires modulations in both cis and trans organization of the lipid bilayer.
Sterol/Sphingolipid-Dependent Cargo Sorting into Transport Vesicles
Apart from the structural changes in membrane organization that initiate vesicle budding, specific lipids are also sorted as cargo into nascent vesicles. Complex sphingolipids and ergosterol (the fungal equivalent of cholesterol) are enriched in secretory vesicles compared with the Golgi membrane from whence they came.27,28 Together, sphingolipids and sterol can be isolated as detergent resistant membranes (DRMs) corresponding to specific membrane microdomains that are sorting platforms for specific membrane proteins.29 The synthesis of the lipid components of these microdomains appears to be integrated with vesicle formation at the Golgi in order to sort and concentrate specific membrane proteins (Fig. 1).
In budding yeast, the biosynthesis of sphingolipids is simple as compared with metazoans and only three “complex” species of inositol phosphate-containing sphingolipids are made.30 After its synthesis in the ER, ceramide passes to the Golgi where mannose and inositol phosphates are sequentially added to produce all complex sphingolipids.30 PI serves as a precursor of inositol phosphate in the Golgi making the maintenance of PI pools extremely important for complex sphingolipid synthesis. In the Golgi, PI is generated by Pis1p (phosphatidyl inositol synthase 1), which couples a phosphatidyl moiety from CDP-DAG (CDP-diacylglycerol) to inositol,31 and by Sac1p-mediated dephosphorylation of PI4P.18 For the latter, inositol phosphate-containing sphingolipids are generated at the expense of PI4P used in DAG production (Fig. 1). Presumably the natural affinity between sterols and sphingolipids spontaneously leads to membrane microdomain formation. The coordinated regulation of PI and sphingolipid metabolism therefore appears to be important for integrating membrane sorting with vesicle formation.
The generation of sterol/sphingolipid microdomains in the Golgi membrane promotes the exocytosis of several well-defined PM transporters as well as glycosylphosphatidylinositol (GPI)-anchored proteins like Gas1p. Unlike other membrane proteins transported to the cell cortex, mutations that perturb ergosterol or sphingolipid metabolism disrupt the trafficking and distribution of the H+-ATPase Pma1p, the Tat2p tryptophan permease, the arginine permease Can1p, the Gap1p general amino acid permease and the uracil permease Fur4p.28,32-35 Gas1p and Pma1p are sorted into membrane microdomains in the ER before reaching the Golgi,36-38 but the other transporters are concentrated into DRMs within the Golgi membrane.33,36,39,40 The original site of sorting into DRMs roughly correlates with the lateral segregation of these proteins within different membrane domains once at the PM. In S. cerevisiae, the PM is compartmentalized into at least three different microdomains.41 Eisosomes or MCC (membrane compartment of Can1p) domains are stable and relatively immobile 300 nm-sized patches on the PM containing Can1p, Fur4p and Tat2p.34,41,42 MCP (membrane compartment occupied by Pma1p) domains contain Pma1p and represent PM regions containing readily diffusible proteins that are excluded from the MCC.41 The third membrane domain, MCT (membrane compartment containing TORC2), consists of punctuate patches containing the TORC2 complex that regulates the actin cytoskeleton and ceramide synthesis.43 Because the resident proteins are stably contained within these membrane domains, the lateral segregation of these proteins at the PM appears to be predetermined at the ER or the Golgi, depending on where the membrane domain originally formed.
PI4P Regulation of Exocytosis during Vesicle Transit between Membranes
Even before their release from the Golgi, nascent vesicles are attached to the type V myosin Myo2p, which is recruited via the Rab GTPases Ypt31p/32p and by PI4P (Fig. 1).44,45 As Myo2p-dependent transport proceeds and vesicles are moved along actin filaments to the bud, the small GTPase Sec4p displaces Ypt31p/32p.45,46 Although important for the proper regulation of actomyosin transport, this GTPase cascade is dispensable if the interaction between Myo2p and PI4P is augmented. By replacing the GTPase-binding C-terminal tail of Myo2p with an additional PI4P-binding PH domain, small GTPases are no longer required for Myo2p-dependent vesicle transport.46 Consistent with these results, increased PI4P levels can also negate the inhibitory effects of overexpressing the C-terminal tail of Myo2p. This tail region competes for Sec4p and Ypt31p/32p binding with the endogenous wild-type Myo2p, but increasing Pik1p production of PI4P restores Myo2p association with vesicles.46
In addition to the recruitment of Myo2p to vesicles, PI4P affects the protein interactions of Ypt31p/32p and Sec4p during the priming of the vesicle-associated subunits of the exocyst complex, which is required for subsequent vesicle docking with the PM.47 A critical event in this priming occurs when the Sec2p GEF (guanine-nucleotide exchange factor), originally recruited to vesicles and bound by Ypt31p/32p, switches its binding to the exocyst subunit Sec15p. With this exchange of binding partners, the Sec2p GEF is then free to activate Sec4p-GTP for the assembly of the other vesicle-associated exocyst complex subunits.47 Although it is not fully understood, a drop in vesicle PI4P levels triggers Ypt31p/32p release of Sec2p, and the subsequent Sec4p-dependent GTPase signaling cascade then ensues.47 Here again, the recruitment and assembly of regulatory protein complexes required for transport seems ultimately to be controlled by a lipid—PI4P.
Lipid Requirements for Exocytic Vesicle Docking and Fusion with the PM
At sites of polarized growth, vesicle docking and membrane fusion with the PM completes exocytosis and the relevant regulatory lipid involved is PI(4,5)P2. The polarized localization of the PM-associated exocyst complex subunits, Exo70p and Sec3p, is dependent on direct interactions with PI(4,5)P2. Sec3p and Exo70p polarization is also dependent on the Rho GTPase Cdc42p (Fig. 1).48-50 Overexpression of the PI4P 5-kinase Mss4p increases PI(4,5)P2 levels and partially rescues the temperature-sensitive growth defects of cdc42–6 cells at elevated temperatures, suggesting that PI(4,5)P2 and Cdc42p define two independent mechanisms for recruiting exocyst complex subunits to the PM.51 However, it is not known if increased PI(4,5)P2 levels can completely bypass the Cdc42p requirement for recruiting Sec3p and/or Exo70p to the PM. In addition, the Sec14p-related PI-transfer protein Sfh5p promotes Cdc42p activity at the PM by extracting PI from exocytic vesicles then presenting it to Stt4p (PI 4-kinase) and Mss4p for conversion into PI(4,5)P2 at the PM.51 The PI(4,5)P2 generated in this way is required for Cdc42p localization to sites of polarized exocytosis, though the exact mechanism is unclear.51 In addition, PI(4,5)P2 is required for localization of both Cdc24p, the PH domain-containing GEF that activates Cdc42p, and the Cdc42p effector Gic2p.52-54 PI(4,5)P2 is therefore the key lipid at the PM for assembling exocytosis regulatory complexes.
Another Rho GTPase, Rho1p, also binds and promotes Sec3p polarized localization, whereas both Rho3p and Cdc42p bind Exo70p to mediate its polarization.48,50,55 Rom2p, the GEF for Rho1p, also binds PI(4,5)P2 through a PH domain that is essential for Rom2p localization to sites of polarized growth and downstream activation of Rho1p.56,57 PI(4,5)P2 is therefore a requirement for multiple different regulators and effectors for vesicle docking at the PM.
Like bilayer asymmetries in the Golgi membrane, lipid asymmetry across the PM bilayer also impacts exocytosis. PS is localized to incipient bud sites where it promotes Cdc42p-dependent bud formation. Deletion of CHO1, encoding PS synthase, results in Cdc42p depolarized localization that is partially rescued by addition of lysoPS into the medium.58 PS, PI and PE (phosphatidylethanolamine) are also required to initiate changes in the sites of polarized exocytosis during the yeast cell cycle, which are needed to support growth of the daughter bud. PS, PI and PE stimulate Cdc42p-GTP turn-over by the GAPs (GTPase-activating proteins) Rga1p/2p, which cause Cdc42p-GDP reorganization at the bud cortex to alter the direction of polarized growth and exocytosis.59 The Lem3p-Dnf1p and Lem3p-Dnf2p flippase complexes facilitate Cdc42p reorganization by flipping PE into the cytoplasmic leaflet of the PM bilayer, where PE activates Cdc42p GAPs.59 The flipping of PE into the cytoplasmic leaflet is also proposed to disrupt electrostatic interactions between a cationic region near the C-terminal end of Cdc42p and the negatively charged PS in the PM, thus aiding both Cdc42p extraction and its recycling from polarized membrane sites.60 In contrast, PI(4,5)P2 in the cytoplasmic leaflet of the PM bilayer appears to inactivate the Rga1p/2p GAPs, though it is not understood how.59
The final event in polarized exocytosis involves SNARE-mediated membrane fusion between the exocytic vesicle and its target at the PM. Fusion requires interactions between Snc1p/2p on vesicles with Sso1p/2p and Sec9p on the PM (Fig. 1). Although in vitro assays show that the SNARE syntaxins, Sso1p/2p, bind PA and PI(4,5)P2 containing liposomes with high specificity, the importance of these lipid interactions during vegetative growth is unclear.61 Membrane trafficking at the PM was proposed to be restricted to, or influenced by, the eisosome/MCC domains within the PM.42 However, it was recently shown that sites of exocytosis or endocytosis are largely found outside the MCC.62 Based on this result, the distribution of SNARE proteins during exocytosis is predicted to be independent of the MCC lateral domains within the PM, though Snc1p appears to reside in DRMs when at the PM.63
Lipid Attachment to the Ends of Small GTPases: Tails that Wag the Dog
Lipid composition and distribution within membranes is clearly important, but lipids are also covalently attached to small GTPases and thereby directly affect their activities.64 Arf-, Rab- and Rho-family GTPases are modified by different lipids, which impart differences in GTPase localization and regulation. Most Rab GTPases are prenylated at their C-terminal ends with two geranylgeranyl groups by the cytoplasmic geranylgeranyl transferase (GGTase) II complex. Most Rho GTPases are prenylated on their C-terminal end with a single geranylgeranyl group by the cytoplasmic GGTase I complex. Arf GTPases are myristoylated near their N-terminus. These lipid modifications are of course required for GTPase membrane attachment, but the lipid attachments also confer targeting specificity sometimes through interactions with escort proteins.
Several proteins that recognize and bind to the di-geranylgeranyl modification determine the targeting of Sec4p and Ypt31p/32p to membranes. Mrs6p is a GGTase II complex chaperone that recognizes newly synthesized Rab GTPases for prenylation and then delivers them to membranes. As a Rab escort protein (REP), Mrs6p cannot retrieve Sec4p or other Rab GTPases after membrane delivery.65 In contrast, Gdi1p, the yeast Rab guanine-nucleotide dissociation inhibitor (GDI), removes Rab GTPases from membranes after they complete GTP hydrolysis. For example, Gdi1p extracts prenylated GDP-bound Sec4p from membranes for recycling back to re-initialize post-Golgi vesicle transport.66 Gdi1p-Sec4p and Gdi1p-Ypt31p/32p complexes are found in inactive cytoplasmic pools and to be activated they require membrane recruitment. Yip1p is an integral membrane protein that appears to recruit Gdi1p-GDP-Rab GTPase complexes to the Golgi. YIP1 genetically interacts with both GDI1 and with genes that encode Golgi-specific Rab GTPases,67 though in vitro Yip1p has a broad affinity for other Rab GTPases with di-geranylgeranyl modification.68 Sec4p is functional if its prenylation motif is replaced with a transmembrane (TM)-spanning domain, but only if the TM domain targets Sec4p to the correct location.69 Based on these findings, the di-geranylgeranyl modification of Sec4p not only imparts general membrane association but also appears to be recognized by receptor complexes that confer compartment specificity. Because correct membrane targeting is necessary for subsequent interactions with effectors, di-geranylgeranyl modification has an important but indirect effect on Rab GTPase activities.
Like the Rab GTPases, prenylated Rho GTPases are also regulated by a GDI that binds and extracts them from membranes.70 Rdi1p, the sole Rho GDI in yeast, in part binds the single geranylgeranyl tail attached to Cdc42p and Rho1p to remove them from the PM.71,72 Oddly enough, the elimination of Rdi1p neither affects cell growth nor the gross cytoplasmic/PM distribution of Cdc42p or Rho1p.73 The apparent solution to this puzzle is that Cdc42p recycling involves parallel mechanisms requiring both Rdi1p and endocytosis, and the elimination of both pathways leads to a rapid loss in Cdc42p polarization.74 It should be noted that C-terminal prenylation of Cdc42p and Rho3p is important for protein-protein interactions with the exocyst complex subunit Exo70p. In vitro purified constitutively activated Cdc42p and Rho3p bind Exo70p only if an intact C-terminal prenylation motif is present.55 The geranylgeranyl modification is therefore important for Rho GTPase signaling despite some functional redundancy with other mechanisms.
Rho3p lipid modification differs from other Rho GTPases in that it is prenylated at its C-terminus with farnesyl, and the fatty acid palmitate is attached in a region near its N-terminus.50 Cdc42p is particularly important during early events in bud formation whereas Rho3p plays a greater role later when the bud is larger. A fusion protein where the palmitoylated N-terminal region of Rho3p is added onto Cdc42p was sufficient to rescue growth defects seen in rho3Δ cells.50 These results suggest that Rho3p and Cdc42p perform similar functions but palmitoylation directs that activity to slightly different locations on the PM, which is particularly important in large-budded cells. Palmitate is also covalently attached to the vesicle SNAREs Snc1p/2p, but the lipid addition does not affect their association with vesicles and a mutation that renders Snc1p incapable of palmitoylation does not affect SNARE function.75 In short, the regulatory role of palmitoylation in exocytosis is clearly important but still not fully understood.
Unlike Rho or Rab GTPases, Arf1p is N-myristoylated and nucleotide exchange is directly coupled with its membrane association.76 In the Arf1p GDP/GTP cycle, Arf1p-GDP is mainly cytoplasmic but its myristoylated N-terminal amphipathic helix causes Arf1-GDP translocation into membranes wherein GDP exchange for GTP occurs.77 Apart from promoting protein complex assembly, the membrane-associated myristoylated Arf1p-GTP induces positive membrane curvature, which is proposed to be critical for the formation of nascent transport vesicles.78 Together these findings suggest that myristoylation couples Arf1p activation and signaling to structural changes in the Golgi membrane for vesicle biogenesis.
PI4P: The Master Regulatory Lipid for Polarized Exocytosis in Yeast
PI4P is perhaps the most important lipid in exocytosis, not only as a precursor for other PIPs but also for membrane targeting and activation of regulatory proteins. PI4P phosphorylation to PI(4,5)P2 is the regulatory modification most pertinent to the final events of exocytosis at the PM, whereas in the Golgi PI4P turn-over appears to be important for vesicle biogenesis. Even during vesicle transit, the reconfiguration of Rab GTPase complexes needed for subsequent events in exocytosis is dependent on vesicle PI4P levels. The regulation of yeast exocytosis is ultimately tied to the fate of PI4P as dictated by the opposing activities of the PI4P phosphatases and the PI 4-kinases, Pik1p and Stt4p.
In the Golgi, the fate of PI4P is mainly governed by the PI4P phosphatase Sac1p and the PI 4-P kinase Pik1p (Fig. 1). Although Sac1p is an ER protein, it shuttles in-and-out of the Golgi and in nutrient-deprived cells, Sac1p remains in the Golgi.79 Sac1p and Pik1p (in a complex with its non-catalytic subunit Frq1p) have a reciprocal relationship: Pik1p/Frq1p is released into the cytoplasm when Sac1p is present and, when Sac1p is retained in the ER, Pik1p/Frq1p associates with the Golgi to stimulate vesicle formation by generating PI4P.79 Sac1p itself has seemingly opposing roles in vesicle biogenesis. Although Sac1p inhibits PI4P-dependent activation of vesicle biogenesis by Pik1p and Sec14p, Sac1p-dependent production of PI promotes complex sphingolipid biosynthesis and membrane sorting into nascent vesicles.27,80 These sphingolipids together with associated sterols are generically enriched in all exocytic vesicles, whether targeted to sites of polarized growth or to the PM in general.28
To reconcile how Sac1p might repress vesicle formation while also facilitating lipid and protein sorting into vesicles, it is proposed that the OSBP homolog Osh4p integrates these opposing Sac1p activities as part of a negative feedback loop.81 OSH4 (also known as KES1) is one of seven yeast OSH genes (OSH1–OSH7) that share overlapping essential functions, including a specific role in polarized exocytosis.82-84 Osh4p is a lipid-binding protein that contains mutually exclusive binding sites for a sterol and PI4P.85,86 A mutation that specifically inhibits sterol binding by Osh4p results in its activation, causing growth defects that can be suppressed by deleting SAC1.84 In fact, Osh4p induces Sac1p phosphatase activity in vitro.87 These findings suggest that PI4P-bound Osh4p is an upstream activator of Sac1p, but Osh4p is inhibited when in the sterol-bound form. In the negative feedback model, Osh4p promotes Sac1p dephosphorylation of PI4P to produce PI pools for complex sphingolipid synthesis. Within the Golgi membrane, newly synthesized sphingolipids concentrate with sterols, which might trigger the inhibitory exchange wherein a sterol replaces PI4P for Osh4p binding. As a result, Sac1p activity is reduced and PI4P levels in the Golgi increase, which is an initiating event for vesicle formation. Vesicle biogenesis is thereby delayed until sphingolipid/sterol microdomains can be generated for cargo sorting. This model is supported by multiple lines of evidence: (1) complex sphingolipid levels are reduced in osh4Δ cells (LeBlanc et al., submitted);88 (2) similar to that observed in sac1Δ cells, the deletion of OSH4 also causes missorting of proteins associated with sterol/sphingolipid membrane domains;88,89 (3) like sac1Δ, the deletion of OSH4 restores PI4P levels in cells with conditional PIK1 or SEC14 mutations;90,91 (4) the deletion of either OSH4 or SAC1 bypasses the essential requirement for SEC14, because increases in Golgi PI4P levels might induce vesicle biogenesis with associated decreases in PC or increased levels of DAG and/or sphingolipids.92 Thus, PI4P turn-over during vesicle biogenesis is in part controlled by Osh4p and its downstream effector Sac1p.
It has been proposed that Osh4p transfers sterols and PI4P between membranes in opposite directions, and PI4P hydrolysis is suggested to drive this vectorial exchange of lipids to generate a sterol gradient between membranes.86 However, sterol transport in both yeast and mammalian cells is not a directed mechanism but rather a process of equilibration, resulting in the steady-state concentration of sterols within cellular membranes enriched in sphingolipid membrane domains (rafts).93,94 Based in part on these findings, and in vivo sterol transport assays,95 it is argued that Osh4p is not a sterol transfer protein.81 Whether or not Osh4p sequesters or transfers PI4P in vivo, is not yet clear but it is another interesting possibility.
Osh proteins and Sac1p are also implicated in PI4P turn-over later in vesicle transport well after post-Golgi vesicle formation. Despite its importance in regulating vesicle budding from the Golgi, Osh4p is not required for vesicle biogenesis. Rather, all proteins of the Osh family must be inactivated to inhibit polarized transport, and the block is manifested only when vesicles dock with the PM but not earlier in exocytosis.83,84 Because PI4P turn-over in vesicles regulates Rab GTPase interactions with both Myo2p and exocyst complex subunits, and Osh4p travels along with exocytic vesicles to the cell cortex, it is proposed that Osh proteins might regulate PI4P sequestration and/or dephosphorylation events even after vesicle release from the Golgi. Multiple findings support this proposed role for Osh proteins during vesicle docking: (1) Osh proteins associate in vivo with exocyst complex subunits and their GTPase regulators (but not Myo2p); (2) genetic interactions reveal functional interactions between Osh proteins and the exocyst complex; (3) Osh proteins are required for Cdc42p and Rho1p polarized localization at the PM; (4) Sec4p association with exocytic vesicles is not Osh-dependent, but the docking of Sec4p-containing vesicles with the PM is Osh-dependent; (5) both OSH4 and SAC1 overexpression cause severe growth defects in conditional MYO2 mutants, potentially due to reductions in PI4P levels.46,83,84
With the complexity of lipid interactions required at every event during polarized exocytosis, it seems impossible for just one lipid to be the key regulator. However, the status of PI4P is particularly important not only for vesicle formation at the Golgi, but during each subsequent step in transport to the PM. Clearly PI4P, along with its protein regulators, is now the focal point for understanding how these different steps during exocytosis are so seamlessly integrated.
Acknowledgments
The work in our laboratory is supported by grants from the Natural Sciences and Engineering Research Council (NSERC) and the Canadian Cancer Society. Many thanks to Nancy Hawkins, Anant Menon and Christopher McMaster for their useful comments on the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/cellularlogistics/article/20490
References
- 1.Harsay E, Bretscher A. Parallel secretory pathways to the cell surface in yeast. J Cell Biol. 1995;131:297–310. doi: 10.1083/jcb.131.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kearns BG, McGee TP, Mayinger P, Gedvilaite A, Phillips SE, Kagiwada S, et al. Essential role for diacylglycerol in protein transport from the yeast Golgi complex. Nature. 1997;387:101–5. doi: 10.1038/387101a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henneberry AL, Lagace TA, Ridgway ND, McMaster CR. Phosphatidylcholine synthesis influences the diacylglycerol homeostasis required for SEC14p-dependent Golgi function and cell growth. Mol Biol Cell. 2001;12:511–20. doi: 10.1091/mbc.12.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skinner HB, McGee TP, McMaster CR, Fry MR, Bell RM, Bankaitis VA. The Saccharomyces cerevisiae phosphatidylinositol-transfer protein effects a ligand-dependent inhibition of choline-phosphate cytidylyltransferase activity. Proc Natl Acad Sci U S A. 1995;92:112–6. doi: 10.1073/pnas.92.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Routt SM, Bankaitis VA. Biological functions of phosphatidylinositol transfer proteins. Biochem Cell Biol. 2004;82:254–62. doi: 10.1139/o03-089. [DOI] [PubMed] [Google Scholar]
- 6.Mousley CJ, Tyeryar KR, Vincent-Pope P, Bankaitis VA. The Sec14-superfamily and the regulatory interface between phospholipid metabolism and membrane trafficking. Biochim Biophys Acta. 2007;1771:727–36. doi: 10.1016/j.bbalip.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Z Naturforsch C. 1973;28:693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- 8.Allan D, Thomas P, Michell RH. Rapid transbilayer diffusion of 1,2-diacylglycerol and its relevance to control of membrane curvature. Nature. 1978;276:289–90. doi: 10.1038/276289a0. [DOI] [PubMed] [Google Scholar]
- 9.Kearns BG, Alb JG, Jr., Bankaitis VA. Phosphatidylinositol transfer proteins: the long and winding road to physiological function. Trends Cell Biol. 1998;8:276–82. doi: 10.1016/S0962-8924(98)01281-1. [DOI] [PubMed] [Google Scholar]
- 10.Xie Z, Fang M, Rivas MP, Faulkner AJ, Sternweis PC, Engebrecht JA, et al. Phospholipase D activity is required for suppression of yeast phosphatidylinositol transfer protein defects. Proc Natl Acad Sci U S A. 1998;95:12346–51. doi: 10.1073/pnas.95.21.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sreenivas A, Patton-Vogt JL, Bruno V, Griac P, Henry SA. A role for phospholipase D (Pld1p) in growth, secretion, and regulation of membrane lipid synthesis in yeast. J Biol Chem. 1998;273:16635–8. doi: 10.1074/jbc.273.27.16635. [DOI] [PubMed] [Google Scholar]
- 12.Poon PP, Nothwehr SF, Singer RA, Johnston GC. The Gcs1 and Age2 ArfGAP proteins provide overlapping essential function for transport from the yeast trans-Golgi network. J Cell Biol. 2001;155:1239–50. doi: 10.1083/jcb.200108075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanagisawa LL, Marchena J, Xie Z, Li X, Poon PP, Singer RA, et al. Activity of specific lipid-regulated ADP ribosylation factor-GTPase-activating proteins is required for Sec14p-dependent Golgi secretory function in yeast. Mol Biol Cell. 2002;13:2193–206. doi: 10.1091/mbc.01-11-0563.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Springer S, Spang A, Schekman R. A primer on vesicle budding. Cell. 1999;97:145–8. doi: 10.1016/S0092-8674(00)80722-9. [DOI] [PubMed] [Google Scholar]
- 15.Benjamin JJ, Poon PP, Lewis SM, Auger A, Wong TA, Singer RA, et al. The yeast Arf GTPase-activating protein Age1 is regulated by phospholipase D for post-Golgi vesicular transport. J Biol Chem. 2011;286:5187–96. doi: 10.1074/jbc.M110.185108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong TA, Fairn GD, Poon PP, Shmulevitz M, McMaster CR, Singer RA, et al. Membrane metabolism mediated by Sec14 family members influences Arf GTPase activating protein activity for transport from the trans-Golgi. Proc Natl Acad Sci U S A. 2005;102:12777–82. doi: 10.1073/pnas.0506156102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stock SD, Hama H, DeWald DB, Takemoto JY. SEC14-dependent secretion in Saccharomyces cerevisiae. Nondependence on sphingolipid synthesis-coupled diacylglycerol production. J Biol Chem. 1999;274:12979–83. doi: 10.1074/jbc.274.19.12979. [DOI] [PubMed] [Google Scholar]
- 18.Hama H, Schnieders EA, Thorner J, Takemoto JY, DeWald DB. Direct involvement of phosphatidylinositol 4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J Biol Chem. 1999;274:34294–300. doi: 10.1074/jbc.274.48.34294. [DOI] [PubMed] [Google Scholar]
- 19.Cleves AE, Novick PJ, Bankaitis VA. Mutations in the SAC1 gene suppress defects in yeast Golgi and yeast actin function. J Cell Biol. 1989;109:2939–50. doi: 10.1083/jcb.109.6.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mousley CJ, Tyeryar KR, Ile KE, Schaaf G, Brost RL, Boone C, et al. Trans-Golgi network and endosome dynamics connect ceramide homeostasis with regulation of the unfolded protein response and TOR signaling in yeast. Mol Biol Cell. 2008;19:4785–803. doi: 10.1091/mbc.E08-04-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–24. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen CY, Graham TR. An arf1Delta synthetic lethal screen identifies a new clathrin heavy chain conditional allele that perturbs vacuolar protein transport in Saccharomyces cerevisiae. Genetics. 1998;150:577–89. doi: 10.1093/genetics/150.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gall WE, Geething NC, Hua Z, Ingram MF, Liu K, Chen SI, et al. Drs2p-dependent formation of exocytic clathrin-coated vesicles in vivo. Curr Biol. 2002;12:1623–7. doi: 10.1016/S0960-9822(02)01148-X. [DOI] [PubMed] [Google Scholar]
- 24.Chen S, Wang J, Muthusamy BP, Liu K, Zare S, Andersen RJ, et al. Roles for the Drs2p-Cdc50p complex in protein transport and phosphatidylserine asymmetry of the yeast plasma membrane. Traffic. 2006;7:1503–17. doi: 10.1111/j.1600-0854.2006.00485.x. [DOI] [PubMed] [Google Scholar]
- 25.Sheetz MP, Singer SJ. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci U S A. 1974;71:4457–61. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson M, Poon PP, Schindler C, Murray LE, Kama R, Gabriely G, et al. The Gcs1 Arf-GAP mediates Snc1,2 v-SNARE retrieval to the Golgi in yeast. Mol Biol Cell. 2006;17:1845–58. doi: 10.1091/mbc.E05-09-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klemm RW, Ejsing CS, Surma MA, Kaiser HJ, Gerl MJ, Sampaio JL, et al. Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network. J Cell Biol. 2009;185:601–12. doi: 10.1083/jcb.200901145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Surma MA, Klose C, Klemm RW, Ejsing CS, Simons K. Generic sorting of raft lipids into secretory vesicles in yeast. Traffic. 2011;12:1139–47. doi: 10.1111/j.1600-0854.2011.01221.x. [DOI] [PubMed] [Google Scholar]
- 29.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 30.Dickson RC, Sumanasekera C, Lester RL. Functions and metabolism of sphingolipids in Saccharomyces cerevisiae. Prog Lipid Res. 2006;45:447–65. doi: 10.1016/j.plipres.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Leber A, Hrastnik C, Daum G. Phospholipid-synthesizing enzymes in Golgi membranes of the yeast, Saccharomyces cerevisiae. FEBS Lett. 1995;377:271–4. doi: 10.1016/0014-5793(95)01361-X. [DOI] [PubMed] [Google Scholar]
- 32.Bagnat M, Chang A, Simons K. Plasma membrane proton ATPase Pma1p requires raft association for surface delivery in yeast. Mol Biol Cell. 2001;12:4129–38. doi: 10.1091/mbc.12.12.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umebayashi K, Nakano A. Ergosterol is required for targeting of tryptophan permease to the yeast plasma membrane. J Cell Biol. 2003;161:1117–31. doi: 10.1083/jcb.200303088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fröhlich F, Moreira K, Aguilar PS, Hubner NC, Mann M, Walter P, et al. A genome-wide screen for genes affecting eisosomes reveals Nce102 function in sphingolipid signaling. J Cell Biol. 2009;185:1227–42. doi: 10.1083/jcb.200811081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lauwers E, André B. Association of yeast transporters with detergent-resistant membranes correlates with their cell-surface location. Traffic. 2006;7:1045–59. doi: 10.1111/j.1600-0854.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 36.Bagnat M, Keränen S, Shevchenko A, Shevchenko A, Simons K. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc Natl Acad Sci U S A. 2000;97:3254–9. doi: 10.1073/pnas.060034697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee MC, Hamamoto S, Schekman R. Ceramide biosynthesis is required for the formation of the oligomeric H+-ATPase Pma1p in the yeast endoplasmic reticulum. J Biol Chem. 2002;277:22395–401. doi: 10.1074/jbc.M200450200. [DOI] [PubMed] [Google Scholar]
- 38.Gaigg B, Timischl B, Corbino L, Schneiter R. Synthesis of sphingolipids with very long chain fatty acids but not ergosterol is required for routing of newly synthesized plasma membrane ATPase to the cell surface of yeast. J Biol Chem. 2005;280:22515–22. doi: 10.1074/jbc.M413472200. [DOI] [PubMed] [Google Scholar]
- 39.Hearn JD, Lester RL, Dickson RC. The uracil transporter Fur4p associates with lipid rafts. J Biol Chem. 2003;278:3679–86. doi: 10.1074/jbc.M209170200. [DOI] [PubMed] [Google Scholar]
- 40.Malínská K, Malínský J, Opekarová M, Tanner W. Visualization of protein compartmentation within the plasma membrane of living yeast cells. Mol Biol Cell. 2003;14:4427–36. doi: 10.1091/mbc.E03-04-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malinsky J, Opekarová M, Tanner W. The lateral compartmentation of the yeast plasma membrane. Yeast. 2010;27:473–8. doi: 10.1002/yea.1772. [DOI] [PubMed] [Google Scholar]
- 42.Walther TC, Brickner JH, Aguilar PS, Bernales S, Pantoja C, Walter P. Eisosomes mark static sites of endocytosis. Nature. 2006;439:998–1003. doi: 10.1038/nature04472. [DOI] [PubMed] [Google Scholar]
- 43.Berchtold D, Walther TC. TORC2 plasma membrane localization is essential for cell viability and restricted to a distinct domain. Mol Biol Cell. 2009;20:1565–75. doi: 10.1091/mbc.E08-10-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lipatova Z, Tokarev AA, Jin Y, Mulholland J, Weisman LS, Segev N. Direct interaction between a myosin V motor and the Rab GTPases Ypt31/32 is required for polarized secretion. Mol Biol Cell. 2008;19:4177–87. doi: 10.1091/mbc.E08-02-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin Y, Sultana A, Gandhi P, Franklin E, Hamamoto S, Khan AR, et al. Myosin V transports secretory vesicles via a Rab GTPase cascade and interaction with the exocyst complex. Dev Cell. 2011;21:1156–70. doi: 10.1016/j.devcel.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santiago-Tirado FH, Legesse-Miller A, Schott D, Bretscher A. PI4P and Rab inputs collaborate in myosin-V-dependent transport of secretory compartments in yeast. Dev Cell. 2011;20:47–59. doi: 10.1016/j.devcel.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mizuno-Yamasaki E, Medkova M, Coleman J, Novick P. Phosphatidylinositol 4-phosphate controls both membrane recruitment and a regulatory switch of the Rab GEF Sec2p. Dev Cell. 2010;18:828–40. doi: 10.1016/j.devcel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He B, Xi F, Zhang X, Zhang J, Guo W. Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. EMBO J. 2007;26:4053–65. doi: 10.1038/sj.emboj.7601834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X, Orlando K, He B, Xi F, Zhang J, Zajac A, et al. Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J Cell Biol. 2008;180:145–58. doi: 10.1083/jcb.200704128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu H, Brennwald P. The function of two Rho family GTPases is determined by distinct patterns of cell surface localization. Mol Cell Biol. 2010;30:5207–17. doi: 10.1128/MCB.00366-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yakir-Tamang L, Gerst JE. A phosphatidylinositol-transfer protein and phosphatidylinositol-4-phosphate 5-kinase control Cdc42 to regulate the actin cytoskeleton and secretory pathway in yeast. Mol Biol Cell. 2009;20:3583–97. doi: 10.1091/mbc.E08-10-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen GC, Kim YJ, Chan CS. The Cdc42 GTPase-associated proteins Gic1 and Gic2 are required for polarized cell growth in Saccharomyces cerevisiae. Genes Dev. 1997;11:2958–71. doi: 10.1101/gad.11.22.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orlando K, Zhang J, Zhang X, Yue P, Chiang T, Bi E, et al. Regulation of Gic2 localization and function by phosphatidylinositol 4,5-bisphosphate during the establishment of cell polarity in budding yeast. J Biol Chem. 2008;283:14205–12. doi: 10.1074/jbc.M708178200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu JW, Mendrola JM, Audhya A, Singh S, Keleti D, DeWald DB, et al. Genome-wide analysis of membrane targeting by S. cerevisiae pleckstrin homology domains. Mol Cell. 2004;13:677–88. doi: 10.1016/S1097-2765(04)00083-8. [DOI] [PubMed] [Google Scholar]
- 55.Wu H, Turner C, Gardner J, Temple B, Brennwald P. The Exo70 subunit of the exocyst is an effector for both Cdc42 and Rho3 function in polarized exocytosis. Mol Biol Cell. 2010;21:430–42. doi: 10.1091/mbc.E09-06-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Audhya A, Emr SD. Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev Cell. 2002;2:593–605. doi: 10.1016/S1534-5807(02)00168-5. [DOI] [PubMed] [Google Scholar]
- 57.Manning BD, Padmanabha R, Snyder M. The Rho-GEF Rom2p localizes to sites of polarized cell growth and participates in cytoskeletal functions in Saccharomyces cerevisiae. Mol Biol Cell. 1997;8:1829–44. doi: 10.1091/mbc.8.10.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fairn GD, Hermansson M, Somerharju P, Grinstein S. Phosphatidylserine is polarized and required for proper Cdc42 localization and for development of cell polarity. Nat Cell Biol. 2011;13:1424–30. doi: 10.1038/ncb2351. [DOI] [PubMed] [Google Scholar]
- 59.Saito K, Fujimura-Kamada K, Hanamatsu H, Kato U, Umeda M, Kozminski KG, et al. Transbilayer phospholipid flipping regulates Cdc42p signaling during polarized cell growth via Rga GTPase-activating proteins. Dev Cell. 2007;13:743–51. doi: 10.1016/j.devcel.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 60.Das A, Slaughter BD, Unruh JR, Bradford WD, Alexander R, Rubinstein B, et al. Flippase-mediated phospholipid asymmetry promotes fast Cdc42 recycling in dynamic maintenance of cell polarity. Nat Cell Biol. 2012;14:304–10. doi: 10.1038/ncb2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mendonsa R, Engebrecht J. Phosphatidylinositol-4,5-bisphosphate and phospholipase D-generated phosphatidic acid specify SNARE-mediated vesicle fusion for prospore membrane formation. Eukaryot Cell. 2009;8:1094–105. doi: 10.1128/EC.00076-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brach T, Specht T, Kaksonen M. Reassessment of the role of plasma membrane domains in the regulation of vesicular traffic in yeast. J Cell Sci. 2011;124:328–37. doi: 10.1242/jcs.078519. [DOI] [PubMed] [Google Scholar]
- 63.Dupré S, Haguenauer-Tsapis R. Raft partitioning of the yeast uracil permease during trafficking along the endocytic pathway. Traffic. 2003;4:83–96. doi: 10.1034/j.1600-0854.2003.40204.x. [DOI] [PubMed] [Google Scholar]
- 64.Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–6. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- 65.Alory C, Balch WE. Molecular evolution of the Rab-escort-protein/guanine-nucleotide-dissociation-inhibitor superfamily. Mol Biol Cell. 2003;14:3857–67. doi: 10.1091/E03-04-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garrett MD, Zahner JE, Cheney CM, Novick PJ. GDI1 encodes a GDP dissociation inhibitor that plays an essential role in the yeast secretory pathway. EMBO J. 1994;13:1718–28. doi: 10.1002/j.1460-2075.1994.tb06436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen CZ, Calero M, DeRegis CJ, Heidtman M, Barlowe C, Collins RN. Genetic analysis of yeast Yip1p function reveals a requirement for Golgi-localized rab proteins and rab-Guanine nucleotide dissociation inhibitor. Genetics. 2004;168:1827–41. doi: 10.1534/genetics.104.032888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Calero M, Chen CZ, Zhu W, Winand N, Havas KA, Gilbert PM, et al. Dual prenylation is required for Rab protein localization and function. Mol Biol Cell. 2003;14:1852–67. doi: 10.1091/mbc.E02-11-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ossig R, Laufer W, Schmitt HD, Gallwitz D. Functionality and specific membrane localization of transport GTPases carrying C-terminal membrane anchors of synaptobrevin-like proteins. EMBO J. 1995;14:3645–53. doi: 10.1002/j.1460-2075.1995.tb00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15:356–63. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 71.Cole KC, McLaughlin HW, Johnson DI. Use of bimolecular fluorescence complementation to study in vivo interactions between Cdc42p and Rdi1p of Saccharomyces cerevisiae. Eukaryot Cell. 2007;6:378–87. doi: 10.1128/EC.00368-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tiedje C, Sakwa I, Just U, Höfken T. The Rho GDI Rdi1 regulates Rho GTPases by distinct mechanisms. Mol Biol Cell. 2008;19:2885–96. doi: 10.1091/mbc.E07-11-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koch G, Tanaka K, Masuda T, Yamochi W, Nonaka H, Takai Y. Association of the Rho family small GTP-binding proteins with Rho GDP dissociation inhibitor (Rho GDI) in Saccharomyces cerevisiae. Oncogene. 1997;15:417–22. doi: 10.1038/sj.onc.1201194. [DOI] [PubMed] [Google Scholar]
- 74.Slaughter BD, Das A, Schwartz JW, Rubinstein B, Li R. Dual modes of cdc42 recycling fine-tune polarized morphogenesis. Dev Cell. 2009;17:823–35. doi: 10.1016/j.devcel.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Couve A, Protopopov V, Gerst JE. Yeast synaptobrevin homologs are modified posttranslationally by the addition of palmitate. Proc Natl Acad Sci U S A. 1995;92:5987–91. doi: 10.1073/pnas.92.13.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Y, Kahn RA, Prestegard JH. Dynamic structure of membrane-anchored Arf*GTP. Nat Struct Mol Biol. 2010;17:876–81. doi: 10.1038/nsmb.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Buosi V, Placial JP, Leroy JL, Cherfils J, Guittet É, van Heijenoort C. Insight into the role of dynamics in the conformational switch of the small GTP-binding protein Arf1. J Biol Chem. 2010;285:37987–94. doi: 10.1074/jbc.M110.134445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beck R, Sun Z, Adolf F, Rutz C, Bassler J, Wild K, et al. Membrane curvature induced by Arf1-GTP is essential for vesicle formation. Proc Natl Acad Sci U S A. 2008;105:11731–6. doi: 10.1073/pnas.0805182105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Faulhammer F, Kanjilal-Kolar S, Knödler A, Lo J, Lee Y, Konrad G, et al. Growth control of Golgi phosphoinositides by reciprocal localization of sac1 lipid phosphatase and pik1 4-kinase. Traffic. 2007;8:1554–67. doi: 10.1111/j.1600-0854.2007.00632.x. [DOI] [PubMed] [Google Scholar]
- 80.Brice SE, Alford CW, Cowart LA. Modulation of sphingolipid metabolism by the phosphatidylinositol-4-phosphate phosphatase Sac1p through regulation of phosphatidylinositol in Saccharomyces cerevisiae. J Biol Chem. 2009;284:7588–96. doi: 10.1074/jbc.M808325200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beh CT, McMaster CR, Kozminski KG, Menon AK. A detour for yeast Oxysterol-binding protein homologues and nonvesicular trafficking. J Biol Chem. 2012;287:11481–8. doi: 10.1074/jbc.R111.338400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beh CT, Cool L, Phillips J, Rine J. Overlapping functions of the yeast oxysterol-binding protein homologues. Genetics. 2001;157:1117–40. doi: 10.1093/genetics/157.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kozminski KG, Alfaro G, Dighe S, Beh CT. Homologues of oxysterol-binding proteins affect Cdc42p- and Rho1p-mediated cell polarization in Saccharomyces cerevisiae. Traffic. 2006;7:1224–42. doi: 10.1111/j.1600-0854.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- 84.Alfaro G, Johansen J, Dighe SA, Duamel G, Kozminski KG, Beh CT. The sterol-binding protein Kes1/Osh4p is a regulator of polarized exocytosis. Traffic. 2011;12:1521–36. doi: 10.1111/j.1600-0854.2011.01265.x. [DOI] [PubMed] [Google Scholar]
- 85.Im YJ, Raychaudhuri S, Prinz WA, Hurley JH. Structural mechanism for sterol sensing and transport by OSBP-related proteins. Nature. 2005;437:154–8. doi: 10.1038/nature03923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de Saint-Jean M, Delfosse V, Douguet D, Chicanne G, Payrastre B, Bourguet W, et al. Osh4p exchanges sterols for phosphatidylinositol 4-phosphate between lipid bilayers. J Cell Biol. 2011;195:965–78. doi: 10.1083/jcb.201104062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stefan CJ, Manford AG, Baird D, Yamada-Hanff J, Mao Y, Emr SD. Osh proteins regulate phosphoinositide metabolism at ER-plasma membrane contact sites. Cell. 2011;144:389–401. doi: 10.1016/j.cell.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 88.LeBlanc MA, Fairn GD, Brice SE, Cowart LA, McMaster CR. Oxysterol binding proteins act as sterol sensors to regulate lipid raft trafficking. 2012; submitted.
- 89.Proszynski TJ, Klemm RW, Gravert M, Hsu PP, Gloor Y, Wagner J, et al. A genome-wide visual screen reveals a role for sphingolipids and ergosterol in cell surface delivery in yeast. Proc Natl Acad Sci U S A. 2005;102:17981–6. doi: 10.1073/pnas.0509107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li X, Rivas MP, Fang M, Marchena J, Mehrotra B, Chaudhary A, et al. Analysis of oxysterol binding protein homologue Kes1p function in regulation of Sec14p-dependent protein transport from the yeast Golgi complex. J Cell Biol. 2002;157:63–77. doi: 10.1083/jcb.200201037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fairn GD, Curwin AJ, Stefan CJ, McMaster CR. The oxysterol binding protein Kes1p regulates Golgi apparatus phosphatidylinositol-4-phosphate function. Proc Natl Acad Sci U S A. 2007;104:15352–7. doi: 10.1073/pnas.0705571104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fang M, Kearns BG, Gedvilaite A, Kagiwada S, Kearns M, Fung MK, et al. Kes1p shares homology with human oxysterol binding protein and participates in a novel regulatory pathway for yeast Golgi-derived transport vesicle biogenesis. EMBO J. 1996;15:6447–59. [PMC free article] [PubMed] [Google Scholar]
- 93.Baumann NA, Sullivan DP, Ohvo-Rekilä H, Simonot C, Pottekat A, Klaassen Z, et al. Transport of newly synthesized sterol to the sterol-enriched plasma membrane occurs via nonveicular equilibration. Biochem. 2005;44:5816–26. doi: 10.1021/bi048296z. [DOI] [PubMed] [Google Scholar]
- 94.Maxfield FR, Menon AK. Intracellular sterol transport and distribution. Curr Opin Cell Biol. 2006;18:379–85. doi: 10.1016/j.ceb.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 95.Georgiev AG, Sullivan DP, Kersting MC, Dittman JS, Beh CT, Menon AK. Osh proteins regulate membrane sterol organization but are not required for sterol movement between the ER and PM. Traffic. 2011;12:1341–55. doi: 10.1111/j.1600-0854.2011.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]