Abstract
Cullin-RING-ligases (CRLs) comprise the largest class of multisubunit E3 ubiquitin ligases, which regulate a broad range of cellular processes. Cullin3 (Cul3) recently emerged as an important regulator of intracellular trafficking, in particular secretion and endosome maturation. Here we summarize and discuss possible functions and substrates of Cul3 in the endocytic system.
Keywords: endocytosis, ubiquitin, Cullin3, Cul3, EGFR, influenza A virus
Cullin-RING-ligases (CRLs) are assembled by the cullin scaffolds, which interact through the C-terminal domain with the RING finger protein Rbx1 to recruit the E2 ubiquitin conjugating enzyme, and through their N-terminus with one of many adaptor proteins that confer substrate specificity.1 Due to their modular arrangement, CRLs regulate a broad range of substrates involved in various cellular functions including cell cycle progression or DNA damage signaling. Cullin3-based E3-ligases (CRL3) use Bric-à-brac, Tramtrack, Broad-complex (BTB)-containing adaptors, but their physiological functions and substrates are only beginning to emerge.
New Evidence Identifies Cul3 as an Endosomal Regulator
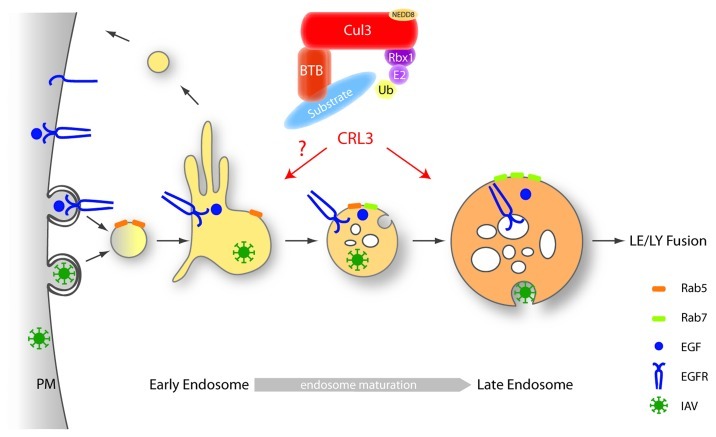
A fraction of Cul3 is specifically activated (neddylated) at the plasma membrane2 and found associated with vesicular markers for intracellular trafficking, suggesting that Cul3-based E3 ligases may regulate membrane-related functions. Indeed, recent data revealed that Cul3 plays important roles in vesicular trafficking, in particular during endosome maturation.3 Depletion of Cul3 by RNA interference (RNAi) affects intracellular trafficking of two well-studied cargos—the influenza A virus (IAV) and the epidermal growth factor receptor (EGFR) (Fig. 1). Without Cul3 activity, IAV internalization into cells appears normal and the virus is able to reach acidic endosomes, which coincide with late endosome (LE)/lysosome (LY) markers. However, IAV is trapped in these compartments, and fails to penetrate into the cytoplasm, indicating that the virus infection cycle is blocked at the endo-lysosomes. Similar to IAV, lysosomal degradation of EGFR is significantly delayed and EGF accumulates in late endosomes, despite normal EGFR activation and Cbl-dependent ubiquitination. While Rab5-containing vesicles appear normal in Cul3-depleted cells, the morphology of Rab7-containing vesicles is highly distorted, increased in size and mostly devoid of intra-luminal vesicles (ILVs) as judged by electron microscopy. Finally, EGFR accumulates at the plasma membrane in Cul3-depleted cells, suggesting a defect beyond late endosomal degradation likely in the receptor recycling processes. Taken together, these observations support a significant role for Cul3-mediated ubiquitination in intracellular trafficking, particular in the maturation process of late endosomes, and suggest that Cul3 may prove an interesting candidate target for development of influenza inhibitors. The observed defects are reminiscent of recent findings that the ubiquitin-dependent p97/Npl4-AAA-ATPase is required for endosomal trafficking.4 Since p97 was shown to bind CRLs,5 Cul3 may function upstream of the p97-segregase to regulate endosome maturation.
Figure 1. Cul3-based E3-ligase complexes regulate endosome maturation. Cul3 is required during the IAV infection cycle, when the virus penetrates from late endosomes into the cytoplasm. Likewise, Cul3 regulates EGFR trafficking, and its depletion delays lysosomal EGFR degradation. Indeed, without Cul3, the morphology of Rab7-containing vesicles is highly distorted, increased in size and mostly devoid of intra-luminal vesicles in Cul3-depleted cells, while Rab5-containing vesicles appear normal. Because Cul3 functions as a scaffolding molecule to assemble distinct E3-ligase complexes with BTB-domain containing substrate-adaptors, these studies imply that Cul3-mediated ubiquitination of unknown substrates regulates maturation of late endosomes. However, defects in earlier steps of endocytic trafficking may be masked by the dominant endosome maturation defects in Cul3-depleted cells.
The Possible Functions of Cul3 in the Endocytic System
What are the physiological substrates ubiquitinated by Cul3 that promote endocytosis? Ubiquitination is known to regulate several processes during uptake of cargo at the plasma membrane and subsequent intracellular trafficking, but the linking of an endocytic function with the relevant E3 ligases and targets has rarely been achieved. In the case of Cul3, a few candidate endocytic activities have been evaluated. Efficient internalization of EGFR was observed in EGF-stimulated cells lacking Cul3, as well as normal autophosphorylation and ubiquitination of EGFR. Likewise, no obvious modification defect of AP2-like adaptor proteins known to be involved in EGFR endocytosis was detected (unpublished results).
Since the endosome maturation process after cargo uptake at the plasma membrane involves several distinct steps, including morphological changes, exchange of membrane components, movement of the endosomes to the perinuclear region, a Rab switch, formation of ILVs, a drop in luminal pH and acquisition of lysosomal components,6 the Cul3-mediated ubiquitination could directly or indirectly affect any of these processes. Indeed, a recent quantitative and systematic approach for detecting the human ubiquitin-modified proteome identified endocytic factors like Rab5c and Rab7a,7 and ubiquitination of the yeast Rab Ypt7 was shown previously to trigger its degradation during membrane fusion. These results indicate that the discovery phase of ubiquitination events in the endocytic pathway is not yet saturated.
Ubiquitination plays a central role in sorting modified proteins into ILVs, which are generated during formation of late endosomes.8 Sorting of cargo into the ILVs is mediated by the endosomal sorting complexes required for transport (ESCRTs). Four ESCRT complexes function consecutively by a mechanism that involves their binding to ubiquitinated cargo via dedicated ubiquitin-binding domains.9 Cul3 could therefore be required to ubiquitinate specific cargo molecules for their targeting to late endosomes. Alternatively, Cul3 may alter the activity of ESCRT components or their regulators. Indeed, the ESCRT components Hrs1 and Tsg101 are known to be regulated through ubiquitination.10,11
Conversely, the deubiquitinating enzymes (DUBs) USP8 (also known as USPY) and AMSH can remove ubiquitin from cargo before entering the ILVs, thereby enabling cargo recycling back to the plasma membrane.12-14 Cul3 is known to regulate the stability of some DUBs,15 and it is thus conceivable that increased levels of USP8 and/or AMSH in Cul3-depleted cells may favor cargo recycling and thereby prevent their ILV uptake. However, the protein levels of USP8 and AMSH seem unchanged upon Cul3-depletion, implying that Cul3 ubiquitination of USP8 and AMSH does not trigger their degradation. Indeed, CRL3 not only targets substrates to proteasomes, but also regulates the activity and/or subcellular localization of proteins by mono-ubiquitination16 or assembling K63-linked chains.17 For example, CRL3 catalyzes monoubiquitination of the COPII-component SEC31, thereby driving the assembly of large COPII coats.16
Perspectives
The human genome encodes for over 200 BTB-proteins, allowing assembly of various Cul3-based E3-ligases complexes in a given cell. Since BTB-adaptors directly interact with their substrate proteins, screening for the relevant BTB-proteins specifically required for intracellular trafficking may be a first step toward identifying substrates. Therefore, the established viral infection and EGFR trafficking assays may provide suitable and quantitative read-outs to screen the available RNAi-libraries targeting all known or predicted BTB-proteins for those that affect distinct endocytic trafficking steps regulated by Cul3-mediated ubiquitination.
Interestingly, Rabankyrin-5 and RhoBTB3 are two BTB-proteins that are already known to be involved in endocytosis. Rabankyrin-5 is a Rab5 effector protein important for the fusion of endosomes.18 RhoBTB proteins lack a classical Rho GTPase function involved in actin cytoskeleton regulation. One of them—RhoBTB3—is required for endosome to Golgi transport by directly binding to Rab9.19 The endocytic defects upon knock-down of those BTB proteins are not obviously shared by a Cul3 depletion phenotype. However, as RNAi-depletion of Cul3 causes rather pleiotropic endocytic defects, it is possible that more subtle deficiencies may be masked by the dominant endosomal maturation defects caused by Cul3-depletion. It would not be surprising, then, if different Cul3 BTB adaptors regulate multiple steps of intracellular trafficking.
Thus, although the published study identified Cul3 as a novel regulator of late endosome maturation, this may only be the tip of the iceberg. Clearly, further work is now required to identify the underlying mechanisms and substrates of Cul3-mediated ubiquitination in endocytic trafficking.
Footnotes
Previously published online: www.landesbioscience.com/journals/cellularlogistics/article/20372
References
- 1.Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6:9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- 2.Meyer-Schaller N, Chou YC, Sumara I, Martin DD, Kurz T, Katheder N, et al. The human Dcn1-like protein DCNL3 promotes Cul3 neddylation at membranes. Proc Natl Acad Sci U S A. 2009;106:12365–70. doi: 10.1073/pnas.0812528106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huotari J, Meyer-Schaller N, Hubner M, Stauffer S, Katheder N, Horvath P, et al. Cullin-3 regulates late endosome maturation. Proc Natl Acad Sci U S A. 2012;109:823–8. doi: 10.1073/pnas.1118744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ritz D, Vuk M, Kirchner P, Bug M, Schütz S, Hayer A, et al. Endolysosomal sorting of ubiquitylated caveolin-1 is regulated by VCP and UBXD1 and impaired by VCP disease mutations. Nat Cell Biol. 2011;13:1116–23. doi: 10.1038/ncb2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexandru G, Graumann J, Smith GT, Kolawa NJ, Fang R, Deshaies RJ. UBXD7 binds multiple ubiquitin ligases and implicates p97 in HIF1alpha turnover. Cell. 2008;134:804–16. doi: 10.1016/j.cell.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30:3481–500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim W, Bennett EJ, Huttlin EL, Guo A, Li J, Possemato A, et al. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol Cell. 2011;44:325–40. doi: 10.1016/j.molcel.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol. 2007;23:519–47. doi: 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–52. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- 10.Polo S, Sigismund S, Faretta M, Guidi M, Capua MR, Bossi G, et al. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature. 2002;416:451–5. doi: 10.1038/416451a. [DOI] [PubMed] [Google Scholar]
- 11.Kim BY, Olzmann JA, Barsh GS, Chin LS, Li L. Spongiform neurodegeneration-associated E3 ligase Mahogunin ubiquitylates TSG101 and regulates endosomal trafficking. Mol Biol Cell. 2007;18:1129–42. doi: 10.1091/mbc.E06-09-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizuno E, Iura T, Mukai A, Yoshimori T, Kitamura N, Komada M. Regulation of epidermal growth factor receptor down-regulation by UBPY-mediated deubiquitination at endosomes. Mol Biol Cell. 2005;16:5163–74. doi: 10.1091/mbc.E05-06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Row PE, Prior IA, McCullough J, Clague MJ, Urbé S. The ubiquitin isopeptidase UBPY regulates endosomal ubiquitin dynamics and is essential for receptor down-regulation. J Biol Chem. 2006;281:12618–24. doi: 10.1074/jbc.M512615200. [DOI] [PubMed] [Google Scholar]
- 14.McCullough J, Clague MJ, Urbé S. AMSH is an endosome-associated ubiquitin isopeptidase. J Cell Biol. 2004;166:487–92. doi: 10.1083/jcb.200401141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sowa ME, Bennett EJ, Gygi SP, Harper JW. Defining the human deubiquitinating enzyme interaction landscape. Cell. 2009;138:389–403. doi: 10.1016/j.cell.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin L, Pahuja KB, Wickliffe KE, Gorur A, Baumgärtel C, Schekman R, et al. Ubiquitin-dependent regulation of COPII coat size and function. Nature. 2012;482:495–500. doi: 10.1038/nature10822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin Z, Li Y, Pitti R, Lawrence D, Pham VC, Lill JR, et al. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell. 2009;137:721–35. doi: 10.1016/j.cell.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Schnatwinkel C, Christoforidis S, Lindsay MR, Uttenweiler-Joseph S, Wilm M, Parton RG, et al. The Rab5 effector Rabankyrin-5 regulates and coordinates different endocytic mechanisms. PLoS Biol. 2004;2:E261. doi: 10.1371/journal.pbio.0020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Espinosa EJ, Calero M, Sridevi K, Pfeffer SR. RhoBTB3: a Rho GTPase-family ATPase required for endosome to Golgi transport. Cell. 2009;137:938–48. doi: 10.1016/j.cell.2009.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]