Abstract
Exosomes, small secreted microvesicles, are implicated in intercellular communication in diverse cell types, transporting protein, lipid and nucleic acid cargo that impact the physiology of recipient cells. Besides the signaling function of exosomes they also serve as a mechanism to dispose obsolete cellular material.1 Particularly exciting is the involvement of exosomal communication in the nervous system, as this has important implications for brain development and function. The properties of exosomes are also beginning to entice the biomedical community since they represent potentially novel avenues for the targeted delivery of customized exosome cargo, such as miRNAs, during disease. Our findings implicating exosomes in trans-synaptic communication emerged from the serendipitous observation that at the Drosophila larval neuromuscular junction (NMJ) the release of a signaling molecule, Wnt1/Wingless (Wg) and its binding partner Evenness Interrupted (Evi)/Wntless (Wls)/Sprint (Srt), were released by motorneurons in association with vesicles, which we postulated to be exosomes.2 In our most recent paper3 using in vivo analysis at the Drosophila NMJ as well as in cultured insect cells we formally demonstrate that Evi rides in exosomes that are released to the extracellular space and identify some of the players involved in their release. In addition, a proteomic analysis of exosomes highlights novel potential function of exosomes.
Keywords: neuromuscular junction, Drosophila, Evi/Wntless/GPR177/mig-14, retromer, local translation, exosome release, Wnt, Wingless, Rab11, Syntaxin 1A, exosomal proteome, RNA-binding proteins
Exosomes refer to small secreted vesicles originating from an endosomal component, the multivesicular body (MVB), and they are emerging as a novel communication pathway between cells. They have been demonstrated to carry, in addition to protein and lipid components, nucleic acids in the form of miRNA and mRNA.4 Exosomal cargo can have significant physiological impact on the recipient cells, and depending on the tissue of origin, exosomes can regulate tumor invasiveness, immune system function, and possibly a large number of other processes.5 Discovered only relatively recently,6 it is no surprise that there has been a steep increase in the number of exosome-related articles over the past decade. Indeed, with the growing awareness about these small vesicles, diverse fields are uncovering surprising new contexts where exosomes are deployed. For example, every tissue fluid analyzed so far has revealed some extent of exosome content (milk, saliva, tears, urine, blood, etc.) and the range of organisms that release small vesicles, that in addition to the specific subclass of exosomes include many other types of microvesicles, encompasses almost all known life forms.7
In the nervous system, the first report on the presence of exosomes in cultured neurons was from Sadoul and colleagues.8,9 When examined by electron microscopy, exosomal release appeared to be confined to the soma and dendrites of cultured cortical neurons, in agreement with other observations of MVB distribution reporting that MVBs are significantly (50×) more abundant in dendritic and somatic compartments, relative to axons.10 A recent study suggests that oligodendrocytes can also release exosomes in an activity dependent manner, and that these exosomes are not only endocytosed by neurons but their contents are also used by neuronal cells.11
In Drosophila we and others2,3,12 have recently unequivocally demonstrated the presence of exosome release both in vivo and in tissue culture. At the larval NMJ, pre- and postsynaptic compartments are continuously expanding to accommodate massive muscle growth during this stage. This process is coordinated by both anterograde and retrograde mechanisms that ensure synchronized synaptic matching.13,14 One of these signaling mechanisms is provided by the release of Wnt-1, Wingless (Wg), which interacts both with pre- and postsynaptic Frizzled2 Wg receptors.14 Mutations in Wg result in poor expansion of the NMJ and in a subset of synaptic boutons that lack postsynaptic machinery.15
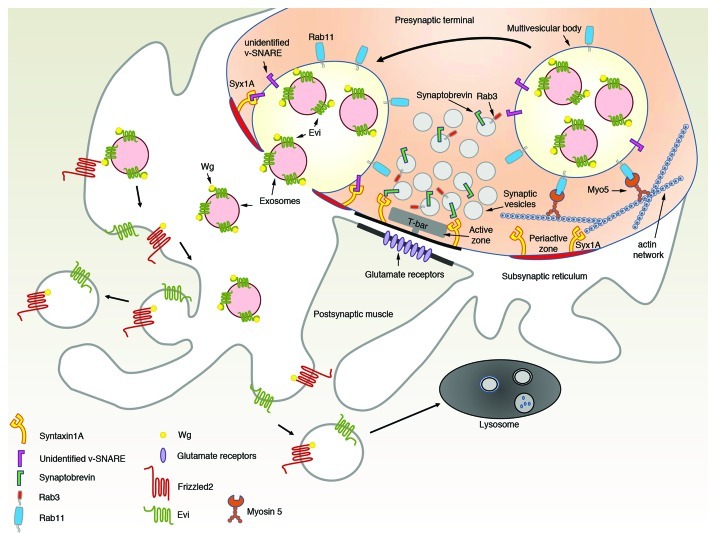
Given that Wg is a hydrophobic molecule, owing to lipid post-translational modifications, an important question has been how secreted Wg is able to traffic in the extracellular space. While searching for these mechanisms we found that Wg is transferred in exosomes through binding to the exosomal protein, Evi, which is released from presynaptic terminals2,14 (Fig. 1). Thus, our data suggest that axons can also support exosomal release. At presynaptic terminals Evi is also found in MVBs, suggesting that these MVBs fuse with the presynaptic membrane. However, the sites of release are likely to be different from the active zones,2 regions of presynaptic terminals involved in synaptic vesicle exocytosis. In contrast, Evi-containing exosomes are likely to be released at periactive zone regions, where these vesicles gain access to the subsynaptic reticulum (SSR),2,3 a complex system of membrane folds at the postsynaptic junctional region (Fig. 1).
Figure 1. Wg rides on exosomes. Schematic representation of Evi-exosome mediated Wg release at Drosophila larval NMJs. Wg carried by Evi-exosomes is sorted into multivesicular bodies at synaptic terminals. Multivesicular bodies fuse with the presynaptic membrane releasing their exosomal content to the cisternae of underlying subsynaptic reticulum, where DFrizzled2 receptors are found. This fusion occurs at sites that are likely distinct from active zones, the periactive zones. In contrast to synaptic vesicles, which use Rab3 and Syntaxin1A for targeting and release of the vesicles at the active zones, the release of exosomes requires Rab11, its effector Myosin5 and the target-SNARE Syntaxin1A. Synaptobrevin is a vesicular-SNARE that functions in synaptic vesicle release and fusion via its interaction with Syntaxin1A, however the v-SNARE on multivesicular bodies has not yet been identified. Potential fates of the released Evi-exosomes are also depicted, however for a more detailed discussion on the role of postsynaptic Evi, see reference 2.
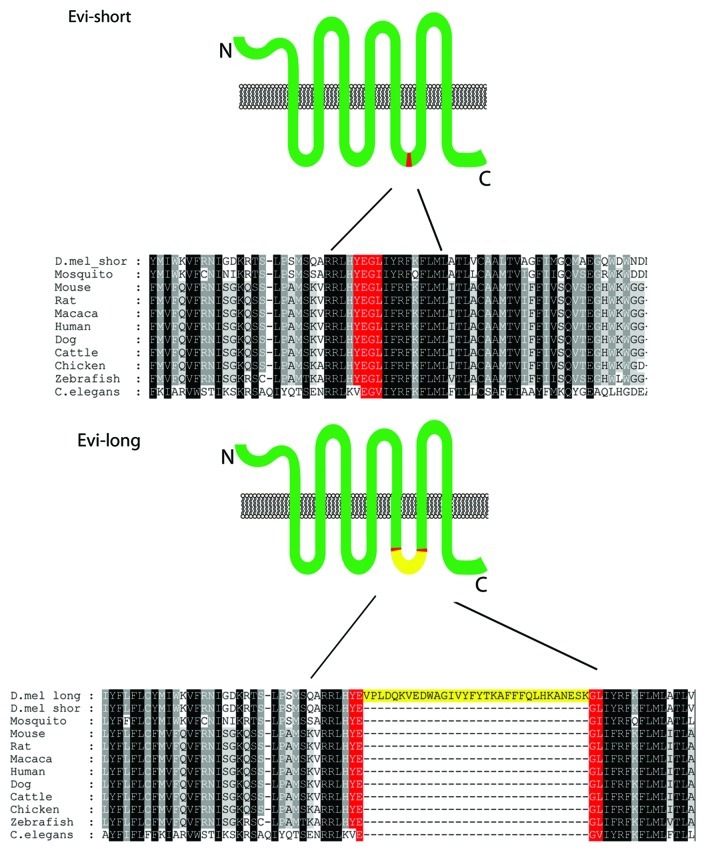
Evi (GPR177 in mammals, mig-14 in C. elegans) is a 7/8-pass transmembrane protein (the exact transmembrane configuration is still awaiting confirmation), which is highly conserved in all examined species. Besides its role in transporting Wg via exosomes,2,3 Evi also functions in the retromer system,16-20 where it is recycled from endosome to to the Golgi apparatus for subsequent rounds of Wg release at the plasma membrane. In what appears to be an evolutionary detour, in some members of the Drosophilidae family, evi mRNA is alternatively spliced to give rise to two isoforms, Evi-long (Wls-A; NP_648445) and Evi-short (Wls-B; NP_729681), with Evi-long in Drosophila melanogaster containing extra 32 amino acids in its predicted last intracellular loop (Fig. 2). Vertebrate and other insect genomes, in contrast, contain only the isoform corresponding to Evi-short in this intracellular loop. This shorter isoform contains a conserved YXXΦ endocytosis motif,21 which was shown to be required for the recycling of Evi from the plasma membrane. Although only four amino acids long, this motif is interrupted by the extra exon found in Evi-long, which therefore lacks this conserved endocytosis domain (Fig. 2). However, expressing Evi-long in wing disc epithelium rescues the Wg secretion defect found in vsp35 retromer mutants or upon expressing Vsp35-RNAi17,18 and interestingly both Evi-long and Evi-short coimmunoprecipitate with VSP3516 suggesting that the endocytic motif is not required for the role of Evi in the retromer. The influence of this motif on the exosomal sorting of Evi isoforms remains to be established.
Figure 2. The short and long of Evi. ClustalW2 alignment of the region near the last intracellular loop of Evi/Wls/GPR177/mig-14, from the species indicated on the left, showing that the alternative splicing of an extra exon in Drosophila melanogaster Evi-long isoform (D.mel long) leads to the disruption of the conserved YXXΦ endocytosis domain.21 All known vertebrate and mammalian species only produce the protein corresponding to the Drosophila melanogaster Evi-short isoform (D.mel shor). Black shading indicates identical amino acids, gray shading denotes conserved physic-chemical properties, while white background corresponds to lack of conservation. Although in this drawing Evi is depicted as a seven pass transmembrane protein, the exact number of membrane spanning domains (7 or 8) is yet to be determined.
In our studies we also began investigating the mechanisms mediating the release of exosomes.3 We established a cell-based assay using Evi-long-GFP as an exosomal marker and the transfer of Evi-exosomes from one cell to another. Candidate genes were then tested in vivo at the NMJ to determine their requirement for the release of Evi-exosomes by motorneuron terminals. We found that the Rab protein Rab11 and the SNARE protein Syntaxin1A (Syx1A) were required for Evi-exosome release both in cell culture and at the NMJ.3 In contrast, Rab35 and Rab27a/Rab27b, which were previously implicated in exosome release from oligodendrocytes22 and HeLa cells,23 respectively, did not have an effect on Evi-exosome release from cultured insect S2 cells or Drosophila larval motorneurons.3 Conversely, Rab11 knock-down in HeLa cells did not affect exosome secretion,23 unlike in Drosophila S2 cells and motorneurons3 or in K562 human erythromyeloblastoid leukemia cells.24 These findings appear to point to a tissue and context-dependent use of Rab-proteins in exosome release, which in turn would argue for the existence of diverse types of exosomes, perhaps with different biological function, which through Rabs might become targeted to alternative release sites. It will be an exciting future endeavor to decipher exosome diversity and whether their release correlates with a dedicated Rab family in terms of function and/or cargo.
Interestingly, Syx1A is also utilized for the release of synaptic vesicles at active zones, although it uses a different Rab (Rab3) for neurotransmitter release.25 Thus, Syx1A might be a common element in the exocytosis of synaptic vesicles and MVBs. However, their specificity for a particular release site (active zone or perisynaptic region) might be conveyed by their interaction with alternative Rabs.
A somewhat surprising finding from our proteomic studies of S2 cell culture derived exosomes was the conserved set of proteins among all reported (vertebrate, mammalian and insect) exosomes, such as heat shock proteins, annexins, coffilin, actin, 14-3-3 proteins, tetraspanins and proteins involved in translation. Interestingly, eukaryotic translation elongation factor 2 (EEF2) and eukaryotic translation elongation factor 1-α1 (EEF1α1) are considered exosomal markers, given the frequency with which they appear in different exosomal proteomes.26 In some studies, both the protein and mRNA have been reported to be present in exosomes.27 A concern is that some of these proteins (such as elongation and initiation factors) might represent contaminants arising during exosome purification.28 EEF2 and EEF1α1 proteins are among the top 0.1% and 1.8%, respectively, of the 5,029 most abundant proteins in mouse NIH 3T3 cells.29 EEF1α1 mRNA is also the most abundant transcript, with EEF2 in the top 0.38%.29 Like other vertebrate studies, we also identified the Drosophila protein orthologs of EEF1α and EEF2 in the Evi-exosome proteome, as well as several other RNA binding/RNA processing proteins. The significance of these observations will have to await future investigation, including ultrastructural localization of these proteins in the intact tissue, and testing whether they are endogenously released in exosomes. However, it is interesting to speculate about the potential function of RNA binding protein release from exosomes at synapses in the nervous system. A key mechanism in the regulation of synaptic strength during synaptic plasticity is the local translation of mRNAs present within ribonucleoprotein (RNP) granules.30 RNPs are trafficked to postsynaptic sites where they are maintained silent until plasticity-inducing stimuli relieves this silencing. A potential regulatory mechanism could be the release of mRNA regulatory proteins by presynaptic terminals through exosomes, which would provide the synaptic specificity that is observed during synaptic plasticity.
In summary, our data confirm that exosomes are evolutionary conserved and are present in vivo in the Drosophila larval nervous system; however this emerging and fascinating field is still in the early stages and a solid consensus on exosome composition and function is yet to emerge.
Acknowledgments
We thank Daniel Tianfang Ge for critical discussions on Evi isoforms. Supported by NIH grant R01 MH0700000 (to V.B.)
Footnotes
Previously published online: www.landesbioscience.com/journals/cellularlogistics/article/21981
References
- 1.Johnstone RM. Exosomes biological significance: A concise review. Blood Cells Mol Dis. 2006;36:315–21. doi: 10.1016/j.bcmd.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, et al. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139:393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koles K, Nunnari J, Korkut C, Barria R, Brewer C, Li Y, et al. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J Biol Chem. 2012;287:16820–34. doi: 10.1074/jbc.M112.342667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40(Database issue):D1241–4. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Théry C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–78. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 7.Deatherage BL, Cookson BT. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect Immun. 2012;80:1948–57. doi: 10.1128/IAI.06014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fauré J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, et al. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006;31:642–8. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G, et al. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci. 2011;46:409–18. doi: 10.1016/j.mcn.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Von Bartheld CS, Altick AL. Multivesicular bodies in neurons: distribution, protein content, and trafficking functions. Prog Neurobiol. 2011;93:313–40. doi: 10.1016/j.pneurobio.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frühbeis C, Fröhlich D, Krämer-Albers EM. Emerging Roles of Exosomes in Neuron-Glia Communication. Front Physiol 2012; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koppen T, Weckmann A, Müller S, Staubach S, Bloch W, Dohmen RJ, et al. Proteomics analyses of microvesicles released by Drosophila Kc167 and S2 cells. Proteomics. 2011;11:4397–410. doi: 10.1002/pmic.201000774. [DOI] [PubMed] [Google Scholar]
- 13.Keshishian H, Kim YS. Orchestrating development and function: retrograde BMP signaling in the Drosophila nervous system. Trends Neurosci. 2004;27:143–7. doi: 10.1016/j.tins.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Koles K, Budnik V. Wnt signaling in neuromuscular junction development. Cold Spring Harb Perspect Biol. 2012 doi: 10.1101/cshperspect.a008045. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Packard M, Koo ES, Gorczyca M, Sharpe J, Cumberledge S, Budnik V. The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell. 2002;111:319–30. doi: 10.1016/S0092-8674(02)01047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belenkaya TY, Wu Y, Tang X, Zhou B, Cheng L, Sharma YV, et al. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev Cell. 2008;14:120–31. doi: 10.1016/j.devcel.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Franch-Marro X, Wendler F, Guidato S, Griffith J, Baena-Lopez A, Itasaki N, et al. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol. 2008;10:170–7. doi: 10.1038/ncb1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Port F, Kuster M, Herr P, Furger E, Bänziger C, Hausmann G, et al. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat Cell Biol. 2008;10:178–85. doi: 10.1038/ncb1687. [DOI] [PubMed] [Google Scholar]
- 19.Harterink M, Port F, Lorenowicz MJ, McGough IJ, Silhankova M, Betist MC, et al. A SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion. Nat Cell Biol. 2011;13:914–23. doi: 10.1038/ncb2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang PT, Lorenowicz MJ, Silhankova M, Coudreuse DY, Betist MC, Korswagen HC. Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev Cell. 2008;14:140–7. doi: 10.1016/j.devcel.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Gasnereau I, Herr P, Chia PZ, Basler K, Gleeson PA. Identification of an endocytosis motif in an intracellular loop of Wntless protein, essential for its recycling and the control of Wnt protein signaling. J Biol Chem. 2011;286:43324–33. doi: 10.1074/jbc.M111.307231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189:223–32. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 2010; 12:19-30; sup pp 1-13. [DOI] [PubMed] [Google Scholar]
- 24.Savina A, Vidal M, Colombo MI. The exosome pathway in K562 cells is regulated by Rab11. J Cell Sci. 2002;115:2505–15. doi: 10.1242/jcs.115.12.2505. [DOI] [PubMed] [Google Scholar]
- 25.Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–47. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 26.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–20. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 28.Chaput N, Théry C. Exosomes: immune properties and potential clinical implementations. Semin Immunopathol. 2011;33:419–40. doi: 10.1007/s00281-010-0233-9. [DOI] [PubMed] [Google Scholar]
- 29.Schwanhäusser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, et al. Global quantification of mammalian gene expression control. Nature. 2011;473:337–42. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- 30.Wang DO, Martin KC, Zukin RS. Spatially restricting gene expression by local translation at synapses. Trends Neurosci. 2010;33:173–82. doi: 10.1016/j.tins.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]