Abstract
Cognitive reserve is thought to reflect life experiences. Which experiences contribute to reserve and their relative importance is not understood. Subjects were 652 autopsied cases from the Rush Memory and Aging Project and the Religious Orders Study. Reserve was defined as the residual variance of the regressions of cognitive factors on brain pathology and was captured in a latent variable that was regressed on potential determinants of reserve. Neuropathology variables included Alzheimer’s disease markers, Lewy bodies, infarcts, microinfarcts, and brain weight. Cognition was measured with six cognitive domain scores. Determinants of reserve were socioeconomic status (SES), education, leisure cognitive activities at age 40 (CA40) and at study enrollment (CAbaseline) in late life. The four exogenous predictors of reserve were weakly to moderately inter-correlated. In a multivariate model, all except SES had statistically significant effects on Reserve, the strongest of which were CA40 (β= .31) and CAbaseline (β= .28). The Education effect was negative in the full model (β= −.25). Results suggest that leisure cognitive activities throughout adulthood are more important than education in determining reserve. Discrepancies between cognitive activity and education may be informative in estimating late life reserve.
Keywords: Cognitive reserve, Alzheimer’s disease, Cerebrovascular disorders, Aging, Neuropsychological test battery, Multivariate analysis
INTRODUCTION
The construct cognitive reserve is frequently invoked to explain discrepancies between brain pathology and brain function. Conceptualized as a malleable trait (Borenstein, Copenhaver, & Mortimer, 2006), reserve is a potential mechanism through which the effects of brain pathology can be modified by experiences and events over the life course (Stern, 2009). Which experiences matter most, though, is not well understood.
Education, occupation, and leisure time activities are the most commonly studied potential markers of reserve. Reviews conclude that there is evidence that more education, greater cognitive demands of work, and involvement in intellectually stimulating activities in leisure time protect against dementia (Fratiglioni & Wang, 2007; Valenzuela & Sachdev, 2006a). However, few studies speak to the issue of how these different potential indicators of reserve relate to each other. For example, are their effects independent? Does one mediate the others? How large is their combined effect? Education is often controlled for in studies of other potential reserve markers; occupational characteristics are controlled less commonly. Results suggest that the effects of occupation are independent of education (Andel, Vigen, Mack, Clark, & Gatz, 2006; Evans et al., 1993; Karp et al., 2004; Schmand, Smit, Geerlings, & Lindeboom, 1997; Valenzuela & Sachdev, 2006a), and that effects of leisure activities are independent of both education (Hultsch, Hertzog, Small, & Dixon, 1999; Lindstrom et al., 2005; Wilson, Mendes De Leon et al., 2002) and occupation (Akbaraly et al., 2009). However, further details on relationships of these variables to each other and to the overall construct of reserve are not well defined.
One reason the question of what contributes to reserve is not often studied has to do with how reserve is modeled—or, rather, not modeled. In a typical study of reserve a variable that is conceptualized as a marker of reserve is examined as a predictor of outcomes such as dementia. If a significant relationship is observed such that the predictor is associated with, for example, lower rates of dementia, the result is interpreted as evidence for reserve. In these analyses there is no measure of reserve itself and effects on reserve are inferred.
We recently published a latent variable modeling approach to measuring reserve based on the idea that reserve can be estimated by the residual term in the regression of cognitive function on brain pathology (Reed et al., 2010). That is, “reserve” may be defined as the difference between cognitive performance as predicted by an individual’s brain pathology and that individual’s observed cognitive performance. Thus, people whose measured cognitive performance is better than predicted by pathology have high reserve whereas those who perform worse than predicted have low reserve. Advantages of this approach include (1) It provides an operational measure of current reserve that is quantitative, continuous, and individually specific; (2) It defines reserve a priori; (3) It enables measurement of change in reserve over time; and (4) Hypotheses about the determinants and effects of reserve can be tested without circularity. It is this last point in particular that makes this approach useful in investigating relationships between multiple potential determinants of reserve. Once reserve is quantified, it is conceptually simple to investigate how multiple potential predictors relate to the outcome variable, reserve.
The original study describing this approach defined the residual term by regressing episodic memory scores on volumetric MRI (atrophy serving as an index of brain pathology). One goal of the present investigation was to replicate and generalize this approach; replicate in the sense of yielding similar results in a different sample with different data; generalize in the sense of creating the residual term (reserve measure) by regressing a different set of cognitive tests on autopsy-derived (rather than MRI) measures of neuropathology. To this end, the general modeling approach was applied to data from two community-based longitudinal studies of cognitive aging, the Religious Orders Study (ROS) (Wilson, Bienias, Evans, & Bennett, 2004) and the Rush Memory and Aging Project (MAP) (Bennett, Schneider, Buchman, et al., 2005). Multiple cognitive measures were regressed on neuropathological measures to replicate and extend the approach of modeling reserve as a residual. The second aim of this study was to use this modeling approach to test substantive hypotheses about the determinants of reserve. Four potential determinants were available, specifically, education, cognitively stimulating leisure time activities at two times of life, and lifetime socioeconomic status (SES). SES is a broad construct with potential to build reserve through multiple mechanisms, including prenatal care, childhood nutritional status, degree of exposure to books, writing, and cultural events, quality of schools, etc. We hypothesized: (1) as single predictors, all three variables are positively correlated with reserve; (2) all four predictors have positive, independent effects on reserve in joint models; and (3) cognitive activities have the strongest independent relationship with reserve (Valenzuela & Sachdev, 2006b; Wilson, Mendes De Leon, et al., 2002).
METHODS
Overview
The analyses presented here use the model presented in Dowling et al. (2011, this issue) of the effects of multiple types of neuropathology on domain-specific neuropsychological function as a base. The two reports use exactly the same participants, neuropsychological measures, and neuropathological measures. The measurement model of the 17 neuropsychological tests is identical, as is the measurement model of the neuropathology. The structural model of the effects of neuropathology on neuropsychological function is the same. The present report extends that model by defining latent measures of cognitive reserve and investigates correlates of reserve, so measured. Methods of Dowling et al. (this issue) that are duplicated in this report are summarized briefly here and the reader is referred to that publication for details.
Participants
Participants were 652 autopsied cases from two prospective, longitudinal studies, MAP (n = 237) (Bennett, Schneider, Buchman, et al., 2005) and ROS (n = 415) (Bennett, Schneider, Arvanitakis, et al., 2006; Bennett, Schneider, Bienias, Evans, & Wilson, 2005; Bennett et al., 2003). Participants were not demented at the time of enrollment. Both studies use the same basic clinical, diagnostic, neuropsychological, and neuropathology methods. Follow-up evaluations take place annually. All protocols were approved by, and informed consent was obtained in accordance with the policies of the Institutional Review Board at Rush University Medical School.
Neuropsychological Factors
The neuropsychological data were scores on 17 widely used clinical neuropsychological tests (detailed in Wilson, Beckett, et al., 2002) obtained at the last examination before death. Best fit was obtained for a model that specified six cognitive factors: (1) episodic memory, (2) semantic memory, (3) working memory, (4) visuospatial ability, (5) perceptual speed, and (6) verbal fluency.
Neuropathology
Neuropathology was assessed following a standardized protocol as described in Bennett, Schneider, Bienias, et al., 2005. A measurement model for neuropathology was developed in a series of factor analyses (Dowling et al., this issue). In brief, the model defines five latent variables: neuritic plaques, diffuse plaques, neocortical neurofibrillary tangles, medial temporal neurofibrillary tangles, and brain weight (adjusted for gender and height). Three observed variables were also used: single summary measures of Lewy bodies, chronic microscopic infarctions, and macroinfarcts.
Predictors of Reserve
Four potential determinants of late life cognitive reserve were tested: Education, SES, Cognitively Stimulating Activities at age 40 (CA40) and Cognitively Stimulating Activities at baseline, that is, at the time of entry to the study (CAbaseline), which averaged approximately age 75 for ROS and approximately age 81 for MAP. SES, CA40, and CAbaseline variables were available only for MAP. Cognitively Stimulating Activity were measured via self-report using a 5-point scale (Wilson, Barnes, & Bennett, 2003), with 1 indicating participation in the activity once or less per year and 5 indicating participation daily or near daily. Activities included reading, writing letters, keeping a journal, visiting a library, and attending a concert. SES was measured using a composite scale consisting of both individual-level and community-level indicators of SES early in life and at midlife. These included participant’s income and occupation in midlife, the occupations and educational level of their parents, literacy rates, and mean SES ranking of heads of household in their census tracts of residence (Wilson et al., 2005).
Data Analysis
Overview
Data analysis and model building proceeded in incremental steps: (1) a latent variable model was developed to characterize dimensions of Alzheimer neuropathology; (2) dimensions underlying neuropsychological tests of cognitive function at the evaluation preceding death were identified; and (3) effects of neuropathology on cognitive function were modeled. These three steps are reported in Dowling et al. (this issue).
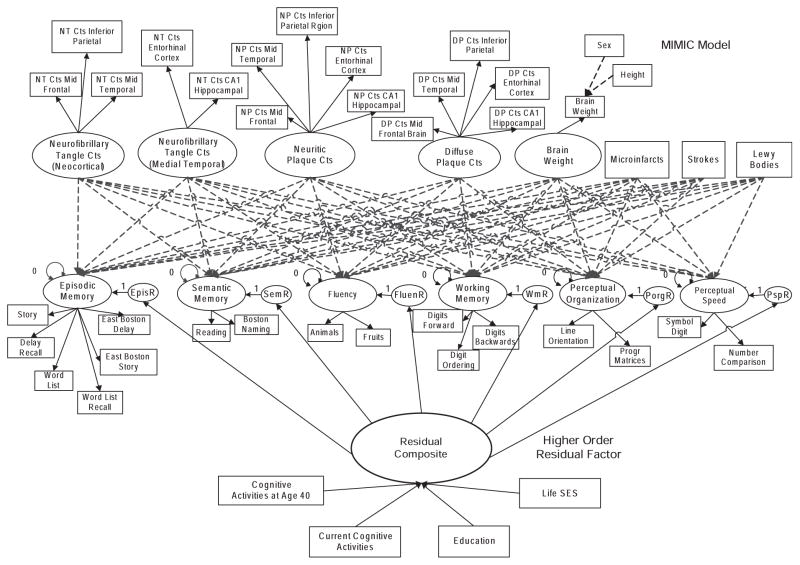
Next, each cognitive factor was decomposed into variance explained by neuropathology and a residual component, which was used to create latent, domain-specific measures of cognitive reserve. Step 4 examined the dimensionality of residuals from different domains, and finally, in step 5, dimensions of reserve were regressed on measures of education, SES, and cognitive activity to address the primary hypotheses of this study. Models created in earlier steps were combined in later steps, such that, in step 5, all observed and latent variables from earlier steps were included and all model parameters were simultaneously estimated. Because the cognitive activity and SES variables included in step 5 were collected in MAP but not in ROS, only MAP data were used in modeling the predictors of reserve. Thus, all available data from both samples was used to model reserve, but hypotheses about how reserve is related to external variables were tested using data from MAP. The overall analytic model is presented in Figure 1.
Fig. 1.
Diagram of the full analytic model. Rectangles are observed variables, and ovals are latent variables. The top left part shows the measurement model for the latent variables that define dimensions of the observed measures of Alzheimer’s disease (AD) neuropathology. The measurement model for cognitive domains is in the middle and shows the latent variables defining dimensions of cognition and their observed indicators. Domain specific residuals are labeled with an “R” (e.g., “EpisR”) and capture the residual variance (reserve) in the corresponding cognitive factor (e.g., Episodic Memory) not explained by neuropathology. The “Residual Composite” is a second-order factor formed by the six domain-specific reserve terms, and was the primary measure of reserve used in this study. The bottom of the Figure diagrams the regressions of Reserve (Residual Composite) on four observed, potential predictors of Reserve. For simplicity, the model does not show the correlations among the exogenous factors and observed variables and the correlations among the disturbances of the cognitive factors assumed in the estimation of the parameters.
Defining components of cognition not related to neuropathology
All six cognitive factors (identified in step 2) were simultaneously regressed on the AD neuropathology dimensions (from step 1) and the observed neuropathology variables. The six residuals were captured as latent variables that represent the variance in each cognitive domain not explained by neuropathology. Further discussion of decomposition cognitive scores to model reserve is in Reed et al. (2010). This process is diagrammed in Figure 1. For example, the latent Episodic Memory factor is defined by six observed (reflexive) indicators and is regressed on (formative) indicators of neuropathology (top of model). The residual variance not explained by neuropathology is captured in an additional latent variable (EpisR). The decomposition of multiple domains was simultaneous, accomplished with one model incorporating all cognitive and neuropathology variables.
Dimensions of residuals of cognitive domains
The dimensional structure of the six cognitive residuals was evaluated using CFA (bottom of Figure 1). This showed that the interrelationships of the residuals across the multiple cognitive domains could be explained by a single second-order factor.
Relationship of residuals to external indicators of reserve
The second-order residual factor (global reserve) was regressed on external variables including years of education, childhood SES, CA40, and CAbaseline. First, we examined relationships of individual predictors with global reserve. Then, we built a multivariate prediction model in incremental steps. Thus, Model 1 included education only. Model 2 added Lifetime SES. Model 3 added CA40 and CAbaseline evaluation as jointly entered independent variables along with education and SES.
In the primary analysis, the relationship of the external variables (SES, etc.) with the six cognitive residuals was constrained to be indirect and mediated by their relationship with the second-order reserve factor. Follow-up analyses permitted direct paths from external variables to the domain-specific reserve along with the indirect path through global reserve. The presence of a significant direct path is indicative of a differential, domain-specific effect of an external variables on reserve.
Latent variable modeling approach
All models were fitted using MPLUS 5.2 (Muthén & Muthén, 1998–2007). The models were tested using sample variance-covariance matrices as input and parameters were estimated using robust maximum-likelihood (MLR) minimization functions to handle non-normality and missing data. In the presence of missing data, MLR estimation generates a likelihood function for each subject using all available data on that subject. This estimator has been shown to produce unbiased parameter estimates and standard errors under missing at random (MAR) and missing completely at random (MCAR) assumptions (Muthén, Kaplan, & Hollis, 1987). The main source of missing data for this study was that cognitive activity and SES variables were not collected in ROS. These data were therefore missing by design, which satisfies MAR requirements, so consequently, results from the missing values analyses should provide unbiased estimates of effects of these variables on reserve. Nonetheless, comparing three predictors of reserve that were acquired in one study (MAP) to a fourth predictor gathered in both studies might raise concerns about methodological artifact. Therefore, we ran the reserve prediction models two ways, one using only MAP predictor data, and the other using MLR estimation of missing data. The final models under these two conditions were highly similar and for the main analyses we present only the results from models that exclude ROS education data.
To increase the reliability of each model solution evaluation, we used multiple indices of fit: the Tucker Lewis fit indexes (TLI), the comparative fit index (CFI), the root-mean-squared error of approximation (RMSEA) and its 90% confidence interval (CI), and the ratio χ2/df (Jöreskog & Sörbom, 1996). We defined a model as acceptable if the following criteria were met: CFI > .90; TLI > .90; RMSEA < .08, 90% CI < .08; and 2 < χ2/df < 5 (Browne & Cudek, 1993; Hu & Bentler, 1999; Schumacker & Lomax, 1996). Pratt’s normalized estimates of relative importance (Pratt, 1987; Thomas, Hughes, & Zumbo, 1998; Thomas, Zhu, & Decady, 2007) were used to compare the relative importance of different independent variables in the model in addition to statistical significance tests.
RESULTS
Sample Characteristics
Participants ranged from cognitively normal (33%) to demented (43%), were quite elderly (average age, 87 years), predominantly female (approximately 60%), and highly educated (Table 1).
Table 1.
Sample demographic and clinical characteristics by last clinical diagnosis
| Sample characteristics | Clinical diagnosis
|
Total (N = 652) | ||
|---|---|---|---|---|
| NCI (N = 214) | MCI (N = 160) | Dementia (N = 278) | ||
| Age at death, mean ± SD | 84 ± 6.58 | 87 ± 6.62 | 89 ± 6.03 | 87 ± 6.67 |
| Education, mean ± SD † | 16.82 ± 3.85 | 16.64 ± 3.71 | 16.77 ± 3.57 | 16.76 ± 3.69 |
| Gender, male (%)† | 42.99 | 40 | 38.49 | 40.34 |
| White non-Hispanic (%)† | 94.39 | 95 | 94.96 | 94.79 |
| MMSE, mean ± SD | 28.22 ± 1.61 | 25.98 ± 3.60 | 14.15 ± 8.49 | 21.41 ± 8.90 |
Note. NCI = no cognitive impairment; MCI = mild cognitive impairment; MMSE = Mini-Mental State Examination.
Analyses of variance F-tests and χ2 tests did not produce statistically significant differences by clinical diagnostic group for education (F = .11, p = .899), gender (χ2 = 1.03, p = .598), and racial composition (White, non-Hispanic) (χ2 = .10, p = .951).
AD neuropathology and cognition dimensions
Modeling of neuropathology measures and cognitive performance is detailed in Dowling et al., this issue. For neuropathology, best fit was achieved using a four factor model that included one dimension of neuritic plaques, one dimension of diffuse plaques, and two dimensions of neurofibrillary tangles, one defined by medial temporal regions (hippocampal and entorhinal) and the second by neocortical regions (mid-frontal, mid-temporal, inferior parietal).
The best fitting model for cognition identified six dimensions: Episodic Memory, Semantic Memory, Working Memory, Perceptual Organization, Processing Speed, and Fluency. The inter-factor correlations were relatively high (.77 to .90), which indicates that there is substantial common variance among the six factors. This was true across all the alternative models examined, and the six factor model clearly provided better fit than did alternate, lower-order factor models, so the six factor model was selected as the best fitting model for the cognitive variables.
Relationships of neuropathology and cognition
Table 2 shows associations of the six cognitive factors with the four AD neuropathology factors and the additional observed neuropathology variables. Neuropathology-cognition relationships are presented in greater detail in Dowling et al. (this issue).
Table 2.
Relationships of cognitive factors with neuropathology variables
| Episodic Memory
|
Semantic Memory
|
Fluency
|
Working Memory
|
Visuospatial Ability
|
Perceptual Speed
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Std. estimate | SE | Std. estimate | SE | Std. estimate | SE | Std. estimate | SE | Std. estimate | SE | Std. estimate | SE | |
| Neuritic plaque factor | −.19 | .10 | −.09 | .10 | −.21 | .11 | −.20 | .12 | −.26 | .12 | −.26 | .10 |
| Neocortical neurofibrillary tangle factor | −.35 | .08 | −.40 | .09 | −.33 | .09 | −.32 | .09 | −.18 | .09 | −.15 | .08 |
| Medial temporal neurofibrillary tangle factor | −.12 | .07 | −.01 | .07 | .03 | .08 | .10 | .08 | .07 | .09 | −.08 | .08 |
| Diffuse plaque factor | .01 | .07 | .02 | .08 | .02 | .08 | .00 | .08 | .00 | .09 | .02 | .07 |
| Micro-infarcts | −.09 | .04 | −.12 | .04 | −.11 | .04 | −.08 | .04 | −.10 | .05 | −.12 | .05 |
| Infarcts | −.16 | .04 | −.15 | .05 | −.18 | .04 | −.17 | .05 | −.19 | .05 | −.19 | .04 |
| Lewy bodies | −.25 | .04 | −.25 | .05 | −.23 | .04 | −.23 | .05 | −.27 | .05 | −.28 | .04 |
| Brain weight | .16 | .04 | .27 | .05 | .17 | .04 | .22 | .05 | .20 | .06 | .16 | .05 |
Note. These results were from the final model that incorporated all cognitive and neuropathology variables along with the putative markers of reserve. Tabled results are standardized regression coefficients. Statistically significant effects are highlighted in bold font.
Standardized coefficients, which can be interpreted as correlations independent of all other effects in the model, ranged from −.15 to −.40. The total variance explained by neuropathology for individual cognitive domains was as follows: Episodic Memory, 47.8%; Semantic Memory, 39.1%; Fluency, 34.9%; Working Memory, 32.3%; Visuospatial Ability, 31.2%; Perceptual Speed, 35.1%.
Dimensionality of residual cognition and association with putative measures of reserve
A single higher-order factor was sufficient to account for residual cognition in the six domains after removing effects of neuropathology variables. Standardized factor loadings from this model (Table 3) were uniformly high, ranging from .83 to .92, providing strong evidence for a unidimensional, global cognitive residual/reserve factor. This latent variable was used as a global measure of reserve and subsequent analyses examined the extent to which variance in this factor could be explained.
Table 3.
Standardized factor loadings for one-dimensional model (global cognitive residual) to explain covariance among first-order cognitive residuals
| 1st order residual | Standardized loading | SE |
|---|---|---|
| EpisR | .85 | .02 |
| SemR | .83 | .04 |
| FluenR | .87 | .02 |
| WmR | .92 | .02 |
| PerSpR | .89 | .02 |
| VisSpR | .90 | .04 |
Note. Standardized loadings can be interpreted as correlations between the global cognitive residual factor and the first-order cognitive residuals.
Thus, the final step in data analysis used the global cognitive residual factor as an operational measure of reserve, and examined how putative markers of reserve (years of education, lifelong SES, CA40, CAbaseline) were related to this actual measure of reserve. These four predictors were modestly intercorrelated: Education correlated with CA40 (r = .41) and also with lifelong SES (r = .28) and CAbaseline evaluation (r = .22). CA40 was correlated with cognitive activity at the baseline evaluation (r = .35). Lifelong SES was weakly correlated with both cognitive activity variables (r’s = .16 and .17).
We first examined relationships of individual potential predictors of reserve with the latent variable measuring global reserve. Significant relationships were found for CA40 (0.321 ± 0.126); standardized coefficients ± S.E.), and CAbaseline (0.36 ± .085), but not lifetime SES (0.123 ± 0.076), p = .10) or education. The independent and joint effects of the markers of reserve on the global reserve measure were explored next (Table 4). Education and lifelong SES were first added as joint independent variables to examine their independent contributions. SES, but not education, had a significant independent association with reserve. The final model included education, SES, CA40, and CAbaseline evaluation as joint independent variables used to explain global reserve. The two cognitive activity variables had relatively strong and independent relationships with reserve. Education was significantly, but interestingly, negatively related to reserve when controlling for the other predictors. Cognitive activity at age 40 and in later life at the time of the baseline evaluation had stronger relationships with reserve, and cognitive activity at age 40 had the strongest relationship, independent of the contribution of cognitive activity at baseline evaluation. The ranking produced by Pratt’s normalized measure of variable relative importance (Pratt, 1987) also indicated that cognitive activity at age 40 made the largest contribution to the total variance explained in the model.
Table 4.
Associations of global cognitive residual factor with education, lifetime SES, cognitive activity at age 40, and cognitive activity at baseline evaluation
| Model | Predictor | Estimate | SE | t | p value | Model R2 | ρ† | δ‡ |
|---|---|---|---|---|---|---|---|---|
| 1 | Education (years) | .003 | .088 | .035 | .972 | .002 | ||
| 2 | Education (years) | −.053 | .096 | −.552 | .581 | .011 | ||
| Life SES | .155 | .082 | 1.878 | .060 | ||||
| 3 | Education (years) | −.254 | .097 | −2.63 | .009 | .141 | .02 | .04 |
| Life SES | .107 | .079 | 1.35 | .177 | .13 | .10 | ||
| Cognitive Activities at age 40 | .313 | .124 | 2.53 | .011 | .28 | .62 | ||
| Cognitive Activities at baseline evaluation | .280 | .092 | 3.03 | .002 | .28 | .56 |
Marginal correlation between the response variable and the predictor.
Normalized relative importance.
Secondary analyses using the full sample examined direct effects of markers of reserve on the domain specific residuals independent of the indirect pathway mediated by the global residual. There was a positive association between cognitive activity at baseline evaluation and the residual for semantic memory (standardized coefficient = .34; SE = .08; p < .001), but a negative relationship with the episodic memory (−.13 ± .06; p = .04) and fluency residuals (−.16 ± .08; p = .04). These were the only significant incremental effects on cognition after controlling for the global residual factor. These results show that the association of semantic memory reserve with cognitive activity at the baseline evaluation is stronger than would be predicted on the basis of the association of this cognitive activity variable with global reserve, whereas reserve in episodic memory and fluency has a weaker relationship with late life cognitive activity.
This study included two different samples, and consequently, we performed analyses to evaluate whether results differed across samples. We used a multiple-group analysis approach to systematically evaluate model invariance across the two samples, and results showed invariance of the dimensional structure of neuropsychological tests, of the structure of AD neuropathology, and of the regressions of neuropsychology dimensions on neuropathology. Finally, an additional analysis included age and sex as predictors of cognitive variables, so that the reserve variable also was independent of these variables. Relationships of reserve with its putative markers did not change.
DISCUSSION
Reserve was modeled as a latent variable defined as the discrepancy between the expected effect of brain pathology on cognitive performance and the level of performance that is actually observed. The model fit well, thus providing evidence of the feasibility of this approach. The analytic models used to create this measure account for most of the methodological complexity of this study. However, once reserve is quantified in this way, investigations of the correlates and effects of reserve are straightforward. The topical analyses of this study are conceptually just a set of multiple regressions that define the independent and combined effects of four observed demographic and life experience variables on cognitive reserve. Among these four variables, the strongest correlate of late life cognitive reserve was cognitively stimulating activities during leisure time at age 40. Cognitive activities at study entry, a time decades later and much closer to the time at which reserve was measured, had a significant, but lesser independent effect on reserve. When modeled along with the other predictors the effects of education on reserve were actually negative.
The first goal of this study was to replicate a prior investigation of modeling reserve as a residual term (Reed et al., 2010). Although the present data came from a different subject cohort and used different neuropsychological tests, the analytic models were conceptually the same and very similar in structure. An important difference between the studies is that the previous study estimated brain pathology using volumetric MRI whereas this study used autopsy based neuropathology measures. Also, the previous study examined reserve in episodic memory, while this study simultaneously modeled reserve in multiple domains. Thus, the fact that the model fit well provides a strong replication of this approach to measuring reserve.
The second goal of this study was to investigate what lifetime experiences are associated with late life cognitive reserve. Reviews have concluded that education helps to build cognitive reserve (Borenstein et al., 2006; Fratiglioni & Wang, 2007; Valenzuela & Sachdev, 2006a, 2006b). In contrast to those studies, which infer that education builds reserve because education reduces the risk of dementia or cognitive decline, the present analysis instead quantified reserve directly and found no relationship of education with reserve when education was the only predictor in the model. A possible interpretation of the discrepancy is to note that education could affect the risk of dementia through multiple pathways (e.g., general health, socioeconomic status, etc.) only one of which is reserve. Unless reserve is directly measured, or alternative causal pathways are carefully modeled it is not convincing to equate education effects with “reserve” effects.
Prior studies, including analyses from ROS and MAP, have reported that cognitively stimulating leisure activities are associated with lower rates of dementia (Akbaraly et al., 2009; Crowe, Andel, Pedersen, Johansson, & Gatz, 2003; Gatz, Prescott, & Pedersen, 2006; Lindstrom et al., 2005; Wilson, Mendes De Leon, et al., 2002) and slower cognitive decline (Hultsch et al., 1999; Wilson, Bennett, Bianias, et al., 2003). Consistent with this, we found moderately strong associations of reserve with cognitively stimulating activities at age 40 and at study entry. In addition, cognitive activities at age 40 had effects on reserve that were stronger than those of cognitive activities at study entry, suggesting that cognitive activity during middle age is especially important to cognitive reserve.
Because baseline cognitive activities were reported contemporaneously whereas activities at age 40 were reported retrospectively the potential effects of recall bias should be considered. To account for the findings, reserve would need to bias reports of past but not present cognitive activities. Although possible, this concern is reduced because all subjects were non-demented at time of entry to the study, and the mean length of time to diagnosis of cognitive impairment or dementia in those who were impaired is over 4 years. Studies of age effects on bias in survey research suggest that increased age is most often associated with under-reporting rather than over reporting (Knäuper & Wittchen, 1994). Regardless of when it was reported to occur, cognitive activity had stronger effects on reserve than did education. This suggests that the effects of education on reserve are either mediated by cognitive activity later in life, or that those activities are simply more influential than is education.
SES is less often studied as a correlate of reserve. Heterogeneity of definition of SES may be one reason that the evidence on the protective effects of SES is mixed (c.f., Gatz et al., 2006; Karp et al., 2004). Here we found that SES, entered as the single predictor, had positive but relatively weak effects on late life reserve and no effect in multivariate models.
Few reports explicitly investigate how multiple potential predictors of reserve relate to each other. Comparisons across studies of effect sizes of different predictors have suggested that the effects of cognitively stimulating activities are similar to (Valenzuela & Sachdev, 2006b) or smaller than the effects of education on cognitive decline and dementia (Fratiglioni & Wang, 2007; Valenzuela & Sachdev, 2006a). Here, we find that education effects are weaker than those of cognitive activities.
A finding that we believe to be novel is that when the other predictors were in the model the effect of education on reserve was negative. This could represent a statistical artifact. However, inspection of both simple and partial regression plots of education versus the latent residual/reserve variable shows patterns that are typical of moderately correlated factors. Nor are SES, cognitively stimulating activities, and education highly collinear. Alternatively, perhaps the cognitive activity scores reflect the effects, incipient or otherwise, of brain pathology whereas education does not. One can construct a set of circumstances that follow from this that could account for the observed result. The basic problem with this account is that it assumes that effects of the degree of pathology as measured at death would be evident in cognitively stimulating activities at age 40, which is implausible.
A more substantive explanation is that reserve is built by exercising one’s mental capacity. Education develops this capacity early in life. After formal education ends, work and leisure cognitive activities provide ongoing mental exercise and stimulation critical to further developing and maintaining reserve. Recent work suggests that cognitive activity during adulthood can compensate for low education on the outcome variable memory performance (Lachman, Agrigoroaei, Murphy, & Tun, 2009). Conversely, failure to use one’s capacity over many years might result in loss of reserve. In this scenario, a level of education higher than expected given a particular level of cognitively stimulating activities would be associated with lower reserve, while educational achievement lower than expected would underestimate reserve.
Finally, it may be that discrepancies between years of education and lifelong CSA are an indication of other traits related to reserve. For example, persons whose cognitive activity exceeds the average for their level of education may have levels of motivation, curiosity, drive or persistence that effectively build reserve. Thus, cognitive activities exceeding the expectation set by education level (or equivalently, education being lower than expected on the basis of cognitive activities) would be positively associated with reserve. Conversely, low cognitive activities relative to education could reflect lower motivation or lower ability. Education in these cases might be more related to circumstance than ability and the difference between education and CSA could reflect traits negatively associated with late life reserve.
The association between cognitive activity and reserve does not directly speak to the issue of genetic versus exposure factors in creating cognitive reserve. There is evidence that cognitive stimulation late in life can increase the thickness of the cortical mantel, which may in turn contribute to reserve (Engvig et al., 2010). However, whether naturally occurring levels of cognitive activity reflect nature, nurture, or both, is not known (Gatz et al., 2006).
The model here explicitly incorporates domain-specific measures of cognitive reserve, for example, episodic memory reserve, semantic memory reserve, etc. Reserve is usually conceptualized as a unitary capacity and, indeed, the finding that a single second-order factor captured most of the variance in the six domain-specific reserve estimates indicates that there is generality to cognitive reserve. However, it is plausible that there is also domain specificity to reserve and that this varies between people. For example, artistic and verbal abilities could be maintained to very different degrees in the face of dementia (Soricelli, 2006). Our results showed a modest positive effect of midlife cognitive activity on semantic memory reserve whereas its effects on episodic memory and fluency reserve were weakly negative. These findings can be interpreted as showing midlife cognitive activities have comparatively strong effects on semantic reserve, and relatively weak effects on episodic memory and fluency reserve. Both semantic memory and current level of cognitive engagement can be conceptualized as reflecting the summation of cognitive activity over many years, and this might contribute to the particular association of these variables. Domain specific reserve warrants further investigation.
Caveats include the basic point that all the findings are correlational and that causal inference should be made with caution. The results are consistent with a model of reserve in which the effects of education are mediated through ongoing life experiences that more directly build reserve. There are other explanations, though. One alternative is simply the reverse causal pattern: people with more reserve do more cognitively stimulating activities. However, this is not an effect that is predicted by the theory of reserve, nor is it likely that higher reserve could somehow cause higher lifetime (including childhood) SES, which was also associated with reserve. A reporting bias, such that people with higher reserve report (but do not do) more cognitively stimulating activities, is possible. Because all the predictors of reserve are gathered from self-report instruments, gathered at entry to the study, it is possible that people who had incipient dementias were already showing the effects in reduced levels of cognitive activity. Because all reports from earlier periods of life are retrospective, they may be influenced by and consequently distorted by current activity levels. Again, however, it is not clear how this would account for the effects of education or SES.
The issue of how to measure reserve is a neglected topic. This study was framed as both an exploration of an approach to modeling reserve and as an opportunity to test substantive hypotheses about how life experience builds reserve. While other analytic approaches might yield similar results, having a direct, quantitative measure of reserve facilitates hypothesis testing. One of the contributions of this work is that we were able to model the joint and independent effects of multiple predictors of reserve, and in this way lend support to the premise that continuing cognitive activity throughout adulthood is protective against dementia. The unexpected pattern of results for education, the beneficial effects of which are generally accepted, requires more investigation. Careful consideration of how to model reserve is needed to advance scientific understanding of this intuitively appealing, but very complex construct.
Acknowledgments
The data for this study were provided by studies supported by the National Institutes of Health, National Institute of Aging (NIA); the Religious Orders Study (P30AG10161, R01AG15819), and the Rush Memory and Aging Project (NIA Grants AG17917 and AG10161), and the Illinois Department of Public Health). Data were provided by the principal investigator (PI) of these projects, David Bennett, MD. The research was supported in part by a NIA conference grant Conference on Advanced Psychometric Methods in Cognitive Aging Research, (R13AG030995) Dan Mungas, PhD, PI., and by NIA grant AG031563, Bruce Reed, PhD, PI. This material was supported with resources and the use of facilities at the Martinez VA medical center. The authors thank the study participants and the staff of the Rush Alzheimer’s Disease Center.
Footnotes
None of the authors have any conflicts of interest to report.
References
- Akbaraly TN, Portet F, Fustinoni S, Dartigues JF, Artero S, Rouaud O, Berr C. Leisure activities and the risk of dementia in the elderly: results from the Three-City Study. Neurology. 2009;73(11):854–861. doi: 10.1212/WNL.0b013e3181b7849b. [DOI] [PubMed] [Google Scholar]
- Andel R, Vigen C, Mack WJ, Clark LJ, Gatz M. The effect of education and occupational complexity on rate of cognitive decline in Alzheimer’s patients. Journal of the International Neuropsychological Society. 2006;12(01):147–152. doi: 10.1017/S1355617706060206. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66(12):1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64(5):834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25(4):163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Mendes de Leon CF, Arnold SE, Bienias JL. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60(12):1909–1915. doi: 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- Borenstein AR, Copenhaver CI, Mortimer JA. Early-life risk factors for Alzheimer disease. Alzheimer Disease and Associated Disorders. 2006;20(1):63–72. doi: 10.1097/01.wad.0000201854.62116.d7. [DOI] [PubMed] [Google Scholar]
- Browne M, Cudek R. Alternate ways of assessing model fit. In: Bollen K, Long J, editors. Testing structural equation models. Thousand Oaks, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Crowe M, Andel R, Pedersen NL, Johansson B, Gatz M. Does participation in leisure activities lead to reduced risk of Alzheimer’s disease? A prospective study of Swedish twins. Journal of Gerontology B Psychological Sciences and Social Sciences. 2003;58(5):P249–P255. doi: 10.1093/geronb/58.5.p249. [DOI] [PubMed] [Google Scholar]
- Dowling NM, Tomaszewski Farias S, Reed BR, Sonnen JA, Strauss ME, Schneider JA, Mungas D. Neuropathological associates of multiple cognitive functions in two community-based cohorts of older adults. Journal of the International Neuropsychological Society. 2011 doi: 10.1017/S1355617710001426. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth Ø, Larsen VA, Walhovd KB. Effects of memory training on cortical thickness in the elderly. Neuroimage. 2010;52(4):1667–1676. doi: 10.1016/j.neuroimage.2010.05.041. [DOI] [PubMed] [Google Scholar]
- Evans DA, Beckett LA, Albert MS, Hebert LE, Scherr PA, Funkenstein HH, Taylor JO. Level of education and change in cognitive function in a community population of older persons. Annals of Epidemiology. 1993;3(1):71–77. doi: 10.1016/1047-2797(93)90012-s. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Wang HX. Brain reserve hypothesis in dementia. Journal of Alzheimer’s Disease. 2007;12(1):11–22. doi: 10.3233/jad-2007-12103. [DOI] [PubMed] [Google Scholar]
- Gatz M, Prescott CA, Pedersen NL. Lifestyle risk and delaying factors. Alzheimer Disease and Associated Disorders. 2006;20(3 Suppl 2):S84–S88. doi: 10.1097/00002093-200607001-00013. [DOI] [PubMed] [Google Scholar]
- Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6(1):1–55. [Google Scholar]
- Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: engaged lifestyle as a buffer of cognitive decline in aging? Psychology and Aging. 1999;14(2):245–263. doi: 10.1037//0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- Jöreskog KG, Sörbom D. LISREL 8: User’s reference guide. Chicago: Scientific Software International; 1996. [Google Scholar]
- Karp A, Kareholt I, Qiu C, Bellander T, Winblad B, Fratiglioni L. Relation of education and occupation-based socioeconomic status to incident Alzheimer’s disease. American Journal of Epidemiology. 2004;159(2):175–183. doi: 10.1093/aje/kwh018. [DOI] [PubMed] [Google Scholar]
- Knäuper B, Wittchen HU. Diagnosing major depression in the elderly: evidence for response bias in standardized diagnostic interviews? Journal of Psychiatric Research. 1994;28(2):147–164. doi: 10.1016/0022-3956(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Lachman ME, Agrigoroaei S, Murphy C, Tun PA. Frequent cognitive activity compensates for education differences in episodic memory. American Journal of Geriatric Psychiatry. 2009;18(1):4–10. doi: 10.1097/JGP.0b013e3181ab8b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom HA, Fritsch T, Petot G, Smyth KA, Chen CH, Debanne SM, Friedland RP. The relationships between television viewing in midlife and the development of Alzheimer’s disease in a case-control study. Brain and Cognition. 2005;58(2):157–165. doi: 10.1016/j.bandc.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Muthén B, Kaplan D, Hollis M. On structural equation modeling with data that are not missing completely at random. Psychometrika. 1987;52(3):431–462. [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 5. Los Angeles, CA: Muthén & Muthén; 1998–2007. [Google Scholar]
- Pratt JW. Dividing the indivisible: using simple symmetry to partition variance explained. In: Pukkila T, Puntanen S, editors. Proceedings of the Second International Conference in Statistics. Tampere, Finland: University of Tampere; 1987. pp. 245–260. [Google Scholar]
- Reed BR, Mungas D, Tomaszewski Farias S, Harvey D, Beckett L, Widaman KF, DeCarli C. Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain. 2010;133(Pt 8):2196–2209. doi: 10.1093/brain/awq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmand B, Smit JH, Geerlings MI, Lindeboom J. The effects of intelligence and education on the development of dementia. A test of the brain reserve hypothesis. Psychological Medicine. 1997;27(6):1337–1344. doi: 10.1017/s0033291797005461. [DOI] [PubMed] [Google Scholar]
- Schumacker RE, Lomax RG. A beginner’s guide to structural equation modeling. Hillsdale, NJ, England: Lawrence Erlbaum Associates, Inc; 1996. [Google Scholar]
- Soricelli RL. Medicine and the arts. A series of self portraits by William Utermohlen. Commentary. Academic Medicine. 2006;81(11):996–997. doi: 10.1097/00001888-200611000-00014. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DR, Hughes E, Zumbo BD. On variable importance in linear regression. Social Indicators Research. 1998;45:253–275. [Google Scholar]
- Thomas DR, Zhu P, Decady YJ. Point estimates and confidence intervals for variable importance in multiple linear regression. Journal of Educational and Behavioral Statistics. 2007;32:61–91. [Google Scholar]
- Valenzuela MJ, Sachdev P. Brain reserve and cognitive decline: a non-parametric systematic review. Psychological Medicine. 2006a;36(8):1065–1073. doi: 10.1017/S0033291706007744. [DOI] [PubMed] [Google Scholar]
- Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychological Medicine. 2006b;36(4):441–454. doi: 10.1017/S0033291705006264. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Bennett DA. Assessment of lifetime participation in cognitively stimulating activities. Journal of Clinical and Experimental Neuropsychology. 2003;25(5):634–642. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychology and Aging. 2002;17(2):179–193. [PubMed] [Google Scholar]
- Wilson RS, Bennett DA, Bienias JL, Mendes de Leon CF, Morris MC, Evans DA. Cognitive activity and cognitive decline in a biracial community population. Neurology. 2003;61(6):812–816. doi: 10.1212/01.wnl.0000083989.44027.05. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Bienias JL, Evans DA, Bennett DA. Religious orders study: overview and change in cognitive and motor speed. Neuropsychology and Cognition. 2004;11(2–3):280–303. [Google Scholar]
- Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. Journal of the American Medical Association. 2002;287(6):742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Scherr PA, Hoganson G, Bienias JL, Evans DA, Bennett DA. Early life socioeconomic status and late life risk of Alzheimer’s disease. Neuroepidemiology. 2005;25(1):8–14. doi: 10.1159/000085307. [DOI] [PubMed] [Google Scholar]