Abstract
Pubertal development is marked by significant decreases in cellular proliferation and neurogenesis in the dentate gyrus of the hippocampal formation. Though it is unclear what mediates these developmental changes in the dentate gyrus, gonadal hormones have been implicated in modulating many neurobiological processes during puberty and various parameters of neurogenesis in adulthood. Thus, it is possible that the gradual and sustained increase in gonadal hormones experienced during puberty plays a role in these changes in neurogenesis. In the present experiments, we first quantified cellular proliferation and neurogenesis using 5-bromo-2′-deoxyuridine (BrdU) and doublecortin (DCX) immunohistochemistry, respectively, in the dentate gyrus of pre-pubertal (30d), mid-pubertal (45d), and adult (90d) male rats. We found the decline in BrdU and DCX cell numbers throughout these ages were coincident with increases in their plasma testosterone levels. We next tested whether exposure to the pubertal rise in gonadal hormones was necessary for this decrease in hippocampal neurogenesis to occur. Thus, we examined cellular proliferation and neurogenesis in intact 30 day (pre-pubertal) and 60 day old (late-pubertal) rats, as well as 60 day old rats that had previously been gonadectomized or sham-gonadectomized at 30 days of age. Although we again found the expected decline in BrdU and DCX cell numbers between 30 and 60 days of age in the intact groups, there were no differences among the 60 day old animals, regardless of gonadal status. These data indicate that the pubertal-related decline in hippocampal cellular proliferation and neurogenesis is independent of the pubertal change in gonadal hormones.
Keywords: Adolescence, Castration, Doublecortin, Hippocampus, Male
INTRODUCTION
Alongside the well established endocrinological and somatic changes associated with puberty (Sizonenko, 1989; Rogol et al., 2002), a burgeoning line of research has revealed that the pubertal brain also undergoes significant transformations, which may mediate some of the cognitive and emotional changes observed during this stage of development (reviewed in; Juraska and Markham, 2004; Crews et al., 2007; Giedd et al., 2009; Casey et al., 2010; Giedd and Rapoport, 2010; Spear, 2010). For instance, studies in both human and non-human animals have shown significant pubertal-related cortical thinning and neuron pruning in select cortical regions (Jernigan et al., 1991; Pfefferbaum et al., 1994; Giedd et al., 1999; Courchesce et al., 2000; Nunez et al., 2002; Giedd, 2004; Gogtay et al., 2004; Markham et al., 2007; Sowell et al., 2007; Knickmeyer et al., 2010), while the volume of limbic areas, such as the amygdala and hippocampus, as well as myelinated fiber tracts, have been reported to increase (Giedd et al., 1996; Giedd et al., 1997; Giedd et al., 1999; De Bellis et al., 2001; Romeo and Sisk, 2001; Isgor et al., 2004; Juraska and Markham, 2004; Suzuki et al., 2005; Cooke, 2010; Knickmeyer et al., 2010; Lebel and Beaulieu, 2011). Though it is still unclear what factor(s) mediate these morphological changes during puberty, a few studies have provided evidence that gonadal hormones may be involved (Nunez et al., 2002; Cooke et al., 2007; Perrin et al., 2008; Raznahan et al., 2010).
In addition to these structural alterations, changes at a more cellular level have been reported in the peripubertal brain as well. One such notable change is the pubertal-related decrease in both cellular proliferation and neurogenesis in the dentate gyrus of the hippocampal formation. In particular, prepubertal males have levels of cellular proliferation and neurogenesis that are twice as high as those found in adults (Heine et al., 2004; Kim et al., 2004; McDonald and Wojtowicz, 2005; Crews et al., 2007; He and Crews, 2007; Cowen et al., 2008; Hodes et al., 2009). Although hippocampal neurogenesis in adulthood has been purported to play a role in learning and memory (Shors et al., 2001; Shors et al., in press) and mood disorders (Samuels and Hen, 2011), the neurobehavioral implications of the change in neurogenesis during puberty remain unknown.
Similar to some of the gross morphological alterations noted above, it is possible that the change in gonadal hormones associated with puberty may mediate the pubertal-related decrease in cell proliferation and neurogenesis in the dentate gyrus. Gonadal hormones in adulthood have been shown to modulate hippocampal neurogenesis (Tanapat et al., 1999; Tanapat et al., 2005; Galea et al., 2006; Spritzer and Galea, 2007; Barker and Galea, 2008; Galea, 2008), suggesting that hormones may also have an impact on this process during puberty. Moreover, exposure to gonadal hormones during puberty does appear to alter cellular proliferation, ultimately contributing to the sex difference in the volume of specific brain nuclei, such as the sexually dimorphic nucleus of the hypothalamus and medial amygdala (Ahmed et al., 2008). However, the relationship between pubertal changes in gonadal hormones and hippocampal neurogenesis has not been fully explored.
In an effort to gain a deeper understanding of pubertal brain development and changes in neurogenesis in the dentate gyrus, two experiments were conducted. In the first experiment using 5-bromo-2′-deoxyuridine (BrdU) and doublecortin (DCX) immunohistochemistry in male rats, we examined the temporal nature of the pubertal decline in cellular proliferation and neurogenesis in the dentate gyrus and measured the testosterone levels of prepubertal, mid-pubertal, and adult rats. In the second experiment, we tested the hypothesis that the pubertal rise in gonadal hormones was responsible for the observed pubertal decrease in hippocampal cell proliferation and neurogenesis. We report here that even though pubertal decreases in BrdU and DCX cell numbers are coincident with an increase in testosterone levels, this decline in hippocampal neurogenesis is independent of the pubertal rise in gonadal hormones.
METHODS
Animals
Male Sprague-Dawley rats were obtained from our breeding colony at Barnard College. All animals were weaned at 21 days of age, housed 2 per cage in clear polycarbonate cages (45 × 25 × 20 cm) with wood chip bedding, and maintained on a 12 h light-dark schedule (lights on at 0900 h). Animals had ad libitum access to food and water and the animal room was maintained at 21±2°C. All procedures were carried out in accordance with the guidelines established by the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee (IACUC) of Columbia University.
Experimental Design
Two experiments were conducted. Experiment 1 examined the effects of pubertal development on cell proliferation and neurogenesis in the dentate gyrus of the hippocampal formation. Experiment 2 investigated the role of the pubertal rise of gonadal hormones on pubertal-related changes in these parameters.
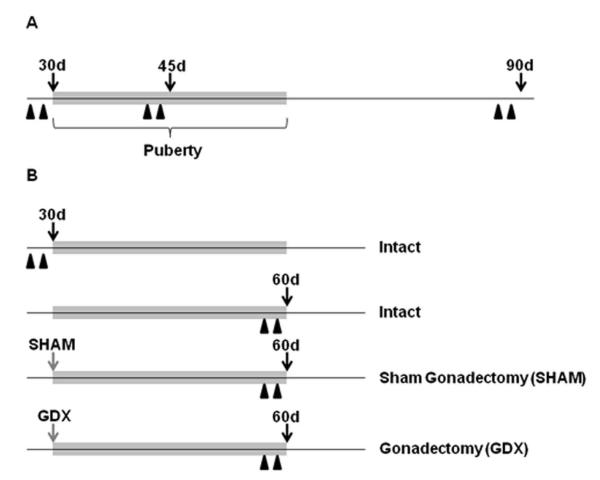
In experiment 1, male rats at 28 (prepubertal), 43 (mid-pubertal) or 88 (adult) days of age received two intraperitoneal (i.p.) injections of BrdU (200 mg/kg in a 0.9% saline vehicle; Sigma, St. Louis, MO) separated by 24h, and were sacrificed 24h after the second injection (i.e., at 30, 45, or 90 days of age; n = 6 per age; Figure 1A). For tissue collection, animals were weighed, given an overdose of sodium pentobarbital (150 mg/kg), and following a cardiac puncture, were perfused with 0.9% heparinized saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Brains were postfixed in 4% paraformaldehyde for 24 h then stored in 20% sucrose at 4°C. Thirty-micron coronal sections were made on a cryostat and stored in cryoprotectant at -20°C until processed for BrdU and DCX immunohistochemistry (see below) and Nissl staining.
Figure 1.
Schematic timelines for the experimental designs in experiments 1 (A) and 2 (B). Black arrows indicate the day tissues were collected, gray arrows the day of surgery, and black arrowheads the BrdU injections (200 mg/kg). The gray bar on each timeline demarcates the approximate time span of pubertal development. Abbreviations: d, days of age; GDX, gonadectomy; SHAM, sham-gonadectomy.
In experiment 2, four groups of rats were assessed: (i) intact prepubertal (28 days of age) males, (ii) intact late-pubertal (58 days of age) males, (iii) late-pubertal (58 days of age) males that had been sham-gonadectomized (SHAM) at 30 days of age, and (iv) late-pubertal (58 days of age) males that had been gonadectomized (GDX) at 30 days of age. During surgery, SHAM and GDX animals were anesthetized with ketamine (100 mg/kg; i.p.) and xylazine (10 mg/kg, i.p.). Similar to experiment 1, these intact, SHAM, and GDX animals at 28 or 58 days of age received two i.p. injections of BrdU (200 mg/kg in a 0.9% saline vehicle) separated by 24h, and sacrificed 24h after the second injection (i.e., at 30, or 60 days of age; n = 6 per group; Figure 1B). Perfusions, tissue collections and sectioning were conducted as described above.
BrdU Immunohistochemistry and Cell Counting
In both experiments, every 8th section throughout the dorsal hippocampal formation (plate 55-70; Paxinos and Watson, 2005) was processed for BrdU immunohistochemistry. To reduce endogenous peroxidase activity prior to staining, tissue sections were treated with 0.6% H2O2 in 0.1M PB for 20 min. Sections were then rinsed three times in 0.1M PB for 5 min each and incubated for 2h at 65°C in 50% formamide and 1X sodium chloride and sodium citrate buffer (SSC, Ambion, Foster City, CA). Sections were then washed two times in 2X SSC pre-heated to 65°C for 2 min each and two times in 0.1M borate buffer (pH 8.5) for 5 min each. Following these washes, sections were incubated in blocking buffer (2% normal goat serum and 0.1% Triton X-100 in 0.1M phosphate buffered saline; PBS) for 1h at room temperature (RT) and then at 4°C in blocking buffer containing the primary antibody (BrdU;1:500; mouse; Roche Diagnostic GmbH, Mannheim, Germany). Forty-eight hours later, sections were incubated in biotinylated goat anti-mouse IgG (1:500; Vector, Burlingame, CA) and then avidin-biotin horseradish peroxidase complex (1:250; Vectastain ABC Kit, Vector) in 0.1M PBS for 1 h each at RT, with three 5 min washes in PBS between each of the incubations. Horseradish peroxidase was visualized with 3,3′diaminobenzidine (DAB) in a 3M sodium acetate buffer containing 0.05% H2O2. Sections were washed, mounted on to Fisher Brand Plus slides (Fisher Scientific, Pittsburgh, PA), dried, counter-stained with cresyl violet, dehydrated in increasing concentrations of alcohol (70%, 95%, and 100%), cleared in xylenes, and coverslipped with DPX Mountant (Sigma).
BrdU-positive cells were counted using a Zeiss Axiovert 200 microscope. Each dentate gyrus was centered under a 10X objective and then the magnification was increased to 40X. Similar to a previous study (Leuner et al., 2009) our data are presented as the estimated number of BrdU-positive cells in the dentate gyrus. Briefly, the total number of BrdU-positive cells was counted in the granule cell layer and subgranular zone (excluding the hilus) of seven bilateral dentate gyri. The sum of each of these bilateral counts was multiplied by eight (the number of intervening sections) and then added to provide the estimate. Additionally, because we measured the cross-sectional area of each dentate gyrus (see below), we also report our data as mean number of BrdU cells per unit area (100mm2).
Cross-Sectional Area Measurements
To assess possible effects of age and gonadal hormone status on the cross-sectional area of the dentate gyrus, adjacent sections to those processed for BrdU were mounted on Fisher Brand Plus slides, dried, stained with 1.5% cresyl violet, cleared in xylenes, and coverslipped with DPX Mountant. Cross-sectional area was measured by capturing an image of the dentate gyrus under a 2.5X objective with an Olympus DP71 camera and then subjected to analysis using ImageJ (Wayne Rasband, National Institutes of Health, Bethesda, MD). For each animal, the complete cross-sectional area of each dentate gyrus was measured from each section and then averaged. Therefore, the data are presented as average cross-sectional area (μm2) per section.
DCX Immunohistochemistry and Cell Counting
For both experiments, six anatomically matched sections from each animal’s dorsal hippocampal formation (plate 58-63; Paxinos and Watson, 2005) were processed for DCX immunohistochemistry. Specifically, free-floating sections were washed in 0.1 M PB and incubated for 10 min in 0.05% H2O2 in 0.1 M PBS. Sections were then washed in 0.1 M PB with 0.1% Triton X-100 (PBT), blocked in 2% normal goat serum (NGS) in PBT for 1 h, and incubated in anti-DCX (1:10,000; guinea pig; Millipore, Billerica, MA) in 2% NGS in PBT for 24 h at 4°C. Sections were then incubated in a goat anti-guinea pig secondary antibody (1:200; Vector) in PBT for 1 h at RT and then in avidin-biotin horseradish peroxidase complex (1:250) in PBT for 1 h at RT. Horseradish peroxidase was visualized with DAB in a 3 M sodium acetate buffer containing 0.05% H2O2. Sections were washed, mounted on to Fisher Brand Plus slides, dried, dehydrated in increasing concentrations of alcohol (70%, 95%, and 100%), cleared in xylenes, and coverslipped with DPX Mountant.
The areal densities (cells per unit area) of DCX-positive cell numbers were quantified in both the upper and lower blades of the dentate gyrus. Cells were counted on a light microscope under a 40X objective (Nikon, Eclipse E400) using an ocular grid measuring 6,250μm2 (please see Figure 5F for approximate grid size and placement). Four bilateral counts were made for each brain section and averaged. The data are presented as mean number of DCX-positive cells per mm2.
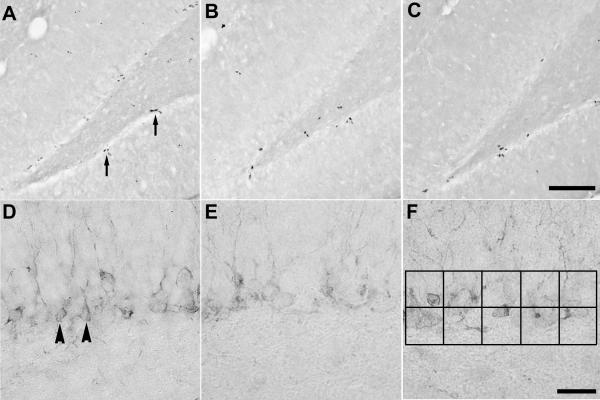
Figure 5.
Representative photomicrographs of BrdU- and DCX-positive cells in the dentate gyrus. Photomicrographs on the top row represent BrdU-positive cells in (A) intact 30 day old males, (B) intact 60 day old males, and (C) 60 day old males that had been gonadectomized at 30 days of age. Photomicrographs on the bottom row represent DCX-positive cells in (D) intact 30 day old males, (E) intact 60 day old males, and (F) 60 day old males that had been gonadectomized at 30 days of age. Arrows in panel A are indicating examples of clusters of BrdU-positive cells, while arrowheads in panel D are indicating examples of DCX-positive cells. The black squares in panel F represent the approximate area (6,250μm2) and placement of the ocular grid used to quantify DCX-positive cell numbers. Scale bars = 100μm and 25μm for the top and bottom row of photomicrographs, respectively.
Testosterone Radioimmunoassay
A testosterone radioimmunoassay was conducted using a Coat-A-Count 125I RIA kit (Siemens Medical Solutions Diagnostics; Malvern, PA) and performed as indicated by the supplier. All samples were run in duplicate and the lower limit of detectability was 0.1 ng/ml and intraassay coefficient of variation was 5.8%.
Statistical Analyses
All data are presented as the mean ± S.E.M. One-way ANOVAs were used for statistical analyses, and significant effects were further analyzed with Tukey’s Honestly Significant Difference tests. Differences were considered significant when P < 0.05. All statistical analyses were performed using GraphPad Prism software (version 5.04).
RESULTS
Experiment 1
As expected, there were significant effects of age on body weight and plasma testosterone levels (F (2,16) = 764.1 and 16.54, respectively, P < 0.05), such that body weights (Table 1) and testosterone concentrations (Table 2) were increased significantly at each of the ages tested. These data confirm that male rats at 30, 45, and 90 days of age are indeed at disparate stages before, during, and after pubertal development.
Table 1.
Mean (± SEM) body weight (g) of rats in experiments 1 and 2. Values that share the same letter are not significantly different from each other.
| Experiment 1 | 30d | 45d | 90d |
|---|---|---|---|
|
|
|||
| 89.3±1.4a | 203.8±4.9b | 506.6±13.8c | |
| Experiment 2 | 30d | 60d | 60d SHAM | 60d GDX |
|---|---|---|---|---|
|
|
||||
| 99.6±5.7a | 349.2±6.0b | 359.2±7.8b | 322.6±9.9c | |
Table 2.
Mean (± SEM) plasma testosterone (ng/ml) levels of rats in experiments 1 and 2. Values that share the same letter are not significantly different from each other.
| Experiment 1 | 30d | 45d | 90d |
|---|---|---|---|
|
|
|||
| 0.12±0.02a | 1.63±0.29b | 3.12±0.63c | |
| Experiment 2 | 30d | 60d | 60d SHAM | 60d GDX |
|---|---|---|---|---|
|
|
||||
| 0.13±0.02a | 1.87±0.30b | 1.49±0.39b | 0.10±0.00a | |
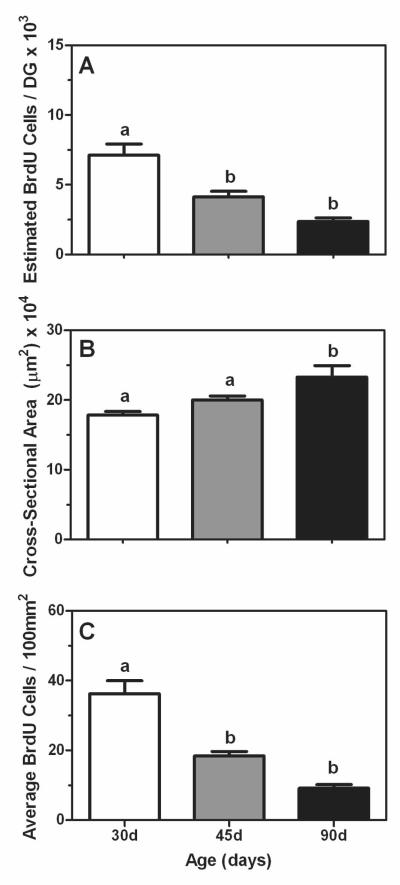
The estimated number of BrdU-positive cells in the dentate gyrus was significantly affected by age (F (2,15) = 17.68, P < 0.05) such that 30 day old animals had a greater number of cells than animals at either 45 or 90 days of age (Figure 2A). Though there was a decrease in cell numbers between the 45 and 90 day old animals, this difference was not statistically significant. Average cross-sectional area of the dentate was also significantly affected by age (F (2,15) = 7.57, P < 0.05). Specifically, 30 and 45 day old animals had significantly smaller cross-sectional areas compared to 90 day old animals (Figure 2B). As these morphological data indicate the area of the dentate is changing during the ages we examined, we further investigated whether the age-related differences in the estimated number of BrdU-positive cells could be accounted for by the changes in cross-sectional area. However, even when expressing the number of BrdU-positive cells per unit area (i.e.,100mm2) we found that 30 day old animals still had a significantly greater number of BrdU cells compared to either the 45 or 90 day old animals (F (2,15) = 29.76, P < 0.05; Figure 2C).
Figure 2.
Mean (±SEM) estimated number of BrdU-positive cells (A), average cross-sectional area (μm2; B), and average number of BrdU-positive cells per 100mm2 (C) in the dentate gyrus of prepubertal (30 days of age, white bars), mid-pubertal (45 days of age, gray bars), and adult (90 days of age, black bars) male rats. Bars that share the same letter are not significantly different from each other.
Using DCX immunohistochemistry, we were able to show these age-dependent changes in cellular proliferation were paralleled by changes in neurogenesis, such that 30 day old animals had a significantly greater number of DCX-positive cells per mm2 compared to the 45 and 90 day old animals (F (2,16) = 12.45, P < 0.05; Figure 3).
Figure 3.
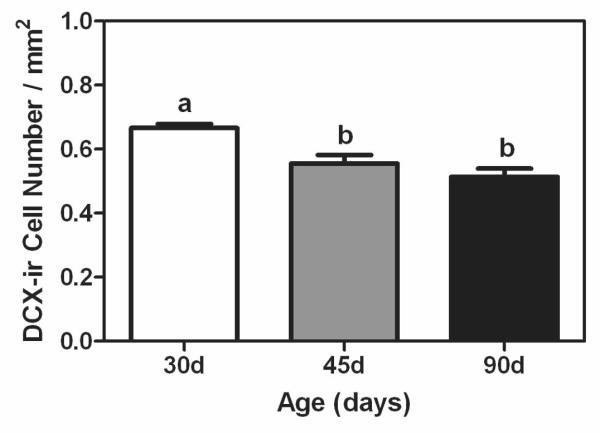
Mean (±SEM) number of DCX-positive cells per mm2 in dentate gyrus of prepubertal (30 days of age, white bar), mid-pubertal (45 days of age, gray bar), and adult (90 days of age, black bar) male rats. Bars that share the same letter are not significantly different from each other.
Experiment 2
One-way ANOVA revealed significant differences between the body weights of these intact and surgically manipulated animals (F (3,22) = 265.3, P < 0.05). Specifically, similar to the data in experiment 1, we found that 30 day old males weighed significantly less than the 60 day old males, independent of their gonadal status (Table 1). However, we also found that 60 day old males that had been gonadectomized at 30 days of age weighed significantly less than the 60 day old males that were intact or sham castrated (Table 1). There was also a difference in plasma testosterone (F (3,22) = 16.81, P < 0.05) in that 30 day old intact and 60 day old gonadectomized males had significantly lower testosterone levels than the 60 day old intact and sham gonadectomized animals (Table 2).
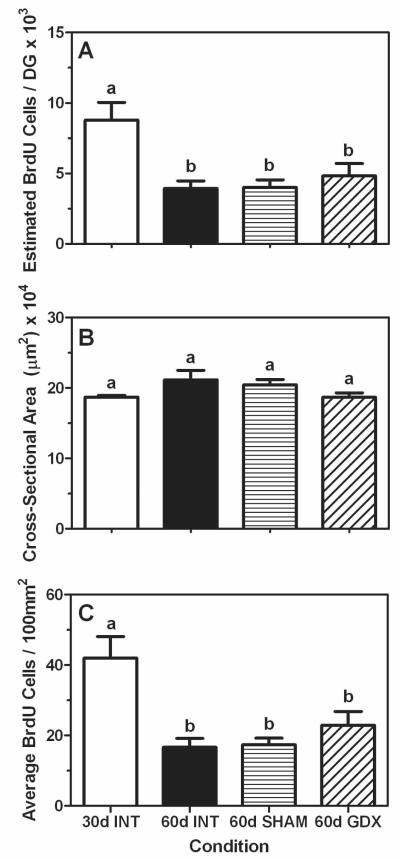
Though we again found a significant pubertal-related decline in estimated BrdU-positive cell numbers (F (3,20) = 6.80, P < 0.05), such that gonadally intact 30 day old animals had a significantly greater number of cells compared to gonadally intact 60 day old males, we did not find any significant differences between intact, sham-gonadectomized, or gonadectomized 60 day old males (Figure 4A and Figure 5A-5C). Furthermore, because there were no significant differences in cross-sectional area per section of the dentate gyrus in any of the groups (P = 0.12, Figure 4B), we observed the same mode of results as those from the cells per section analysis when the data are expressed as BrdU-positive cells per 100mm2 (F (3,20) = 8.25, P < 0.05, Figure 4C).
Figure 4.
Mean (±SEM) estimated number of BrdU-positive cells (A), average cross-sectional area (μm2; B), and average number of BrdU-positive cell number per 100mm2 (C) in dentate gyrus of intact prepubertal (30 days of age INT, white bars), intact late-pubertal (60 days of age INT, black bars), sham-gonadectomized late-pubertal (SHAM, 60 days of age, bars with horizontal hash lines), and gonadectomized (GDX, 60 days of age, bars with diagonal hash lines) male rats. Bars that share the same letter are not significantly different from each other.
Similar to experiment 1, our DCX data parallel the BrdU data such that the 30 day old animals had a significantly greater number of DCX-positive cells per mm2 compared to all of the groups at 60 days of age, regardless of their gonadal status (F (3,19) = 14.98, P < 0.05; Figure 6 and Figure 5D-5F).
Figure 6.
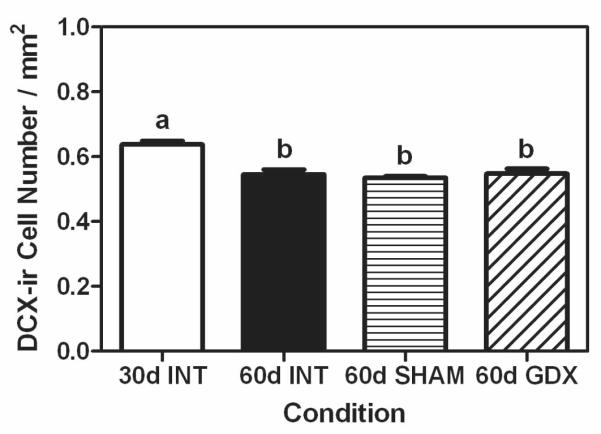
Mean (±SEM) number of DCX-positive cells per mm2 in dentate gyrus of prepubertal (30 days of age INT, white bars), intact late-pubertal (60 days of age INT, black bars), sham-gonadectomized late-pubertal (SHAM, 60 days of age, bars with horizontal hash lines), and gonadectomized (GDX, 60 days of age, bars with diagonal hash lines) male rats. Bars that share the same letter are not significantly different from each other.
DISCUSSION
These results indicate that the significant decline in cellular proliferation and neurogenesis in the dentate gyrus during puberty are independent of the changes in gonadal hormones observed during this stage of development. Thus, while some modifications of the pubertal brain are clearly dependent on the exposure to gonadal hormones, such as the increase in medial amygdala volume (Cooke et al., 2007; Ahmed et al., 2008), cell pruning in the visual cortex (Nunez et al., 2002), and decreases in androgen receptor expression in the hypothalamus (Romeo et al., 2000), our data indicate that certain neurodevelopmental processes can occur during puberty in the absence of gonadal hormones. Similar to our current findings, Andersen and colleagues reported that the pubertal-related decrease in dopamine receptor density in the striatum of male rats is also independent of the pubertal rise in gonadal hormones (Andersen et al., 2002). Therefore, it appears that factors in addition to gonadal hormones shape the pubertal brain, but the specific intracellular and/or extracellular signals that mediate the substantial change in neurogenesis during puberty remain unknown.
Given that gonadal hormones can modulate hippocampal neurogenesis in adulthood (Galea et al., 2006; Galea, 2008), that androgen receptors are present in the dentate gyrus (Tabori et al., 2005; Hajszan et al., 2007; Feng et al., 2010), and testosterone levels and neurogenesis during puberty are temporally related, it was surprising that we found no effect of gonadectomy on either cell proliferation or neurogenesis. However, an earlier experiment did indicate that although testosterone could increase the survival of newly born neurons in the dentate gyrus of adult male rats, testosterone has little effect on cellular proliferation (Spritzer and Galea, 2007). Similarly, the stress-induced suppression of hippocampal cellular proliferation in male rats following social defeat could not be reversed by supplementing the socially defeated adults with exogenous testosterone (Buwalda et al., 2010). Thus, along with our current data, it appears that testosterone does not influence hippocampal cellular proliferation in males during adulthood (Spritzer and Galea, 2007; Buwalda et al., 2010) or during pubertal development (experiment 2). Our present data also extend the previous observation that the presence or absence of the pubertal rise in gonadal hormones does not significantly affect the survival of newly born cells in the dentate gyrus of either male or female rats (Ahmed et al., 2008).
Because DCX is a marker of immature neurons (Rao and Shetty, 2004; von Bohlen und Halbach, 2007) and our BrdU and DCX data parallel one another, these results suggest that the changes in cellular proliferation during puberty are in part reflective of changes in the birth of new neurons. Therefore, it will be important to further investigate the impact of both pubertal development and gonadal hormones on post-proliferative steps of neurogenesis, such as migration and synaptic integration. It would also be interesting to assess the proportional contribution of glial and/or vascular cells to these pubertal-related changes in respect to the number of proliferating cells and whether these types of cells are differentially affected by the presence or absence of gonadal hormones during this developmental transition.
In addition to the pubertal decrease in BrdU- and DCX-positive cell numbers, we also observed a slight, but significant, increase in the cross-sectional area of the dentate gyrus between 45 and 90 day old intact males (experiment 1). These results are agreement with a previous study that reported that various sub-fields of the hippocampal formation, including the dentate gyrus, show continued growth between 56 and 77 days of age (Isgor et al., 2004). Though it is paradoxical that the size of a brain region increases while the cells within that same region exhibit a decrease in their proliferative potential, these data indicate that the pubertal development of the dentate gyrus goes beyond changes in just neurogenesis. Future studies will need to address the mechanisms that mediate this structural change in the dentate gyrus and whether these mechanisms require the presence of gonadal hormones.
It is interesting to note that a recent study showed that chronic stress, and subsequent exposure to stress-related hormones, leads to suppressed neurogenesis in adults, but increases in adolescent animals (Toth et al., 2008). These data suggest that particular neurochemicals may have disparate effects on the brain before and after pubertal maturation. Along these lines, brain derived neurotrophic factor (BDNF) has been demonstrated to promote neurogenesis in the hippocampal formation of rats both in vitro and in vivo (Scharfman et al., 2005; Li et al., 2009). However, a previous study in rats noted that hippocampal levels of BDNF protein increase during puberty (Kozisek et al., 2008). Hence, the inverse relationship between neurogenesis and BDNF during this stage of development suggests that factors that regulate neurogenesis in adulthood may not operate in the same manner as they do during puberty.
In conclusion, we did not find support for our hypothesis that the pubertal rise in gonadal hormones was responsible for the observed decrease in hippocampal cell proliferation and neurogenesis during puberty. It appears, therefore, that factors other than gonadal hormones shape the pubertal change in hippocampal neurogenesis, but what these factors are remain unknown. However, given the role of neurogenesis in such processes as learning and memory, emotionality and responsiveness to antidepressants in adulthood (Shors et al., 2001; Santarelli et al., 2003; Samuels and Hen, 2011), it will be imperative to continue to study factors that alter neurogenesis during puberty and the ramifications of these changes on a developing organism’s neurobehavioral function.
Acknowledgements
We would like to thank Page Buchanan for excellent animal care. This work was supported in part from a grant to Barnard College from the Undergraduate Science Education Program of the Howard Hughes Medical Institute.
REFERENCES
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11:995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Thompson RF, Krenzel E, Teicher MH. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology. 2002;27:683–691. doi: 10.1016/s0306-4530(01)00069-5. [DOI] [PubMed] [Google Scholar]
- Barker JM, Galea LAM. Repeated estradiol administration alters different aspects of neurogenesis and cell death in the hippocampus of female, but not male, rats. Neuroscience. 2008;152:888–902. doi: 10.1016/j.neuroscience.2007.10.071. [DOI] [PubMed] [Google Scholar]
- Buwalda B, van der Borght K, Koolhaas JM, McEwen BS. Testosterone decrease does not play a major role in the suppression of hippocampal cell proliferation following social defeat stress in rats. Physiol Behav. 2010;101:719–725. doi: 10.1016/j.physbeh.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Duhoux S, Cohen MM. Adolescence: what do transmission, transition, and translation have to do with it? Neuron. 2010;67:749–760. doi: 10.1016/j.neuron.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke BM. Synaptic reorganization of the medial amygdala during puberty. J Neuroendocrinol. 2010;23:65–73. doi: 10.1111/j.1365-2826.2010.02075.x. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Jordan CL, Breedlove SM. Pubertal growth of the medial amygdala delayed by short photoperoids in the Siberian hamster, Phodopus sungorus. Horm Behav. 2007;52:283–288. doi: 10.1016/j.yhbeh.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesce E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Neuroradiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Cowen DS, Takase LF, Fornal CA, Jacobs BL. Age-dependent deline in hippocampal neurogenesis is not altered by chronic treatment with fluoxetine. Brain Res. 2008;1228:14–19. doi: 10.1016/j.brainres.2008.06.059. [DOI] [PubMed] [Google Scholar]
- Crews FT, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan M, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cerebral Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Feng Y, Weijdegard B, Wang T, Egecioglu E, Fernandez-Rodriguez J, Huhtaniemi I, Stener-Victorin E, Billig H, Shao R. Spatiotemporal expression of androgen receptors in the female rat brain during the oestrus cycle and the impact of exogenous androgen administration: a comparison with gonadally intact males. Mol Cell Endocrinol. 2010;321:161–174. doi: 10.1016/j.mce.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Galea LAM. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev. 2008;57:332–341. doi: 10.1016/j.brainresrev.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Galea LAM, Spritzer MD, Barker JM, Pawluski JL. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus. 2006;16:225–232. doi: 10.1002/hipo.20154. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the NY Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Prog Neuro-Psychopharmacol Biol Psychiat. 1997;21:1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, Lenroot RK. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J Am Acad Child Adol Psychiat. 2009;48:465–470. doi: 10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4-18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rappoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, Milner TA, Leranth C. Sex steroids and the dentate gyrus. Prog Brain Res. 2007;163:399–415. doi: 10.1016/S0079-6123(07)63023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Crews FT. Neurogenesis decreases during brain maturation from adolescence to adulthood. Pharmacol Biochem Behav. 2007;86:327–333. doi: 10.1016/j.pbb.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Joels M, Lucassen PJ. Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamic-pituitary-adrenal axis activation. Neurobiol Aging. 2004;25:361–375. doi: 10.1016/S0197-4580(03)00090-3. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Yang L, van Kooy J, Santollo J, Shors TJ. Prozac during puberty: distinctive effects on neurogenesis as a function of age and sex. Neuroscience. 2009;163:609–617. doi: 10.1016/j.neuroscience.2009.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–648. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114:2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- Juraska JM, Markham JA. The cellular basis for volume changes in the rat cortex during puberty: white and gray matter. Ann NY Acad Sci. 2004;1021:431–435. doi: 10.1196/annals.1308.058. [DOI] [PubMed] [Google Scholar]
- Kim Y-P, Kim H, Shin M-S, Chang H-K, Jang M-H, Shin M-C, Lee S-J, Lee H-H, Yoon J-H, Jeong I-G, Kim C-J. Age-dependence of the effect of treadmill exercise on cell proliferation in the dentate gyrus of rats. Neurosci Lett. 2004;355:152–154. doi: 10.1016/j.neulet.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Styner M, Short SJ, Lubach GR, Kang C, Hamer R, Coe CL, Gilmore JH. Maturational trajectories of cortical brain development through the pubertal transition: unique species and sex differences in the monkey revealed through structural magnetic resonance imaging. Cerebral Cortex. 2010;20:1053–1063. doi: 10.1093/cercor/bhp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozisek ME, Middlemas D, Bylund DB. The differential regulation of BDNF and TrkB levels in juvenile rats after four days of escitalipram and desipramine treatment. Neuropharmacology. 2008;54:251–257. doi: 10.1016/j.neuropharm.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci. 2011;31:10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Glasper ER, Gould E. Thymidine analog methods for studies of adult neurogensis are not equally sensitive. J Comp Neurol. 2009;517:123–133. doi: 10.1002/cne.22107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Jiang L, Zhang X, Chen H. In-vitro effects of brain-derived neurotrophic factor on neural progenitor/stem cells from rat hippocampus. Neuroreport. 2009;20:295–300. doi: 10.1097/WNR.0b013e32832000c8. [DOI] [PubMed] [Google Scholar]
- Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- McDonald HY, Wojtowicz JM. Dynamics of neurogenesis in the dentate gyrus of adult rats. Neurosci Lett. 2005;385:70–75. doi: 10.1016/j.neulet.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Sodhi J, Juraska JM. Ovarian hormones after postnatal day 20 reduce neuron number in the rat primary visual cortex. J Neurobiol. 2002;52:312–321. doi: 10.1002/neu.10092. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier Academic Press; New York: 2005. [Google Scholar]
- Perrin JS, Herve P-Y, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veilletter S, Pausova Z, Paus T. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. J Neurosci. 2008;28:9519–9524. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number of dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19:234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Lee Y, Stidd R, Long R, Greenstein D, Clasen L, Addington A, Gogtay N, Rapoport JL, Giedd JN. Longitudinally mapping the influence of sex and androgen signaling of the dynamics of human cortical maturation in adolescence. Proc Natl Acad Sci. 2010;107:16988–16993. doi: 10.1073/pnas.1006025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogol AD, Roemmich JN, Clark PA. Growth at puberty. J Adol Health. 2002;31:192–200. doi: 10.1016/s1054-139x(02)00485-8. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Diedrich SL, Sisk CL. Effects of gonadal steroids during pubertal development on androgen and estrogen receptor-a immunoreactivity in the hypothalamus and amygdala. J Neurobiol. 2000;44:361–368. [PubMed] [Google Scholar]
- Romeo RD, Sisk CL. Pubertal and seasonal plasticity in the amygdala. Brain Res. 2001;889:71–77. doi: 10.1016/s0006-8993(00)03111-5. [DOI] [PubMed] [Google Scholar]
- Samuels BA, Hen R. Neurogenesis and affective disorders. Eur J Neurosci. 2011;33:1152–1159. doi: 10.1111/j.1460-9568.2011.07614.x. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Anderson ML, Curlink DM, II, Nokia MS. Use it or lose it: how neurogenesis keeps the brain fit for learning. Behav Brain Res. doi: 10.1016/j.bbr.2011.04.023. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Mlesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Sizonenko PC. Physiology of puberty. J Endocrinol Invest. 1989;12:59–63. [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping adolescent brain maturation using structural magnetic resonance imaging. In: Romer D, Walker EF, editors. Adolescent Psychopathology and the Developing Brain. Oxford University Press; Oxford: 2007. pp. 55–84. [Google Scholar]
- Spear LP. The Behavioral Neuroscience of Adolescence. Norton; New York: 2010. [Google Scholar]
- Spritzer MD, Galea LAM. Testosterone and dihydrotestosterone, but not eastradiol, enhance survival of new hippocampal neurons in adult male rats. Dev Neurobiol. 2007;67:1321–1333. doi: 10.1002/dneu.20457. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Hagino H, Nohara S, Zhou S-Y, Kawasaki Y, Takahashi T, Matsui M, Seto H, Ono T, Kurachi M. Male-specific volume expansion of the human hippocampus during adolescence. Cerebral Cortex. 2005;15:187–193. doi: 10.1093/cercor/bhh121. [DOI] [PubMed] [Google Scholar]
- Tabori NE, Stewart LS, Znamensky V, Romeo RD, Alves SE, McEwen BS, Milner TA. Ultrastructural evidence that androgen receptors are located at extra nuclear sites in the rat hippocampal formation. Neuroscience. 2005;130:151–163. doi: 10.1016/j.neuroscience.2004.08.048. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Gould E. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J Comp Neurol. 2005;481:252–265. doi: 10.1002/cne.20385. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;15:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G, Levit O, Zangen A. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 2008;107:522–532. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O. Immunohistological markers for staging neurogenesis in adulth hippocampus. Cell Tissue Res. 2007;329:409–420. doi: 10.1007/s00441-007-0432-4. [DOI] [PubMed] [Google Scholar]