Abstract
OBJECTIVE
To compare continuous positive airway pressure (CPAP) vs. traditional mechanical ventilation (MV) at 24 h of age as predictors of neurodevelopmental (ND) outcomes in extremely low birth weight (ELBW) infants at 18-22 mo corrected gestational age (CGA).
METHODS
Infants ≤ 1000g birth weight born from January 2000 through December 2006 at two hospitals at the Cincinnati site of the National Institute of Child Health and Human Development Neonatal Research Network were evaluated comparing CPAP (N = 198) vs. MV (N = 109). Primary outcomes included the Bayley Score of Infant Development Version II (BSID-II), presence of deafness, blindness, cerebral palsy, bronchopulmonary dysplasia and death.
RESULTS
Ventilatory groups were similar in gender, rates of preterm prolonged rupture of membranes, antepartum hemorrhage, use of antenatal antibiotics, steroids, and tocolytics. Infants receiving CPAP weighed more, were older, were more likely to be non-Caucasian and from a singleton pregnancy. Infants receiving CPAP had better BSID-II scores, and lower rates of BPD and death.
CONCLUSIONS
After adjusting for acuity differences, ventilatory strategy at 24 h of age independently predicts long-term neurodevelopmental outcome in ELBW infants.
INTRODUCTION
Mechanical ventilation (MV) in an extremely low birth weight (ELBW) neonate, has been linked to poor neurodevelopmental (ND) outcomes1. Laptook et al.2 demonstrated that these risks were greater with increased duration of MV. Van Marter et al.3 showed the odds ratio for development of bronchopulmonary dysplasia (BPD) was more than twice as high if MV was initiated during day of life 1 than at day of life 4-7.
The authors determined whether MV's effect on ND is a result of its relationship to BPD and other associated neonatal comorbidities, or whether MV alone is an independent risk factor for poor ND outcomes. They compared the potential impact of MV vs. continuous positive airway pressure (CPAP) at 24 h of age on ND outcomes at 18-22 mo corrected gestational age (CGA), and analyzed other important neonatal morbidities to ensure that any relationship between mode of ventilation and ND outcome was independent of comorbid conditions.
MATERIAL and METHODS
Cohort data were extracted from the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) Generic Database4 which includes data from 15 different academic institutions on infants ≤ 1500g birth weight admitted to a network center within 14 days of birth or who were live-born but died in a network center delivery room. Follow up data were similarly obtained from the NICHD NRN ELBW Follow-Up Study at 18-22 mo CGA. Our cohort included infants born July 2000 - December 2006 at two participating hospitals at the Cincinnati site of the NICHD NRN, which were selected because the intimate knowledge of their neonatal intensive care unit (NICU) management strategies allowed the authors to determine with confidence that no unique institutional changes in management strategy for major neonatal morbidities occurred during the study period. The lack of definitive, evidence-based algorithm regarding the best mode of early respiratory support in the study cohort, meant the decision to place an infant on CPAP or MV was at the discretion of the attending neonatologist.
Infants included in the study: 1) Weighed ≤ 1000g at birth (e.g ELBW infants), 2) Were alive at 24 h of age, 3) Required either CPAP or MV at 24 h of age, 4) Had follow up ND assessment and Bayley Scale of Infant Development Version II (BSID-II)5 examinations recorded as part of the 18-22 mo CGA follow up visit. Infants placed on CPAP in the delivery room were eligible. Infants requiring immediate delivery room intubation or infants who did not require ventilatory support at 24 h of age were excluded.
During the study period 916 infants ≤1000g were delivered. One hundred fifty six died in the first 12 h of life, 393 were intubated during initial resuscitation and 60 required no ventilatory support at 24 h. Of the 307 infants meeting inclusion criteria at 24 h of life, 45 subsequently died in the NICU, 24 had no NRN follow up visit data recorded and 30 were missing BSID-II scores. 208 infants met all inclusion and exclusion criteria and were considered in the final assessment (Figure 1).
Fig. 1.
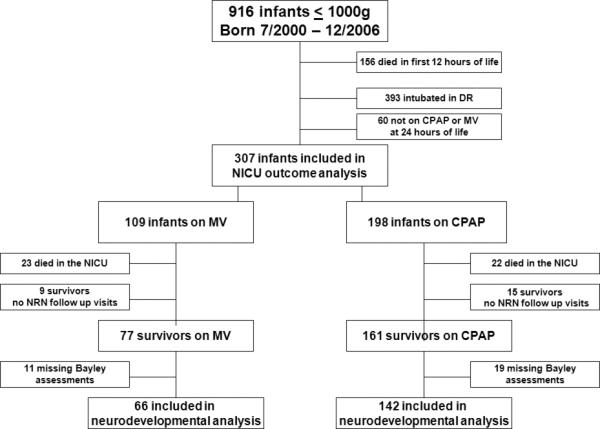
Flow diagram illustrating categorization of ventilatory groups
Primary ND outcomes included: BSID-II Mental Development Index (MDI) and Psychomotor Development Index (PDI), rates of CP, deafness, blindness, BPD and death. BSID-II examination was administered at the 18-22 mo CGA follow up visit by a nationally recognized gold standard examiner, and in the presence of the patients’ primary caretaker to ensure optimal performance. Patients who could not complete the BSID-II examination due to ND deficits received a score of 49. MDI or PDI scores > 85 were defined as no ND disability. MDI or PDI scores < 70 were defined as significant ND disability. Other primary outcomes were defined as follows: Blindness - bilateral lack of functional vision. Deafness - hearing loss requiring amplification in both ears. Cerebral palsy - static encephalopathy with delayed achievement of motor milestones, abnormalities in muscle tone in at least one extremity, and dysfunctional control of movement or posture. Cerebral palsy was further categorized as moderate or severe using the Amiel-Tison criteria6. BPD - need for supplemental oxygen when ≥36 wk CGA.
Secondary outcomes used to assess potential differences in common neonatal morbidities between groups included growth data at 36 wk CGA and 18-22 mo CGA, occurrence of seizure or use of seizure medications during initial neonatal hospitalization, length of NICU stay, rates of necrotizing enterocolitis (NEC), retinopathy of prematurity (ROP), Stage 3 or 4 ROP, patent ductus arteriosus (PDA) treated with indomethacin or by surgical ligation, grade III-IV intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL) and late onset sepsis. Secondary outcomes were defined as: NEC - Bell's classification ≥ IIa.7 ROP - international classification of ROP8, and ROP Stage 3 or 4 in either eye as documented by clinical ophthalmological examination. Late onset sepsis - occurring ≥ 72 h of life. IVH - Papile's criteria for grade ≥ III. PVL - determined by head ultrasound performed after 2 wk of age (If no head ultrasound was performed then data regarding PVL was not recorded). PDA - clinical or echocardiographic evidence for flow through the ductus.
Antenatal risk factors (Table 1) were defined as: Prolonged preterm rupture of membranes (PPROM) - rupture occurring > 24 h prior to delivery. Antenatal hemorrhage - maternal bleeding documented after 20 wk gestation in a pregnant woman with placenta previa, abruption or threatened abortion which resulted in vaginal bleeding or occult retro-placental clot. Antenatal antibiotics - antibiotics administered to the mother during the hospitalization that resulted in the delivery of the infant. Antenatal steroids - steroids given to the mother in an attempt to accelerate development of the fetal lungs in preparation for preterm delivery and extrauterine life. Tocolytics - medication given to the mother in an attempt to stop preterm labor. Gestational age was determined by best obstetric dating methods (last menstrual period or first trimester ultrasonography). Race was categorized as Caucasian or non-Caucasian due to the demographic composition of the referral area for the study hospitals. Trained research nurses obtained all growth measurements at pre-specified intervals.
Table 1.
Antenatal Risk Factors and Demographics
| CPAP N=198 | MV N=109 | P-value | |
|---|---|---|---|
| PPROM | 42 (21.2%) | 16 (14.7%) | 0.16 |
| Antenatal Hemorrhage | 36 (18.2%) | 25 (22.9%) | 0.32 |
| Antenatal Antibiotics | 125(63.1%) | 61 (56.0%) | 0.22 |
| Antenatal Steroids | 180 (90.9%) | 93 (85.3%) | 0.14 |
| Tocolytics | 104 (52.8%) | 65 (59.6%) | 0.25 |
| Cesarean section | 125 (63.1%) | 79 (72.5%) | 0.10 |
| Caucasian Race | 105 (53.0%) | 84 (77.1%) | <.0001 |
| Male Gender | 85 (42.9%) | 44 (40.4%) | 0.66 |
| 5 minute Apgar <5 | 5 (2.5%) | 13 (11.9%) | 0.001 |
| Multiple Gestation | 43 (21.7%) | 37 (33.9%) | 0.02 |
| Gestational age (wks) | 26.7 (1.9) | 26.1 (1.7) | 0.003 |
| Birth Weight (gm) | 810 (133) | 774 (133.5) | 0.02 |
| Birth OFC (cm) | 23.4 (1.7) | 23.4 (1.5) | 0.99 |
Categorical variables reported as N (%); Continuous variables reported as mean (SD)
PPROM = premature prolonged rupture of membranes; OFC = occipital-frontal circumference
Statistical Analysis
Unadjusted bi-variable analyses compared mechanical ventilation and CPAP groups to assess confounders, covariates and outcomes. Categorical variables were analyzed by χ2 and Fisher's Exact test. Continuous variables were analyzed with Student's t test. Multivariable logistic regression analyses assessed independent risk factors for outcomes of interest while controlling for any potential confounders. A stepwise approach to model selection included variables in the models only if they were significantly related to the outcome or the risk factor at a significance level of p < 0.20 in the bi-variable analyses (Tables 1, 2 and 3). Variables were retained in the model if they maintained a significance level of p<0.10. Because the number of events was limited for some outcomes, it was important to pick the most parsimonious regression model. The best fit models were confirmed using a combination of the Hosmer-Lemeshow test statistic, as well as the Deviance and Pearson's chi-square statistic. A variable for NICU site remained in all models to control for potential site/practice variability. Gestational age, birth weight or both remained in all models to control for level of prematurity. Final results were statistically significant if p <0.05.
Table 2.
Primary Outcomes by Mode of Ventilatory Support
| CPAP N = 142 | MV N = 66 | P - value | |
|---|---|---|---|
| PDI > 85 | 130 (91.6) | 51 (77.3) | 0.004 |
| PDI < 70 | 5 (3.5) | 7 (10.6) | 0.06* |
| Mean (SD) PDI scores | 95.7 (12.3) | 89.9 (16.8) | 0.02 |
| MDI > 85 | 90 (63.4) | 40 (60.6) | 0.70 |
| MDI < 70 | 10 (7.0) | 10 (15.1) | 0.06 |
| Mean (SD) MDI scores | 89.0 (14.4) | 87.5 (18.1) | 0.99 |
| BPD† | 80 (40.4) | 60 (55.1) | 0.01 |
| Death† | 22 (11.1) | 23 (21.1) | 0.02 |
| PDI < 70 or death‡ | 27 (16.5%) | 30 (33.7%) | 0.002 |
| MDI < 70 or death‡ | 32 (19.5%) | 33 (37.1%) | 0.002 |
| CP | 5 (3.1) | 8 (10.4) | 0.03* |
| Mod/Severe CP | 1 (0.6) | 3 (3.9) | 0.10* |
Reported as N (%).
No blindness occurred in either group. One case of hearing impairment occurred in each group
Fisher's Exact Test
CPAP group N=198, MV group N=109 to include infants who died prior to NICU discharge
CPAP group N=164, MV group N=89
Table 3.
Secondary Outcome Measures by Mode of Ventilatory Support
| CPAP N = 198 | MV N = 109 | P - value | |
|---|---|---|---|
| Total days MV @ 36wk CGA‡ | 2 (0-12.5) | 10 (5-38) | <.0001 |
| Given Surfactant | 47 (23.7%) | 101 (92.7%) | <.0001 |
| Length of stay in NICU‡ | 76 (55-98) | 77 (49-102) | 0.83 |
| NEC | 24 (12.1%) | 10 (9.2%) | 0.43 |
| ROP* | 125 (71.4%) | 72 (83.7%) | 0.03 |
| ROP Stage 3 or 4 either eye* | 22 (12.6%) | 16 (18.6%) | 0.19 |
| Late onset sepsis | 52 (26.3%) | 35 (32.1%) | 0.28 |
| IVH - Grade III-IV | 7 (3.5%) | 16 (14.7%) | 0.0004 |
| PVL | 2 (1.0%) | 6 (5.5%) | 0.03† |
| PDA | 88 (44.4%) | 64 (58.7%) | 0.02 |
| Indomethacin treatment | 74 (84.1%) | 55 (85.9%) | 0.75 |
| Surgical ligation | 11 (12.5%) | 5 (7.8%) | 0.35 |
| Among NICU survivors | CPAP N = 176 | MV N = 86 | P - value |
|---|---|---|---|
| Length of stay in NICU‡ | 76.5 (59-98.5) | 89 (74-106) | 0.004 |
| History of seizure activity | 4 (3.0%) | 3 (4.5%) | 0.69 |
| On seizure meds | 1 (0.9%) | 1 (1.6%) | 1.0 |
| 36 wk CGA wt (gm) | 2033 (436) | 1976 (414) | 0.35 |
| 36 wk CGA OFC (cm) | 31.0 (1.7) | 31.0 (1.6) | 0.78 |
| 18mo CGA wt (kg) | 10.73 (1.18) | 10.78 (1.34) | 0.77 |
| 18mo CGA OFC (cm) | 46.8 (1.5) | 46.9 (1.5) | 0.18 |
Categorical variables reported as N (%); Continuous variables reported as mean (SD)
Median and interquartile range reported
Among survivors: CPAP N=176 MV N=86
**Information available for CPAP group N=110 and MV group N=58
Fisher's Exact Test
NEC = necrotizing enterocolitis; ROP = retinopathy of prematurity; IVH = intraventricular hemorrhage; PVL = periventricular leukomalacia; PDA = patent ductus arteriosus; OFC = occipital-frontal circumference
RESULTS
Although many of the antenatal risk factors were similar between groups, the MV group was more likely than the CPAP group to be Caucasian, to have a 5 minute Apgar < 5, and to be the product of multiple gestation. Small statistically significant differences in weight and gestational age between groups were of questionable clinical significance (Table 1).
The CPAP group had improved neurodevelopment compared to the MV group as evidenced by fewer with MDI < 70 and PDI < 70 as well as more with PDI > 85 and a higher mean PDI. The number of PDI <70 showed a strong trend in favor of the CPAP group. Statistically significant differences in the rates of CP, BPD and death as well as in combined outcomes of MDI < 70 or death and PDI < 70 or death all favored the CPAP group. There were no statistically significant differences between groups regarding rate of deafness, blindness, moderate or severe CP, MDI score >85 or mean MDI score (Table 2).
Groups did not differ by length of NICU stay, rates of NEC or late onset sepsis, history of seizure activity or use of seizure medications, or by growth parameters postulated to have an impact on neurodevelopment7 either at 36 wk CGA or at the 18-22 mo ELBW follow-up visit. Rates of ROP were lower in the CPAP group but rates of Stage 3 or 4 ROP were not significantly different between groups. Rates of grade III-IV IVH and PVL were significantly lower in the CPAP group. Although the rate of PDA was lower in the CPAP group, rates of medical vs. surgical management of PDA were remarkably similar between groups. Usage of surfactant was lower in the CPAP group as was the total number of days of MV.
Multivariable logistic regression analyses assessed the independent relationship between ventilation mode and ND outcomes such as: MDI < 70, PDI <70, MDI < 70 or death and PDI < 70 or death, as well as non ND outcomes of BPD and BPD or death. After controlling for factors found to be statistically significant in the multivariable regression model the mode of ventilation was an independent risk factor for composite outcomes of MDI < 70 or death and PDI < 70 or death (Table 4). Infants receiving MV at 24 h of life were twice as likely as their CPAP counterparts to have the composite outcome of MDI < 70 or death and the composite outcome of PDI < 70 or death In addition to the effects on ND composite outcomes, MV at 24 h of life was independently associated with a greater than twofold increase in odds of developing BPD or (the composite outcome of BPD or death (Table 4).
Table 4.
Multivariable Logistic Regression for MV vs. CPAP
| Outcome | aOR | 95% CI | P-value | N |
|---|---|---|---|---|
| MDI <70a | 1.6 | 0.52, 4.76 | 0.42 | 208 |
| PDI <70b | 2.4 | 0.61, 9.41 | 0.21 | 208 |
| BPDc | 2.3 | 1.15, 4.42 | 0.02 | 262 |
| MDI <70 or deathd | 2.2 | 1.07, 4.67 | 0.03 | 253 |
| PDI <70 or deathe | 1.9 | 0.92, 3.79 | 0.09 | 253 |
| BPD or deathf | 2.3 | 1.21, 4.33 | 0.01 | 307 |
aOR = adjusted odds ratio reported for each outcome regarding adjusted comparison of MV vs. CPAP
Variables included in model: gestational age, sepsis, PDA, multiple birth, NICU site.
Variables included in model: gestational age, sepsis, race, PDA, NICU site.
Variables included in model: gestational age, birth weight, gender, PROM, highest % FiO2 (24 hrs), PDA, sepsis, NICU site.
Variables included in model: gestational age, birth weight, gender, 5-minute Apgar, sepsis, BPD, multiple birth, NICU site.
Variables included in model: birth weight, gender, 5-minute Apgar, sepsis, antenatal steroids, multiple birth, NICU site.
Variables included in model: gestational age, birth weight, gender, highest % FiO2 (24 hrs), PDA, sepsis, NICU site.
DISCUSSION
Safety and efficacy of early CPAP intervention have been established in prior studies9-11. More recent, prospective, randomized controlled studies by Morley and Finer12-13 provide additional evidence of the safety of delivery room CPAP intervention, however they lack information regarding long-term ND outcomes. Our study complements the Morley study by only analyzing those infants who did not require immediate intubation for resuscitation. However, our study challenges the results of the Morley and Finer studies by finding a significant decrease in mortality and in BPD in the CPAP group. Several studies in the literature support our findings that early CPAP provides a reduction in BPD14. In a recent retrospective analysis, early CPAP intervention while avoiding mechanical ventilation led to improved developmental quotients in the CPAP group at 18 mo CGA. Other beneficial trends were noted in the CPAP group including decreased rates of IVH, and improved length and OFC10. Our results support the reduction of BPD with early CPAP as well as suggest a potential long term ND benefit
Our ability to equalize acuity of illness between MV and CPAP groups at 24 h of age was inherently limited by the retrospective study design. Great consideration was given to remediate this weakness. Excluding infants who required immediate intubation at birth, as well as those who did not require respiratory support at 24 h of age, intentionally removed the groups of infants at highest and lowest risk for poor ND, biasing the study toward the null hypothesis. These exclusion criteria resulted in ventilatory groups that were similar in many prenatal risks. Statistically significant differences in gestational age (4 day difference between groups) and birth weight (one ounce difference between groups) were felt to be of minimal clinical consequence. Five minute Apgar scores also differed between groups but the subjective nature of the score as well as components that are typically scored lower in a premature and/or extremely low birth weight population regardless of level of acuity (tone, irritability, color) mean that a low Apgar score is limited in predicting morbidity, mortality and long term outcomes15.
Other studies of neonatal outcomes have frequently utilized neonatal acuity scoring models. We chose not to pursue this option for several reasons. First, our data were insufficient to complete the most recently modified Clinical Risk Index for Babies (CRIB-II)16, or the Score for Neonatal Acute Physiology Perinatal Extension Version II (SNAPPE-II)17. More importantly, even if complete data were available for these acuity scoring models, the models were designed to predict in-hospital morality, not long-term morbidity and neurodevelopment and they were validated for use in infants that weigh more and are greater gestational age than our cohort. Since these models were not validated in our study population nor are they validated when partitioned into individual components, they were not included in the final study analysis.
The present study suggests that effects of early ventilation choice on neurodevelopment are independent of many known antenatal and neonatal risk factors for poor outcome. Biologically plausible explanations for the benefits of CPAP are found in animal studies. Loeliger, et al.18 compared brain histopathology of premature baboons managed with mechanical ventilation and either early or delayed weaning to CPAP to normal gestation controls and found significant histological differences in the white matter of the premature brains. Pathologic damage including increased astrocytic density, capillary cuffing and persistent radial glia, increased proportionally with length of mechanical ventilation. These changes result when cytokines and other inflammatory mediators are released presumably in response to mechanical ventilation.
This inflammatory hypothesis is supported by evidence that activated phagocytes are higher in the blood of preterm mechanically ventilated infants than in their non-ventilated controls19. Further evidence supporting the inflammatory hypothesis exists directly and indirectly in the literature. Capoluongo et al.20 when comparing mechanical ventilation to high frequency oscillatory ventilation, demonstrated that serum inflammatory cytokines were elevated in the mechanically ventilated group reinforcing the idea that cytokine release is promoted by ventilator associated lung injury. Serum cytokines could thus act on distal targets such as the developing brain potentially explaining the association between MV and ND.
CONCLUSION
The nature of retrospective study design does not allow for perfect matching of study cohorts. However, our groups were generally well balanced in terms of known antenatal risk factors for poor ND outcome. Where statistically significant differences between groups were noted, multivariable logistic regression applied to clarify the relationship between variables showed that the differences between CPAP and MV groups in composite outcomes of MDI < 70 or death, and PDI < 70 or death favored the CPAP group independent of other known neonatal morbidities. This finding supports the present hypothesis that ventilatory modality at 24 h of age independently predicts long term neurodevelopmental outcome in extremely low birth weight infants at 18-22 mo CGA follow up.
The authors are hopeful that future prospective randomized controlled trials comparing CPAP and MV and including detailed ND analysis will provide additional confirmation that CPAP is the preferred ventilatory strategy whenever possible in the early neonatal period.
Acknowledgments
Funding: Data collection funded by grant 5U10HD027853; Eunice Kennedy Shriver National Institute for Child Health and Human Development
Footnotes
Disclosure/Conflict of Interest: Study authors have nothing to disclose in regards to this study.
Human Subject Protection The study design was reviewed and approved by the Institutional Review Board of Cincinnati Children's Hospital Medical Center, Cincinnati, OH. 45229 USA
Contributors:
All listed authors were involved in the study design, data analysis, and manuscript preparation for this study.
Conflict of Interest: None
Role of Funding Source: None
REFERENCES
- 1.Walsh MC, Morris BH, Wrage LA, Vohr BR, Poole WK, Tyson JE, et al. Extremely low birthweight neonates with protracted ventilation: mortality and 18-month neurodevelopmental outcomes. J Pediatr. 2005 Jun;146(6):798–804. doi: 10.1016/j.jpeds.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 2.Laptook AR, O'Shea TM, Shankaran S, Bhaskar B. Adverse neurodevelopmental outcomes among extremely low birth weight infants with a normal head ultrasound: prevalence and antecedents. Pediatrics. 2005 Mar;115(3):673–80. doi: 10.1542/peds.2004-0667. [DOI] [PubMed] [Google Scholar]
- 3.Van Marter LJ, Allred EN, Pagano M, Sanocka U, Parad R, Moore M, et al. Do clinical markers of barotrauma and oxygen toxicity explain interhospital variation in rates of chronic lung disease? The Neonatology Committee for the Developmental Network. Pediatrics. 2000 Jun;105(6):1194–201. doi: 10.1542/peds.105.6.1194. [DOI] [PubMed] [Google Scholar]
- 4.Wright LL, McNellis D. National Institute of Child Health and Human Development (NICHD)-sponsored Perinatal Research Networks. Semin Perinatol. 1995 Apr;19(2):112–23. doi: 10.1016/s0146-0005(05)80031-x. [DOI] [PubMed] [Google Scholar]
- 5.Bayley N. Bayley Scales of Infant Development-II. Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- 6.Amiel-Tison C. Neuromotor status. In: Taeusch HW, Yogman MW, editors. Follow-up management of the high-risk infant. Little, Brown and Company; Boston, MA: 1987. [Google Scholar]
- 7.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006 Apr;117(4):1253–61. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 8.The Committee for the Classification of Retinopathy of Prematurity An International Classification of Retinopathy of Prematurity. Arch Ophthalmol. 1984;102(8):1130–4. doi: 10.1001/archopht.1984.01040030908011. [DOI] [PubMed] [Google Scholar]
- 9.Lindner W, Pohlandt F. Oxygenation and ventilation in spontaneously breathing very preterm infants with nasopharyngeal CPAP in the delivery room. Acta Paediatr. 2007 Jan;96(1):17–22. doi: 10.1111/j.1651-2227.2006.00009.x. [DOI] [PubMed] [Google Scholar]
- 10.Wintermark P, Tolsa JF, Van Melle G, Forcada-Guex M, Moessinger AC. Long-term outcome of preterm infants treated with nasal continuous positive airway pressure. Eur J Pediatr. 2007 May;166(5):473–83. doi: 10.1007/s00431-006-0272-3. [DOI] [PubMed] [Google Scholar]
- 11.Narendran V, Donovan EF, Hoath SB, Akinbi HT, Steichen JJ, Jobe AH. Early bubble CPAP and outcomes in ELBW preterm infants. J Perinatol. 2003 Apr-May;23(3):195–9. doi: 10.1038/sj.jp.7210904. [DOI] [PubMed] [Google Scholar]
- 12.Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB. Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med. 2008 Feb 14;358(7):700–8. doi: 10.1056/NEJMoa072788. [DOI] [PubMed] [Google Scholar]
- 13.Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010 May 27;362(21):1970–9. doi: 10.1056/NEJMoa0911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel D, Greenough A. Does nasal CPAP reduce bronchopulmonary dysplasia (BPD)? Acta Paediatr. 2008 Oct;97(10):1314–7. doi: 10.1111/j.1651-2227.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- 15.American Academy of Pediatrics, Committee on Fetus Newborn, American College of Obstetricians Gynecologists, Committee on Obstetric Practice The Apgar Score. Pediatrics. 2006;117(4):1444–7. [Google Scholar]
- 16.De Felice C, Del Vecchio A, Latini G. Evaluating illness severity for very low birth weight infants: CRIB or CRIB-II? J Matern Fetal Neonatal Med. 2005 Apr;17(4):257–60. doi: 10.1080/14767050500072557. [DOI] [PubMed] [Google Scholar]
- 17.Gagliardi L, Cavazza A, Brunelli A, Battaglioli M, Merazzi D, Tandoi F, et al. Assessing mortality risk in very low birthweight infants: a comparison of CRIB, CRIB-II, and SNAPPE-II. Arch Dis Child Fetal Neonatal Ed. 2004 Sep;89(5):F419–22. doi: 10.1136/adc.2003.031286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loeliger M, Inder T, Cain S, Ramesh RC, Camm E, Thomson MA, et al. Cerebral Outcomes in a Preterm Baboon Model of Early Versus Delayed Nasal Continuous Positive Airway Pressure. Pediatrics. 2006;118(4):1640–53. doi: 10.1542/peds.2006-0653. [DOI] [PubMed] [Google Scholar]
- 19.Dammann O, O'Shea TM. Cytokines and perinatal brain damage. Clin Perinatol. 2008 Dec;35(4):643–63. v. doi: 10.1016/j.clp.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capoluongo E, Vento G, Santonocito C, Matassa PG, Vaccarella C, Giardina B, et al. Comparison of serum levels of seven cytokines in premature newborns undergoing different ventilatory procedures: high frequency oscillatory ventilation or synchronized intermittent mandatory ventilation. Eur Cytokine Netw. 2005 Sep;16(3):199–205. [PubMed] [Google Scholar]


