Abstract
Tissue macrophages (Mø) and dendritic cells (DC) are thought to derive from hematopoietic stem cells, which exist in the bone marrow and generate intermediate precursor populations with increasingly restricted lineage potentials. There exists several precursors committed to the Mø and DC lineages; these cells exhibit distinct tropism and function and respond differentially in pathophysiologic conditions. In this review, we consider experimental contexts in which Mø and DC responses in tissue are not only dictated by the local environment, but also by the quantity and quality of newly recruited lineage precursor cells. Consequently, we discuss whether therapeutic control of Mø and DC responses in tissue may be achieved through manipulation of their lineage precursors.
Key Words: Host defense, Immune response, Mononuclear phagocyte system
Introduction
Macrophages (Mø) and dendritic cells (DC) represent key components of the mononuclear phagocyte system and are critically important for mediating protective immune responses in tissues. These cells have noteworthy functions: they phagocytose infected or invading pathogens using Fc receptors [1]; they identify pathogens or cellular stress using pattern recognition receptors [2]; they shape inflammatory processes by secreting cytokines and other factors [3], and they play an essential role in adaptive immunity by presenting antigen to T cells [4]. Despite their protective roles, tissue Mø and DC also contribute actively to the progression of chronic inflammatory conditions. For example, Mø and DC exhibit complex regulatory functions mediated through growth factors, chemokines, cytokines, angiogenesis promoting factors and proteolytic enzymes, which can act to promote disease progression [5,6,7].
A critical question is whether it is possible to ablate deleterious Mø or DC responses while sparing protective ones. Answering this question requires knowledge of the mechanisms that control the unfolding of Mø and DC activity in vivo. To do so, extensive work is underway to identify how tissue-resident Mø and DC execute their functions locally, how they become activated and respond to their microenvironments, and perhaps more fundamentally, where they come from and what is the identity of their lineage precursors.
This review is focused on the in vivo dynamics of Mø and DC precursor cell responses in the mouse. It is motivated by the recent identification of discrete populations which derive from self-renewing hematopoietic stem cells (HSC) located in specialized niches of the bone marrow and relocate through the blood to constitute tissue-resident Mø and DC populations [8]. Remarkably, there are different precursors of Mø and DC and these cells appear to be endowed with distinct effector functions. Consequently, the qualities of Mø and DC in tissue may be influenced, at least in part, by the identity of the precursor cells from which they derive.
Initially, we will present information on the ontogeny of Mø and DC and on the migration of defined precursor cells in physiologic conditions. We will then review several pathologic conditions that alter the homeostasis of precursor populations. We will discuss how these alterations can affect the course of diseases and, consequently, whether the control of Mø and DC responses in tissue requires manipulation of their hematopoietic precursors.
Ontogeny and Function of Tissue Mononuclear Phagocytes
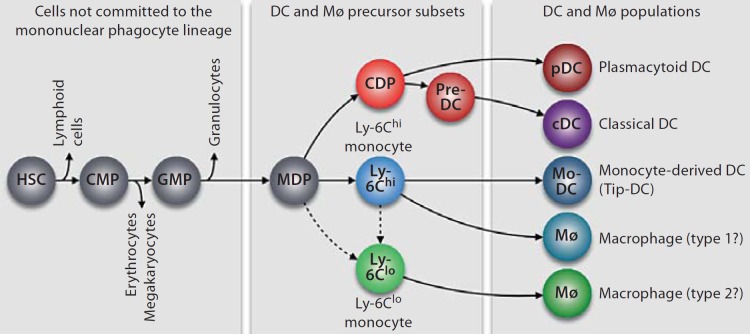
Most tissue Mø and DC in an adult individual originate from self-renewing HSC, which are located in specialized niches of the bone marrow [8,9]. HSC are thought to go through successive and irreversible developmental checkpoints, which lead to the generation of intermediate precursor populations that increasingly lose self-renewal capacity and become restricted to one lineage [10,11,12]. The last progenitor cell that Mø and DC are known to have in common is called an MDP (Mø and DC progenitor) [13]. Figure 1 illustrates that this cell can give rise to common dendritic cell progenitors (CDP) and Ly-6Chi and Ly-6Clo monocytes [14,15,16,17,18]. The molecular control of mononuclear phagocyte development from hematopoietic precursors is discussed in detail elsewhere [19,20].
Fig. 1.
Ontogeny of mononuclear phagocytes. The origin of DC and Mø populations can be tracked back to uncommitted HSC that reside in the bone marrow. Differentiation toward the mononuclear phagocyte lineage involves the generation of cell intermediates, which progressively lose their capacity to produce other lineages. Known cell intermediates include: common myeloid progenitors (CMP), granulocyte and Mø progenitors (GMP) and MDP. MDP represent the most committed progenitor that DC and Mø have in common. They give rise to pDC and cDC via a CDP and a pre-DC intermediate. MDP also give rise to two subsets of blood monocytes (Ly-6Chi and Ly-6Clo monocytes). Upon extravasation in tissue, Ly-6Chi monocytes can differentiate into inflammatory Mø or monocyte-derived DC (Mø/DC), which resemble cDC, or TNF-α and iNOS-producing DC (Tip-DC). Ly-6Clo monocytes, which may derive from Ly-6Chi monocytes or MDP, can differentiate into Mø in tissue.
The ontogenic relationship between the two monocyte subsets is currently debated. It is frequently proposed that Ly-6Clo cells derive from their Ly-6Chi counterparts. In line with this notion, cell depletion and cell transfer studies indicate not only that MDP can produce both Ly-6Chi and Ly-6Clo monocytes in vivo [15] but also that Ly-6Chi cells recirculate into the bone marrow where they can convert into Ly-6Clo cells [15,21,22]. However, the molecular cues that drive Ly-6Chi→Ly-6Clo monocyte conversion remain largely unknown. Alternatively, it has been proposed that Ly-6Clo monocytes can be generated independently of their Ly-6Chi counterparts [23] based on observations that the numbers of circulating Ly-6Clo monocytes were not affected after antibody-based depletion of Ly-6Chi monocytes. However, the existence of a direct precursor→product relationship between MDP and Ly-6Clo monocytes – i.e. without a Ly-6Chi monocyte intermediate – has not been established. Thus, additional studies are required to conclusively understand the relationship between monocyte subsets. The transcription factor Nur77 has been shown to control the production of Ly-6Clo monocytes, but neither of MDP nor Ly-6Chi monocytes [24]. This finding might become instrumental in future ontogenic studies.
There is recent indication that CDP and monocyte subtypes have distinct tropism and functional fates in vivo. Therefore, it appears important to investigate these cells separately and to define their respective impacts on Mø and DC responses. CDP are known to differentiate into classical DC (cDC) via a circulating pre-DC precursor and into plasmacytoid DC (pDC) [14,16,17,18,25]. CDP have lost the potential to generate monocytes; thus DC produced in steady state typically do not originate from a monocytic precursor. Ly-6Chi and Ly-6Clo monocytes themselves are heterogeneous and committed, at least to some extent, to separate functions [14]. Ly-6Chi monocytes produce tumor necrosis factor-α (TNF-α), interleukin-1 and proteolytic enzymes – and therefore are termed inflammatory monocytes – and can differentiate into DC under inflammatory conditions. In contrast, Ly-6Clo monocytes have been shown to promote healing and type 2 responses and to differentiate into Mø [19,26,27]. Ly-6Chi and Ly-6Clo monocytes show distinct tropisms, both in steady state and inflammation, as discussed in the sections below.
Tissue Mø and DC populations are distributed throughout the body but are typically found in higher numbers at strategic sites, such as the skin and mucosal surfaces, which are entry points for pathogens, and secondary lymphoid organs, where adaptive immune responses are initiated. Mø are specialized tissue-resident cells, which display phagocytic functions and clear dying cells and cellular debris in the steady state. Mø populations also express a complex repertoire of cell surface receptors, which are used to recognize pathogens and to mount effector responses. Mø are considered to be plastic cells because the responses that they mount depend on the cues that they receive: classic activation of Mø – e.g. by interferon-γ – generates type 1 inflammatory responses, whereas alternative activation – e.g. by interleukin-4 and interleukin-13 – induces type 2 responses; both types of activation are implicated in physiologic and pathologic processes [7,28,29,30]. The body also comprises heterogeneous populations of DC, which are described in detail elsewhere [31,32,33] and can be divided at least into cDC and pDC as mentioned above. cDC are critical for the induction of adaptive immunity. Some classical features of these cells are their capacity to migrate and concentrate in T cell areas in lymph nodes, form processes so as to probe their environment and induce CD4 and CD8 T cell-adaptive responses [34]. cDC are also responsible for maintaining T cell tolerance to self-antigens [35]. pDC are distinguishable from cDC based on their capacity to produce interferon-α and interferon-β, which are antiviral compounds mediating a wide range of effects; however, pDC can also present antigen and control T cell responses [36]. The study of Mø and DC has benefited tremendously from the generation of monoclonal antibodies, which recognize cell surface markers – e.g. F4/80 on Mø, CD11c on cDC and PDCA on pDC. However, the phenotypes of Mø and DC populations may not always be constant and/or fully discriminatory. Consequently, the separation between these cell types can be challenging when using phenotypic markers only [37]. Here, we will attempt to refer to Mø and DC based on their origins and functions.
Migration of Mø and DC Precursors in Physiologic Conditions
In steady state, monocytes and pre-DC produced in the bone marrow are released into the circulation and can eventually extravasate in distant lymphoid and nonlymphoid tissues. The process of extravasation typically associates with an irreversible program of differentiation and generates tissue-resident Mø and DC populations with specialized functions [8]. Pre-DC, Ly-6Chi and Ly-6Clo monocytes have different tropisms and fates in steady state. The use of intravital optical imaging techniques to study cell behavior in vivo [38] has indicated that Ly-6Clo monocytes continuously patrol the vasculature in the steady state [39]. The cells are also thought to redistribute widely throughout the body and participate in the renewal of resident Mø populations [19]. Some Mø have long tissue half-lives and thus their replenishment is slow. Ly-6Chi monocytes, for the most part, are believed to remain in the circulation or return to the bone marrow in the absence of inflammatory stimuli [40] (however, they can produce DC under defined inflammatory conditions as described in the next section). Pre-DC seed lymphoid and nonlymphoid tissues, where they continue to divide and differentiate into CD11chi MHCIIhi DC [25,41]. Tissue Mø rarely proliferate whereas DC can do so [42]. Importantly, however, notable exceptions to these rules exist and are discussed below.
Intestine
Mø and DC of the intestinal mucosa have two main functions. Not only do they participate in the maintenance of tolerance to harmless food antigens and commensal microorganisms, but they also respond to harmful pathogens by initiating and controlling innate and adaptive immune responses. Cataloging mononuclear cells from the lamina propria based on surface markers defines populations with different origins, turnover rates and functional properties [43]. For example, it has been proposed that CDP and pre-DC give rise to intestinal DC, which are characterized with a CD103+ CX3CR1– phenotype [41,44], whereas Ly-6Chi monocytes – which usually do not enter extramedullary tissue in steady state – generate lamina propria Mø, which are CD103– CX3CR1+ CD11b+ F4/80+ (and sometimes also referred to as DC) [15,44,45]. CD103– CX3CR1+ cells form transepithelial dendrites to the intestinal lumen, sample luminal antigens and likely modulate immune responses to invading pathogens locally [46]. Conversely, CD103+ DC migrate to lymph nodes and participate in the activation of T cell responses [47,48]. Thus, current knowledge suggests that mononuclear cells in the lamina propria have different origins and exhibit separate functions and that manipulation of either CDP or Ly-6Chi monocytes may be used to control selective intestinal immune activities.
Lung
The lung – together with the skin, which is discussed below – represents a major entry point to the body for environmental pathogens. Accordingly, Mø are found in numerous amounts in the alveolar space, they represent key contributors to pathogen clearance and can suppress local inflammatory processes [49,50,51,52]. In steady state alveolar Mø have been proposed to be constituted of circulating Ly-6Clo monocytes, which extravasate and differentiate in the lung parenchyma and then migrate into alveolar spaces [53]. Along with Mø, the lung is the home to pDC and at least two cDC populations (CD103– CD11bhi and CD103+ CD11blo). Both CD103– and CD103+ cDC have a relatively short lifespan in the lung and migrate to bronchial lymph nodes; however, they may have different origins and be endowed with different functions. For example, it has been proposed that Ly-6Clo monocytes can produce CD103– DC [54,55], which express MHC II and can activate antigen-specific CD4+ T cells [56], whereas pre-DC may produce CD103+ DC [41], which resemble lymphoid tissue CD8+ DC because they express MHC I, produce interleukin-12, and efficiently cross-present antigen to CD8+ T cells [32,57,58]. Interestingly, both nonlymphoid tissue CD103+ DC [59] and lymphoid tissue CD8+ DC [60] have an increased ability to interact with CD8 T cells because they express the chemokine receptor XCR1 while CD8+ T cells actively secrete the cognate ligand – XCL1 (lymphotactin 1) – upon antigen activation.
However, some questions remain concerning the origin, phenotypic plasticity and functional diversity of the diverse lung DC and Mø populations. For example, the studies mentioned above suggest that Ly-6Clo monocytes can produce both CD103– DC and Mø populations, which play opposing roles in lung pathology. Specifically, CD103– DC are involved in the perpetuation of airway effector responses and inflammation [56,61], whereas the pulmonary Mø may suppress inflammatory activities [49,50,51,52]. Thus, the mechanisms that control the fate of Ly-6Clo monocytes in tissue require further study.
Skin
Langerhans cells (LC) are DC that reside in the epidermis and serve to protect the host against the external environment [62]. In contrast to Mø and DC populations in most other tissues, most LC are not replaced with circulating precursors under steady-state conditions. LC remain of host origin in mice transplanted with congenic bone marrow and parabiotic mice fail to mix LC populations [63]. Instead, precursor cells that are recruited in the epidermis prior to birth have been postulated to give rise to LC [64]. It has been suggested that the recruited precursor cells expand locally, most notably during the first week after birth, and then renew locally and independently of the bone marrow throughout life under steady-state conditions via low-level proliferation. LC can also proliferate vigorously in response to changes in their microenvironment [64,65]. Only in response to severe injury are LC repopulated with circulating Ly-6Chi monocytes [66]. The unusual development and renewal of LC may occur because the epidermis is avascular and is not easily accessible by circulating precursors in adult life. Whether or not these findings have consequences for the regulation of skin immunity requires further investigation. For example, it appears important to determine whether Ly-6Chi monocyte-derived LC – which accumulate in an inflammatory context – differ functionally from preexisting LC, and whether harnessing one or the other population may serve to manipulate skin immune responses.
Brain
Microglia are Mø that reside in the brain and spinal cord. The cells are separated from the rest of the body by endothelial cells that form the blood-brain barrier [67]. They scavenge the central nervous system for damaged neurons and infectious agents; however, they are also associated with the pathogenesis of severe neurodegenerative diseases [68,69]. Similarly to LC but in contrast to many other Mø populations, microglia are thought to renew independently from the bone marrow under steady-state conditions [70] and arise from circulating Ly-6Chi monocytes only under critical pathological conditions [71]. Fate mapping studies indicate that microglia in the adult brain derive essentially from primitive hematopoietic cell progenitors that arise before embryonic day 8 in the extraembryonic yolk sack [72]. The yolk sack produces progenitor cells, which likely migrate to the brain via newly formed blood vessels. Resident microglia populations are then maintained locally throughout life. Consequently, it will be useful to define whether monocyte-derived Mø and preexisting microglia participate differently in the protection of the nervous system and/or in the induction or progression of neurodegenerative diseases. Emerging evidence suggests distinct functions for these cells. For example, the progression of experimental autoimmune encephalitis may be driven predominantly by Ly-6Chi monocyte-derived Mø [73]. Also, recovery from spinal cord injury likely involves blood-derived Mø, which display anti-inflammatory functions [74].
Spleen
The spleen contains various populations of Mø and DC [75]. These include SIGN-R1+ marginal zone Mø and CD169+ marginal metallophilic Mø, which are positioned in the reticular framework of the marginal zone and protect the host by ingesting and eliminating blood-borne pathogens, red pulp CD163+ Mø, which participate in blood filtration, the removal of old erythrocytes and the recycling of iron, and white pulp CD11chi DC, which are strategically located in T cell zones and initiate adaptive immunity by presenting blood-borne antigens to T cells. The known (and unknown) functions of these populations are reviewed in more detail in an accompanying review of the same issue. Elegant experiments using adoptive transfer and fate mapping of progenitors into nonirradiated animals showed that MDP produce splenic DC as well as marginal zone and red pulp Mø populations [13]. As expected for DC in general, splenic DC do not descend from monocytes [13,15] but instead from CDP, which are downstream of MDP and differentiate into cDC through a pre-DC intermediary [25]. However, the mechanisms involved in the orchestration and specialization of the various splenic Mø and DC functions remain largely unknown.
Unexpectedly, the resting spleen also accumulates MDP-derived circulating Ly-6Chi and Ly-6Clo monocytes, which do not differentiate into Mø or DC locally [76]. These bona fide monocytes are found in clusters located in the parenchyma of the red pulp, are phenotypically distinct from resident Mø and DC, and outnumber their blood equivalents. The splenic monocytes are important because they can reenter blood circulation and be deployed to distant sites in response to inflammation (see ‘Migration of Mø and DC Precursors in Pathophysiologic Conditions’). The spleen thus represents a unique monocyte reservoir that the body exploits to control inflammation. The mechanisms that retain splenic monocytes in an undifferentiated state as well as the compensatory mechanisms that regenerate the splenic niche after release of the reservoir cells remain to be defined.
Migration of Mø and DC Precursors in Pathophysiologic Conditions
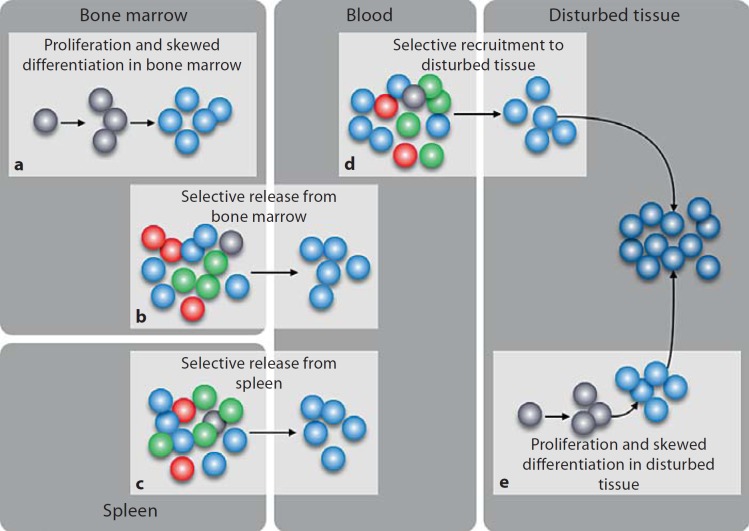
Pathophysiologic conditions are often associated with profoundly altered homeostasis of mononuclear phagocytes (fig. 2). Below we discuss several conditions in which precursors of Mø or DC are involved.
Fig. 2.
Alteration of Mø/DC precursor responses in pathophysiologic conditions. This diagram reviews five scenarios in which mononuclear phagocyte precursors are involved. The responses mediated by these cells can affect tissue Mø and DC both quantitatively and qualitatively, and eventually have an impact on the course of the disease. The quantity of Mø/DC is controlled at least in part by the number of precursor cells that are mobilized, whereas the quality of Mø/DC is affected when defined precursor populations are mobilized. The diagram, which depicts HSC (grey dots), Ly-6Chi monocytes (light blue dots), Ly-6Clo monocytes (green dots), CDP/pre-DC/DC (red dots) and Mø/DC (dark blue dots), highlights how Ly-6Chi monocyte responses can be amplified in vivo. However, similar mechanisms of action – which are not shown here – may apply to other precursor populations. a Dormant HSC residing in the bone marrow can be stimulated (e.g. by TLR ligands) to enter into a limited number of cell divisions and may differentiate toward the myloid lineage [85, 86]. This amplification process may produce Ly-6Chi monocytes. b Select bone marrow precursor populations can be released in circulation [88]. For example, CCR2 signaling triggers the release of bone marrow Ly-6Chi monocytes. c The spleen serves as a reservoir site for monocytes, which can be mobilized into circulation [76]. Defined stimuli may trigger the exit of Ly-6Chi monocytes selectively. d Select precursor populations can be recruited to tissue [3, 96, 101, 109]. For example, production of MCP-1 by a disturbed tissue acts to recruit Ly-6Chi monocytes. e A small fraction of HSC and progenitor cells exit the bone marrow and traffic through extramedullary tissues. These cells can be stimulated locally (e.g. by TLR ligands) to give rise to mononuclear phagocytes [92]. This amplification process may produce Ly-6Chi monocytes and Mø/DC in inflamed tissues.
Inflamed Lymph Node
Lymph nodes that drain infected or inflamed tissue are hubs of DC activity. DC that have captured antigen in tissue migrate to the draining lymph node via afferent lymph vessels and accumulate in T cell areas in a sequence of steps that involves chemokines and adhesion molecules [31]. Antigen presentation occurs through physical interaction with many motile scanning T cells and results in full-fledged activation of the rare lymphocytes, which present a T cell receptor that is specific for the antigen presented by the DC [77]. DC that reside in resting tissue normally derive from bone marrow CDP, which are developmentally distinct from monocytes [15,16,17]. Consequently, the professional antigen-presenting DC that migrate to draining lymph nodes should not have a monocyte origin.
Instead, it was recently demonstrated that gram-negative bacteria also recruit Ly-6Chi monocytes from blood to lymph nodes and that upon recruitment these cells become ‘authentic’ lymph node DC [33]. The mobilization of Ly-6Chi monocytes and their differentiation toward monocyte-derived DCs is potently activated by Toll-like receptor (TLR)4 signaling. Other TLR ligands might be less efficient at inducing differentiation of monocytes towards ‘authentic’ DC [33], although they may work at different concentrations. The route of entry of monocyte-derived DC into lymph nodes has yet to be defined [34]. The cells may first enter the inflamed nonlymphoid tissue adjacent to the lymph node and only then enter the latter using an afferent lymph vessel; alternatively, the cells may enter directly from blood via high endothelial venules. The monocyte-derived DC recruited to lymph nodes can be distinguished from CDP-derived DC because they selectively express the marker DC-SIGN/CD209a. These cells are nevertheless described as authentic DC because they concentrate in T cell areas, share many of the phenotypic attributes of DC and demonstrate efficient cross-presenting activity [33]. Monocyte-derived DC become the dominant antigen-presenting cell in lymph nodes in some experimental conditions and thus may represent a critical and long-lasting component of the DC response.
Infected and Injured Tissues
Whether tissue responds to insults with preexisting resident Mø or DC only, or instead recruits new circulating cells, is context dependent. The degree of tissue malfunction likely represents an important parameter that guides the magnitude of the inflammatory response [78]. A mild tissue malfunction may involve local Mø or DC only, whereas extensive malfunction will require additional cells, perhaps equipped with distinct effector functions. Studies of inflammatory reactions in the skin can be used to illustrate this point whereby an experimental model of atopic dermatitis triggers local proliferation of LC but does not involve infiltration by monocytes [64]; instead, both UV irradiation and graft-versus-host disease, which kill resident LC, stimulate massive infiltration of the epidermis by circulating Ly-6Chi monocytes [79].
It is well known that infected and injured tissues can accumulate inflammatory leukocytes, including Ly-6Chi monocytes. For example, the latter cells are recruited to Listeria monocytogenes-infected spleen and liver, where they mediate antimicrobial activity [3]. Upon recruitment to target sites, Ly-6Chi monocytes are called Tip-DC because they produce TNF-α and inducible nitric oxide synthase (iNOS) [80]. The release of these factors plays an essential role in the early control of infection by the bacteria and spares noninfected sites. Ly-6Chi monocytes also migrate toward sites infected by other pathogens, including influenza [81] and West Nile virus [82], as well as to injured sites [83,84] (see ‘Ischemic Myocardium’). The chemokine receptor CCR2 represents a prototypical cell surface molecule expressed by Ly-6Chi monocytes, which serves to guide the cells toward tissues that produce the inflammatory ligand MCP-1 [14].
Ly-6Clo monocytes normally patrol the resting endothelium of blood vessels but can rapidly extravasate to an abnormal tissue site as well and, in some cases, be mobilized even before their Ly-6Chi counterparts [39]. Gene expression profiling studies indicate that Ly-6Clo cells recruited early to a site of infection differentiate into Mø, whereas Ly-6Chi cells that follow acquire inflammatory DC functions [39]. Future studies should define the consequences of the initial Ly-6Clo monocyte response. Finally, circulating DC, and likely pre-DC, can also engage in rolling interactions with the vasculature and infiltrate tissue in response to inflammation [31].
The examples mentioned above indicate that inflammatory stimuli dictate tissue responses by recruiting select Mø and/or DC precursors to tissue. In addition, inflammatory stimuli can amplify defined precursor populations, even before their recruitment to tissue. For example, HSC express TLRs, which recognize bacterial or viral molecules. TLR ligation induces proliferation of quiescent bone marrow HSC [85] and skews maturation toward myeloid/mononuclear phagocyte lineages ex vivo [86]. TLR ligands can also act on HSC indirectly; bone marrow mesenchymal stem cells stimulated by TLR4 ligands produce MCP-1, which is a chemokine that guides neighboring Ly-6Chi (CCR2+) monocytes into the bloodstream [87]. The process thus augments the availability of Ly-6Chi monocytes in the circulation [88]. Finally, TLR4 ligands also stimulate HSC and progenitor cells outside the bone marrow. Indeed, a small fraction of HSC enter circulation each day to survey peripheral organs before returning to bone marrow cavities [89,90,91], and HSC transiting in tissue can respond actively to inflammatory signals by proliferating and producing DC locally [92]. It will be important to define to what extent such amplification processes further contribute to immune responses mediated by precursor cells in tissue. Also, since HSC and progenitor cells patrol healthy tissue, it will be interesting to test whether, in addition to their contribution during inflammation, they replenish tissue-resident Mø and DC in steady-state conditions.
Helminths Infection
An uncommon scenario occurs in response to infection with the helminth Litomosoides sigmodontis. Larvae transmitted into the skin by mites parasited by the nematode migrate to the thoracic pleural cavity where they mature, breed and induce a potent type 2 Mø response. In this context, local Mø amplification occurs without recruitment of circulating precursors to the pleural cavity; instead, the nematode stimulates rapid in situ proliferation of tissue-resident Mø [93]. This distinct type of response is imposed by the pathogen, and not by the tissue environment, because intrathoracic injection of thyoglicollate stimulates the recruitment of circulating monocytes (and neutrophils) as expected. Proliferation of Mø in the pleural cavity requires IL-4 signaling, as indicated by a failure of IL-4-deficient mice to mount a full-fledged Mø response. Also, administration of bioactive IL-4 (IL4c) [94] elicits Mø proliferation in tissue. It is possible that the type 2 inflammatory response induced by L. sigmodontis requires the appropriate combination of two variables: (1) the absence of Ly-6Chi monocytes, which would exhibit potentially damaging (type 1) effector functions and (2) the presence of Ly-6Clo monocyte-derived resident Mø, which proliferate in response to IL-4, sequester parasites and mediate (type 2) wound repair functions. These and future studies should help to understand better the regulation of type 1 versus type 2 Mø responses and their associated diseases [7,28].
Ischemic Myocardium
Myocardial infarction (MI) is characterized by an interruption of blood supply and death of cardiac myocytes. Healing of the ischemic myocardium requires monocytes and Mø, which represent the most abundant cell infiltrates within the first 2 weeks. The mononuclear phagocytes scavenge and degrade dead cardiac myocytes and promote the formation of granulation tissue and remodeling. These functions need to be properly executed to preserve the heart's left ventricular geometry. If not, the heart undergoes left ventricular dilatation, which leads to heart failure and poor prognosis [95].
Two distinct phases of monocyte participation after MI have been identified in experimental mouse models [96]. Modulation of CCR2 (ligand: MCP-1) and CX3CR1 (ligand: CX3CL1, fractalkine) chemokine receptor expression in the ischemic myocardium triggers the sequential recruitment Ly-6Chi and Ly-6Clo monocytes, respectively. A first phase (days 1–4 after MI) is dominated by Ly-6Chi monocytes. These cells are not only phagocytic but also produce proteolytic enzymes and inflammatory mediators and thus serve to digest damaged tissue. A second phase (days 5–10 after MI) is dominated by Ly-6Clo monocytes. The latter have attenuated inflammatory activity and promote healing because they facilitate angiogenesis and promote the deposition of collagen and accumulation of reparative myofibroblasts [96]. The biphasic monocyte response has also been captured in human patients with acute MI [97].
Sufficient infarct healing requires a balanced and coordinated biphasic response. Indeed, experimental ablation of either monocyte phase [96] or an imbalance of endogenous monocyte subset ratios as seen in hypercholesterolemic ApoE–/– mice [98] disturbs the resolution of inflammation and enhances left ventricular remodeling. Thus, healing after MI is an example of orchestrated mobilization of monocyte subtypes.
Where do recruited monocytes come from? Studies on the origins of mononuclear phagocytes in the ischemic myocardium indicate that the injury triggers the mobilization of reservoir splenic monocytes [76]. Activation and release into the circulation of the reservoir cells is mediated at least in part by the hormone angiotensin II, levels of which increase in serum after MI, and the angiotensin type 1 receptor, which is expressed by the monocytes. Accordingly, treatment with angiotensin-converting enzyme inhibitors attenuates the release of reservoir monocytes after MI and affects the outcome of disease [99]. These initial studies indicate the relevance of understanding the fluxes of monocyte populations in vivo and the molecular cues that control migration of these cells.
Atherosclerotic Plaque
Atherosclerosis is a chronic disease that is profoundly influenced by inflammatory leukocytes. Among them, Mø participate actively in the initiation and progression of the disease. Lesional Mø produce proteases and inflammatory products, which modulate plaque development and can eventually cause the rupture of a plaque and provoke myocardial infarction and stroke [5].
In vivo fate mapping experiments in the ApoE–/– mouse model have revealed that growing atherosclerotic lesions continuously accumulate monocytes and that hypercholesterolemia further augments the flux of circulating monocytes to lesions [100]. It is also known that systemically elevated concentrations of cholesterol and lipids induce a progressive and selective increase of circulating Ly-6Chi monocytes in mice [101]. Such expansion is controlled, at least in part, through loss of interaction between cell surface proteoglycan-bound ApoE and ABCA1/ABCG1 in HSC and progenitor cells, which decreases cholesterol efflux and promotes cell proliferation and monocyte production [102].
Ly-6Chi monocytes efficiently adhere to activated endothelium, preferentially accumulate into lesions and are centrally involved in promoting atherosclerosis [101,103]. Ly-6Chi monocyte infiltration in atheromata is driven by the CCR2, CCR5 and CX3CR1 chemokine receptors and their cognate ligands [103,104]. Upon recruitment, Ly-6Chi monocytes differentiate into lesional Mø and foam cells [101], though some cells can acquire a DC phenotype [103]. Also, hypercholesterolemia-associated monocytosis in ApoE–/– mice dramatically enlarges the size of the splenic reservoir and selectively expands the Ly-6Chi monocyte subset [101]. This process may promote the increased flux of inflammatory monocytes into lesions and accelerate disease progression. Thus, the amplification and lesional accumulation of Ly-6Chi monocytes represent key events of the inflammatory response in experimental atherosclerosis. Ly-6Clo monocytes do not expand in atherosclerotic mice and infiltrate lesions much less frequently than their Ly-6Chi counterparts. Whether Ly-6Clo monocytes or pre-DC play a decisive function in atherosclerosis requires additional investigation.
Tumor Stroma
Tumor-associated Mø (TAM) are common host cells in the stroma of solid tumors. Their presence typically promotes malignant progression and shortens survival, whereas their selective ablation can reduce cancer growth and prolong survival [6,105]. Clinical studies have largely validated the initial findings in mice by showing that the presence of TAM in several types of human cancer correlates with adverse outcomes and shorter survival [106,107]. Thus, TAM represent exciting new targets for the therapy of various types of cancer.
Experimental evidence using different animal models of cancer indicates that Ly-6Chi monocytes are the main source of TAM [106,108,109]. These cells are coopted by tumors and promote malignant progression in different ways, which include stimulation of angiogenesis, enhancement of tumor cell migration, promotion of tumor cell intravasation at the primary site and extravasation at metastatic sites, and suppression of antitumor T cell immunity [6]. Accumulation of Ly-6Chi monocytes in tumors also associates with an increased turnover of Mø (or TAM) [108]. Taken together, these data indicate that circulating precursors should significantly influence the TAM repertoire in growing tumors.
A population of circulating monocytes called TEM (for TIE2-expressing monocytes) has also been reported in humans and mice; these cells accumulate in perivascular and hypoxic areas of tumors and contribute to tumor angiogenesis [110]. TEM express a TIE2+ VEGF+ CCR2– CCR5+ phenotype, and thus may correspond to Ly-6Clo monocytes [19]. Blockade of the TIE2 ligand angiopoietin-2 disables TEM, which has been used to inhibit tumor growth and metastasis in several genetic mouse models of cancer [111].
Myeloid-derived suppressor cells (MDSC) [112], which are defined operationally by their capacity to suppress T cell responses and often characterized as CD11b+ Gr-1+ (F4/80lo CD11clo CD31lo CD34lo) cells, overlap phenotypically with neutrophils and Ly-6Chi (Gr-1+) monocytes. Thus, the functions of MDSC may be attributed in part to Ly-6Chi monocytes (and inversely). Studies focusing on MDSC have identified that the number of these cells in the circulation increases in patients with various forms of cancer and is correlated with clinical stage. MDSC are also known to accumulate in tumors, where they may acquire a CD11b+ F4/80+ Gr-1– phenotype, which is reminiscent of the one of TAM [113]. Remarkably, tumor-bearing hosts that produce GM-CSF and IL-6 stimulate the production of MDSC in the bone marrow [114]. These findings may open new ways to suppress tumor-promoting immunity.
Perspectives for Fundamental Research and Therapeutic Interventions
Research on the origins of Mø and DC over the past decade has been successful on at least three levels: (i) it has decoded ontogenic pathways to hematopoietic stem cells; (ii) it has determined some of the rules that guide precursor cell migration and fate, and (iii) it has identified how some precursor cell responses can profoundly impact certain diseases. Certainly, new questions have arisen, some of which are listed in the sections above. The appendix also compiles several open inquiries which may be worthwhile investigating. Finally, whether current and future knowledge of the underpinnings of mononuclear responses can be exploited in therapy represents an interesting challenge. Remarkably, the notion that Ly-6Chi (CCR2+) monocytes are central effectors of inflammation has been recently harnessed for therapy in various experimental models. It has been demonstrated that administration of anti-CCL2 antibodies prevents the recruitment of Ly-6Chi monocytes to metastatic tumors and prolongs the survival of tumor-bearing mice [109]. Lipid nanoparticles incorporating a CCR2-silencing short-interfering RNA (siRNA) were also shown to accumulate efficiently into Ly-6Chi monocytes upon in vivo injection. The procedure prevented Ly-6Chi monocyte accumulation to lesions and reduced cancer progression [115]. Therapeutic CCR2-siRNA silencing was also shown to prolong normoglycemia in diabetic mice, to lower the number of Mø in atheromata and to reduce atherosclerosis in hypercholesterolemic mice. Thus targeting of the Ly-6Chi monocyte axis – or its human equivalent – may become a useful therapeutic component of various chronic inflammatory diseases. The contributions of Ly-6Clo monocytes and CDP to pathologic conditions remain generally less well understood, though harnessing them for therapy may represent interesting additional options.
Appendix – Open Questions
(1) What are the individual contributions of CDP, Ly-6Chi and Ly-6Clo monocytes in disease? These three subsets are functionally heterogeneous and generate different Mø and DC populations, yet future studies should inform about their roles, specifically in pathologic conditions.
(2) Do other precursors of Mø and DC exist? Current knowledge does not exclude the possibility that unidentified distinct precursor cells commit to other important Mø and/or DC lineages.
(3) Ly-6Chi monocytes can be produced in the bone marrow and stored in the spleen and both bone marrow and splenic monocytes can be recruited to sites of inflammation. Are these cells different and do they mediate separate functions? Also, how is the splenic reservoir repopulated following monocyte mobilization?
(4) Which factors can be used to manipulate HSC and progenitor cell commitment and thus to generate a selective Mø or DC subset in a therapeutic setting?
(5) Is it possible to amplify Ly-6Clo monocyte responses and would such an amplification help to suppress inflammation? The number of Ly-6Clo monocytes remains remarkably stable in the circulation, even in subjects undergoing acute or chronic inflammatory reactions. Can one augment Ly-6Clo monocyte numbers therapeutically, e.g. by increasing their production from committed (perhaps unknown) progenitors or by inducing Ly-6Chi→Ly-6Clo monocyte conversion?
(6) What is the role of Ly-6Clo monocytes in patrolling the endothelium? Does this behavior merely favor early extravasation to tissue in some inflammatory conditions, or do monocytes and endothelial cells also continuously exchange meaningful information?
(7) How does the body dispose of innate immune cells that have immigrated into tissue? Data suggest that inflammatory processes drive a massive influx of cells to tissue. However, it is not fully defined whether these cells die locally or instead acquire tissue education and redistribute elsewhere.
(8) What is the nature of Mø and DC that reconstitute tissues once inflammation has resolved? Do these cells resemble Mø and DC that were present before the onset of inflammation or are they different? If the new cells are different, can these differences be beneficial or detrimental to the host?
Acknowldegments
M.E. is part of the International PhD program ‘Cancer and Immunology’ at the University of Lausanne, Switzerland, and supported by a PhD fellowship from the Boehringer Ingelheim Fonds and an AACR Centennial Pre-Doctoral Fellowship in Cancer Research. The work of M.J.P. is supported by the National Institutes of Health grants R01-AI084880, P50-CA086355 and N01-HV08235/HHSN268201000044C.
References
- 1.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 2.Janeway CAJ, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 3.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 6.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128:415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 11.Manz MG, Miyamoto T, Akashi K, Weissman IL. Prospective isolation of human clonogenic common myeloid progenitors. Proc Natl Acad Sci USA. 2002;99:11872–11877. doi: 10.1073/pnas.172384399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwasaki H, Akashi K. Myeloid lineage commitment from the hematopoietic stem cell. Immunity. 2007;26:726–740. doi: 10.1016/j.immuni.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 14.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 15.Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, Carotta S, O'Keeffe M, Bahlo M, Papenfuss A, Kwak JY, Wu L, Shortman K. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol. 2007;8:1217–1226. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- 17.Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- 18.Auffray C, Fogg DK, Narni-Mancinelli E, Senechal B, Trouillet C, Saederup N, Leemput J, Bigot K, Campisi L, Abitbol M, Molina T, Charo I, Hume DA, Cumano A, Lauvau G, Geissmann F. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. 2009;206:595–606. doi: 10.1084/jem.20081385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 20.Chow A, Brown BD, Merad M. Studying the mononuclear phagocyte system in the molecular age. Nat Rev Immunol. 2011;11:788–798. doi: 10.1038/nri3087. [DOI] [PubMed] [Google Scholar]
- 21.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 22.Yrlid U, Jenkins CD, MacPherson GG. Relationships between distinct blood monocyte subsets and migrating intestinal lymph dendritic cells in vivo under steady-state conditions. J Immunol. 2006;176:4155–4162. doi: 10.4049/jimmunol.176.7.4155. [DOI] [PubMed] [Google Scholar]
- 23.Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, Narni-Mancinelli E, Lauvau G. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol. 2008;86:398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- 24.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C– monocytes. Nat Immunol. 2011;12:778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 27.Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121:2437–2445. doi: 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 29.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 31.Alvarez D, Vollmann EH, von Andrian., UH Mechanisms and consequences of dendritic cell migration. Immunity. 2008;29:325–342. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 33.Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, Jeffrey KL, Anthony RM, Kluger C, Nchinda G, Koh H, Rodriguez A, Idoyaga J, Pack M, Velinzon K, Park CG, Steinman RM. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143:416–429. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 35.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 36.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 37.Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10:453–460. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pittet MJ, Weissleder R. Intravital imaging. Cell. 2011;147:983–991. doi: 10.1016/j.cell.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 40.Yona S, Jung S. Monocytes: subsets, origins, fates and functions. Curr Opin Hematol. 2010;17:53–59. doi: 10.1097/MOH.0b013e3283324f80. [DOI] [PubMed] [Google Scholar]
- 41.Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, Price J, Yin N, Bromberg J, Lira SA, Stanley ER, Nussenzweig M, Merad M. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009;206:3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat Immunol. 2007;8:578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- 43.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 44.Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, Stanley ER, Nussenzweig M, Lira SA, Randolph GJ, Merad M. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, Fehling HJ, Hardt WD, Shakhar G, Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 46.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 47.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holt PG, Oliver J, Bilyk N, McMenamin C, McMenamin PG, Kraal G, Thepen T. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med. 1993;177:397–407. doi: 10.1084/jem.177.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gonzalez-Juarrero M, Shim TS, Kipnis A, Junqueira-Kipnis AP, Orme IM. Dynamics of macrophage cell populations during murine pulmonary tuberculosis. J Immunol. 2003;171:3128–3135. doi: 10.4049/jimmunol.171.6.3128. [DOI] [PubMed] [Google Scholar]
- 51.Careau E, Bissonnette EY. Adoptive transfer of alveolar macrophages abrogates bronchial hyperresponsiveness. Am J Respir Cell Mol Biol. 2004;31:22–27. doi: 10.1165/rcmb.2003-0229OC. [DOI] [PubMed] [Google Scholar]
- 52.Bedoret D, Wallemacq H, Marichal T, Desmet C, Quesada Calvo F, Henry E, Closset R, Dewals B, Thielen C, Gustin P, de Leval L, Van Rooijen N, Le Moine A, Vanderplasschen A, Cataldo D, Drion PV, Moser M, Lekeux P, Bureau F. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J Clin Invest. 2009;119:3723–3738. doi: 10.1172/JCI39717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Landsman L, Jung S. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. J Immunol. 2007;179:3488–3494. doi: 10.4049/jimmunol.179.6.3488. [DOI] [PubMed] [Google Scholar]
- 54.Landsman L, Varol C, Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. J Immunol. 2007;178:2000–2007. doi: 10.4049/jimmunol.178.4.2000. [DOI] [PubMed] [Google Scholar]
- 55.Jakubzick C, Tacke F, Ginhoux F, Wagers AJ, van Rooijen N, Mack M, Merad M, Randolph GJ. Blood monocyte subsets differentially give rise to CD103+ and CD103– pulmonary dendritic cell populations. J Immunol. 2008;180:3019–3027. doi: 10.4049/jimmunol.180.5.3019. [DOI] [PubMed] [Google Scholar]
- 56.Beaty SR, Rose CEJ, Sung SS. Diverse and potent chemokine production by lung CD11bhigh dendritic cells in homeostasis and in allergic lung inflammation. J Immunol. 2007;178:1882–1895. doi: 10.4049/jimmunol.178.3.1882. [DOI] [PubMed] [Google Scholar]
- 57.del Rio ML, Rodriguez-Barbosa JI, Kremmer E, Forster R. CD103– and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J Immunol. 2007;178:6861–6866. doi: 10.4049/jimmunol.178.11.6861. [DOI] [PubMed] [Google Scholar]
- 58.GeurtsvanKessel CH, Willart MA, van Rijt LS, Muskens F, Kool M, Baas C, Thielemans K, Bennett C, Clausen BE, Hoogsteden HC, Osterhaus AD, Rimmelzwaan GF, Lambrecht BN. Clearance of influenza virus from the lung depends on migratory langerin+CD11b– but not plasmacytoid dendritic cells. J Exp Med. 2008;205:1621–1634. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35:323–335. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dorner BG, Dorner MB, Zhou X, Opitz C, Mora A, Guttler S, Hutloff A, Mages HW, Ranke K, Schaefer M, Jack RS, Henn V, Kroczek RA. Selective expression of the chemokine receptor XCR1 on cross-presenting dendritic cells determines cooperation with CD8+ T cells. Immunity. 2009;31:823–833. doi: 10.1016/j.immuni.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 61.Julia V, Hessel EM, Malherbe L, Glaichenhaus N, O'Garra A, Coffman RL. A restricted subset of dendritic cells captures airborne antigens and remains able to activate specific T cells long after antigen exposure. Immunity. 2002;16:271–283. doi: 10.1016/s1074-7613(02)00276-5. [DOI] [PubMed] [Google Scholar]
- 62.Ginhoux F, Merad M. Ontogeny and homeostasis of Langerhans cells. Immunol Cell Biol. 2010;88:387–392. doi: 10.1038/icb.2010.38. [DOI] [PubMed] [Google Scholar]
- 63.Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, Weissman IL, Cyster JG, Engleman EG. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chorro L, Sarde A, Li M, Woollard KJ, Chambon P, Malissen B, Kissenpfennig A, Barbaroux JB, Groves R, Geissmann F. Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J Exp Med. 2009;206:3089–3100. doi: 10.1084/jem.20091586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giacometti L, Montagna W. Langerhans cells: uptake of tritiated thymidine. Science. 1967;157:439–440. doi: 10.1126/science.157.3787.439. [DOI] [PubMed] [Google Scholar]
- 66.Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM, Stanley ER, Randolph GJ, Merad M. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11:775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- 68.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 69.Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer's disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 71.Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 72.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 74.Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, Rolls A, Mack M, Pluchino S, Martino G, Jung S, Schwartz M. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6:e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 76.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mempel TR, Henrickson SE, Von Andrian., UH T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 78.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 79.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 80.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 81.Aldridge JRJ, Moseley CE, Boltz DA, Negovetich NJ, Reynolds C, Franks J, Brown SA, Doherty PC, Webster RG, Thomas PG. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc Natl Acad Sci USA. 2009;106:5306–5311. doi: 10.1073/pnas.0900655106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Getts DR, Terry RL, Getts MT, Muller M, Rana S, Shrestha B, Radford J, Van Rooijen N, Campbell IL, King NJ. Ly6c+ ‘inflammatory monocytes' are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. J Exp Med. 2008;205:2319–2337. doi: 10.1084/jem.20080421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mildner A, Mack M, Schmidt H, Bruck W, Djukic M, Zabel MD, Hille A, Priller J, Prinz M. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. 2009;132:2487–2500. doi: 10.1093/brain/awp144. [DOI] [PubMed] [Google Scholar]
- 85.Takizawa H, Regoes RR, Boddupalli CS, Bonhoeffer S, Manz MG. Dynamic variation in cycling of hematopoietic stem cells in steady state and inflammation. J Exp Med. 2011;208:273–284. doi: 10.1084/jem.20101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 89.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 90.Bhattacharya D, Czechowicz A, Ooi AG, Rossi DJ, Bryder D, Weissman IL. Niche recycling through division-independent egress of hematopoietic stem cells. J Exp Med. 2009;206:2837–2850. doi: 10.1084/jem.20090778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mazo IB, Massberg S, von Andrian., UH Hematopoietic stem and progenitor cell trafficking. Trends Immunol. 2011;32:493–503. doi: 10.1016/j.it.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, von Andrian., UH Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Milner JD, Orekov T, Ward JM, Cheng L, Torres-Velez F, Junttila I, Sun G, Buller M, Morris SC, Finkelman FD, Paul WE. Sustained IL-4 exposure leads to a novel pathway for hemophagocytosis, inflammation, and tissue macrophage accumulation. Blood. 2010;116:2476–2483. doi: 10.1182/blood-2009-11-255174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 96.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tsujioka H, Imanishi T, Ikejima H, Kuroi A, Takarada S, Tanimoto T, Kitabata H, Okochi K, Arita Y, Ishibashi K, Komukai K, Kataiwa H, Nakamura N, Hirata K, Tanaka A, Akasaka T. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol. 2009;54:130–138. doi: 10.1016/j.jacc.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 98.Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, Libby P, Pittet M, Weissleder R, Nahrendorf M. Impaired infarct healing in atherosclerotic mice with Ly-6Chi monocytosis. J Am Coll Cardiol. 2010;55:1629–1638. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leuschner F, Panizzi P, Chico-Calero I, Lee WW, Ueno T, Cortez-Retamozo V, Waterman P, Gorbatov R, Marinelli B, Iwamoto Y, Chudnovskiy A, Figueiredo JL, Sosnovik DE, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res. 2010;107:1364–1373. doi: 10.1161/CIRCRESAHA.110.227454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P, Weissleder R. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci USA. 2006;103:10340–10345. doi: 10.1073/pnas.0604260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo C-L, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6Chi and Ly6Clo monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 105.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.DeNardo DG, Brennan D, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, Jirstrom K, West BL, Coussens LM. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, Bast MA, Rosenwald A, Muller-Hermelink HK, Rimsza LM, Campo E, Delabie J, Braziel RM, Cook JR, Tubbs RR, Jaffe ES, Lenz G, Connors JM, Staudt LM, Chan WC, Gascoyne RD. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med. 2010;362:875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 109.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 111.Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, Politi LS, Gentner B, Brown JL, Naldini L, de Palma M. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19:512–526. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 112.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, Calabrese F, Basso G, Zanovello P, Cozzi E, Mandruzzato S, Bronte V. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 115.Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann JF, Marinelli B, Panizzi P, Lee WW, Iwamoto Y, Milstein S, Epstein-Barash H, Cantley W, Wong J, Cortez-Retamozo V, Newton A, Love K, Libby P, Pittet MJ, Swirski FK, Koteliansky V, Langer R, Weissleder R, Anderson DG, Nahrendorf M. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]