Abstract
During the early weeks of human gestation, hematopoietic cells first emerge within the extraembryonic yolk sac (primitive hematopoiesis) and secondarily within the truncal arteries of the embryo. This second wave includes the stem cells giving rise to adult-type lymphohematopoiesis. In both yolk sac blood islands and embryonic aorta, hematopoietic cells arise in the immediate vicinity of vascular endothelial cells. In vitro hematopoietic differentiation of endothelial cells stringently sorted from human embryonic and fetal blood-forming tissues has demonstrated that primitive endothelium lies at the origin of incipient hematopoiesis. These anatomically and temporally localized blood-forming endothelial cells are ultimately derived from a rare subset of mesodermal angio-hematopoietic stem cells, or hemangioblasts. The evidence for an early progenitor of blood-forming cells within the walls of human embryonic blood vessels concurs with parallel data obtained from lower vertebrate, avian, and murine models. Importantly, converging results have recently been obtained with in vitro differentiated human embryonic stem cells, in which we have modeled primitive and definitive hematopoiesis via an endothelium-like developmental intermediate.
Introduction
Efficient delivery of oxygen is essential for the development of embryonic tissue rudiments. Organogenesis is therefore preceded by the production of erythrocytes and the formation of their conduits. Hematopoiesis must adapt to the rapidly changing anatomy of the embryo, and proceeds transiently in the extraembryonic yolk sac, expands briefly in the liver, and is ultimately stabilized in the thymus and marrow of long bones during the second trimester of human gestation. Although hepatic and medullary rudiments are dependent on colonization by extrinsic hematopoietic stem cells (HSCs) in order to initiate hematopoiesis (Le Douarin et al. 1975), yolk sac blood cells are derived directly from extraembryonic mesoderm. Yolk sac hematopoiesis is initially predominated by large nucleated erythroblasts expressing strictly embryonic and fetal hemoglobins, and primitive macrophages.
Although adult-type definitive erythropoiesis, myelopoiesis, and megakaryopoiesis can also arise during the latter part of yolk sac development (McGrath and Palis, 2005, Palis et al. 1999), adult-type hematopoietic progenitors independently arise from the embryo proper in the region of the aorta/gonad/mesonephros region. Importantly, aorta/gonad/mesonephros-derived hematopoietic progenitors are distinguished from those derived from yolk sac by their unique ability to generate long-term engrafting HSCs and lymphoid differentiating potential (Dzierzak, 2003, Tavian et al. 2001).
Another hallmark of yolk sac hematopoiesis is the simultaneous emergence of hematopoietic and endothelial cells within blood islands, leading to joint formation of both blood and blood vessels. This early intermingling of angiogenesis with hematopoiesis was long recognized by observers of the chicken yolk sac, who hypothesized almost a century ago that a common mesodermal stem cell, or hemangioblast, generates both endothelial and blood cells (Sabin 1920, Murray 1932). It was originally hypothesized that the yolk sac is the sole provider of HSC for not only extraembryonic, but also hepatic, thymic, and medullary hematopoiesis. In the last quarter of the 20th century, however, a novel phase of de novo HSC production was characterized and demonstrated to arise within the embryo proper (reviewed in Godin and Cumano, 2002, Dzierzak, 2003). A striking anatomic proximity between vascular cells and emerging HSCs was similarly observed. Intraembryonic HSCs were noted to arise on the luminal aspect of endothelial cells in the dorsal aorta and vitelline artery. Diverse lineage-marking experiments based on metabolic labeling of endothelium in avian embryos in ovo (Jaffredo et al. 1998), purification of mouse embryonic endothelium by flow cytometry (Nishikawa et al. 1998), or tagging of angio-hematopoietic cells in transgenic mice (North et al. 2002) have all suggested the conclusion that discrete subsets of vascular endothelial cells transiently exhibit blood-forming potential during vertebrate development, as originally suggested by Sabin (1920).
Developmental studies with the use of human embryonic and fetal tissue have similarly demonstrated the origin of human adult-type HSC from endothelium with hematogenous potential (Tavian et al. 1996, 1999, 2001). These experiments were performed on first-trimester human fetal tissue (made available following interruption of early pregnancies with RU486) and also with human embryonic stem cell (hESC) differentiation models. Pioneering work with mouse embryonic stem cells (mESCs) originally demonstrated that these pluripotent cells have a remarkable capacity to differentiate into hematopoietic and endothelial progenitors in a manner that closely models embryonic hematopoiesis (Keller et al. 1993, Wiles and Keller, 1991, Vittet et al. 1996). Differentiation of mESCs and hESCs in suspension cultures results in the spontaneous formation of cellular clusters termed embryoid bodies (EB), containing spontaneously differentiating ectodermal, endodermal, and mesodermal components. Mouse embryonic stem cells can readily differentiate to hematopoietic cells in culture (Keller et al. 1993, Wiles and Keller, 1991), recapitulating yolk sac hematopoiesis through a hemangioblastic intermediate, and can even produce yolk sac-like blood islands (Keller et al. 1993) in differentiating EB. The validity of ESCs as a model for adult-type hematopoiesis is more controversial because long-term engrafting HSCs have been difficult to demonstrate from differentiated ESCs. Nonetheless, ectopic expression of homeobox genes (e.g., HOXB4), cell cycle activation genes (e.g., STAT5), or mesoderm-potentiating genes (e.g., CDX4) in mESCs produces HSCs capable of engrafting irradiated hosts (Kyba et al. 2002, 2003, Wang et al. 2005). These retroviral gene-transfer models suggest that generation of adult-type HSCs from ESCs may, in fact, be possible if the normal physiologic stimuli that induce these genes in embryonic hematopoietic progenitors are elucidated.
Gordon Keller's group first provided evidence for the existence of a clonogenic hemangioblast by characterizing a bipotential yolk sac-type progenitor, termed the blast colony-forming cell from mESCs (Choi et al. 1998), and also murine embryos (Huber et al. 2004). These transient vascular endothelial growth factor (VEGF)-responsive progenitors presumptively arise in mammalian yolk sac blood islands as a subset of flk-1/KDR+ (VEGFR2) mesoderm cells and can be enriched with this marker from developing mEB (Kabrun et al. 1997). Hemangioblasts are interesting not only from a developmental perspective, but their derivation and culture from hESCs may also represent a potential unlimited source of both HSCs and endothelial cell progenitors for human tissue engineering. The derivation of multipotent human hemangioblasts from hESCs has not yet been reported and remains an elusive and sought-after goal. More importantly, because human fetal tissue is generally difficult to obtain, the obscure basic cellular and genetic mechanisms of human angio-hematopoiesis initiation and development could be opened to thorough investigation in hESC models. Overall, because hematopoiesis remains an extremely active cell production process throughout life, with hundreds of billions new cells produced daily in the adult human, there is little doubt that deciphering incipient blood cell production, in normal development or in cultured embryonic stem cells, will give clues as to the molecular control of hematopoiesis at postnatal stages.
Multilineage Blood Stem Cells Develop from the Aorta in the First-Month Human Embryo
Thousands of hematopoietic cells are clustered on the endothelium of the dorsal aorta and vitelline artery between the 27th and 40th days of human development (Tavian et al. 1996, 1999). These endothelium-adherent cells are phenotypically primitive hematopoietic progenitors (CD45+, CD34+, CD31+, CD38−, lineage markers negative, GATA-2+, GATA-3+, c-myb+, SCL/Tal1+, c-kit+, flk-1/KDR+) (Tavian et al. 1996,1999, Labastie et al. 1998). Furthermore, these cells can initiate long-term hematopoietic cultures and yield a multilineage progeny of myeloid and lymphoid cells (Tavian et al. 1996, 1999, 2001). Conversely, the precirculation yolk sac analyzed in the same conditions produced only myeloid cells (Tavian et al. 2001). These results suggested that the human blood system develops from two qualitatively independent stocks of HSCs. The human yolk sac only generates progenitors with limited developmental ability, whereas the first and only stem cells endowed with multilineage lymphomyeloid potential emerge autonomously in the aorta. These intraembryonic progenitors are likely responsible for the colonization of the liver rudiment and, in turn, give rise to blood cells in the adult (Figure 1) (reviewed in Tavian and Peault, 2005).
Figure 1.
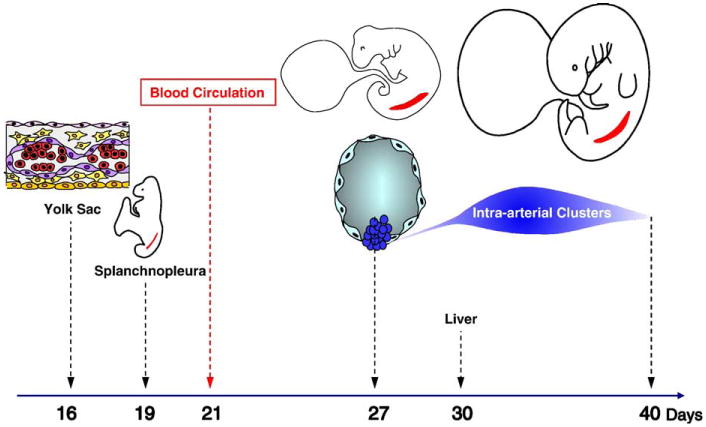
Incipient hematopoiesis in the first month of human development. Endothelial cells (purple) and blood cells (red) emerge concomitantly in the extraembryonic yolk sac from ∼day 16 of development. The paraaortic splanchnopleure, inside the embryo proper, contains the potential to generate hematopoietic cells from at least day 19 of development, although this potential is not expressed before day 27, when multipotent HSCs sprout from the ventral endothelium of the aorta. Intraaortic HSC clusters are present until day 40. The liver rudiment is colonized from day 30 by HSCs derived from intraaortic HSC clusters.
To ascertain the intrinsic origin of HSCs that arise within the 5-week human embryo, the hematopoietic potential of presumptive territories of truncal arteries was assayed at earlier stages of development. Hematopoietic potential was detected in the caudal paraaortic splanchnopleura, an endomesodermal layer that includes the two aortic rudiments, as early as day 21 (i.e., 6 days before the appearance of phenotypically recognizable HSCs). As development progresses, the dorsal aortae fuse and HSCs first appear as scattered groups of CD34+CD45+ cells firmly attached to the endothelial cell layer. Intimate physical contact between emerging intraaortic HSCs and differentiated ventral endothelial cells was confirmed by semithin section observation (Tavian et al. 1999). This analysis strongly suggested that definitive blood cell progenitors arise from an endothelium-like ancestor cell, or hemogenic endothelium.
Hemogenic Endothelium in the Human Embryo and Fetus
To investigate their possible role as generators of blood cells, we purified vascular endothelial cells from the human yolk sac, dorsal aorta, embryonic liver, and fetal bone marrow, and their hematopoietic potential was tested in vitro. Cells with an endothelial cell phenotype were stringently purified by flow cytometry with the use of surface expression of CD34 and CD31, but absence of CD45. The absence of contaminating (CD45+) hematopoietic cells among sorted cells was confirmed by reverse transcriptase-polymerase chain reaction analysis. When co-cultured with the MS-5 mouse stromal cell line (which supports the multilineage, long-term development of human HSCs), a fraction of human CD31+CD34+CD45− embryonic and fetal endothelial cells produced blood cells and established long-term hematopoietic cultures. This was demonstrated not only for endothelial cells sorted from human yolk sac and embryonic aorta, but also, unexpectedly, from the embryonic liver and fetal bone marrow. These data suggest that endothelium-borne hematopoietic cells may not be restricted to the embryonic aorta (Oberlin et al. 2002). The frequency of blood-forming endothelial cells in hematopoietic organs was estimated by performing limiting-dilution culture of sorted cells in the presence of MS-5 stroma and was observed to be closely correlated with the blood-forming status of the tissue of origin, at a given stage of ontogeny. The relative number of blood-forming endothelial cells in the dorsal aorta region, for example, was highest at days 27 to 28, marking the onset of HSC production in this territory. Blood-forming endothelial cell frequency in this territory then decreases dramatically to become barely detectable after day 40, at which time HSC clusters have disappeared from the dorsal aorta. Importantly, no hematopoietic potential was ever detected among CD34+ CD45− endothelial cells purified from fetal thymus, spleen, or other nonhematopoietic tissues such as the heart, fetal aorta, pancreas, lung, or umbilical cord (Oberlin et al. 2002).
It should be kept in mind that hematopoiesis from vessel walls was likely an ancestral mechanism of blood cell generation in evolution, inasmuch as, in addition to converging studies in bird and mouse embryos (Jaffredo et al. 1998, Nishikawa et al. 1998, North et al. 2002, Bollerot et al. 2005), similar hematogenous embryonic aortic cells have been characterized in amphibian and fish embryos (Ciau-Uitz et al. 2000, Gering and Patient, 2005). Important genetic analyses performed in the same lower vertebrates have confirmed that molecular signaling through Hedgehog, Notch, and VEGF is essential to the development of both adult hematopoietic cells and the dorsal aorta, further associating the emergence of HSCs with the aortic blood-forming endothelium (Brown et al. 2000, Gering and Patient, 2005).
Ultimate Origin of the Blood-Forming Endothelial Cells of the Human Embryonic Aorta
Hematopoietic commitment was not observed in aortic ventral endothelial cells prior to the emergence of HSC clusters at their contact (day 27 of development) (Oberlin et al., 2002, and unpublished observations). At earlier stages, hematopoietic potential was detectable in the paraaortic truncal region of the embryo, but this potential was confined within CD34-, nonendo-thelial cells. We therefore hypothesized that the ventral wall of the aorta is colonized, shortly before day 27, by a population of hemangioblastic progenitors, which give rise locally to blood-forming endothelial cells. We are currently evaluating several candidate markers of such human hemangioblasts (reviewed in Tavian et al. 2005). Among these candidates, flk-1/KDR, which is expressed by all developing blood vessels in extra- and intraembryonic tissues, is also detected in a population of non-endothelial, CD34- cells present in the splanchnopleura as early as day 21 of development. As development proceeds, these cells migrate dorsally in the direction of the aorta and flk-1/KDR is later detected in both aortic endothelial cells and associated HSCs. It is therefore possible that a population of human embryonic hemangioblasts, originated in the splanchnopleura, is marked by flk-1/KDR expression (Cortes et al. 1999). However, it remains to be definitively demonstrated experimentally that all pre-HSCs migrate from the splanchnopleura into the embryonic aorta, rather than derive from it.
Human Embryonic Stem Cells Model the Hemangioblastic Origins of Lymphohematopoiesis
We have recently described methods for the modeling of human hematopoietic genesis with the use of in vitro differentiated hESCs. Human ESCs were differentiated into human EB (hEB) capable of spontaneously generating hematopoietic cells (Zambidis et al. 2005). Hematopoiesis in these conditions appears to arise through distinct hemato-endothelial, primitive, and definitive stages in a manner that seems to recapitulate human yolk sac development. Although the technical aspects of hEB formation and hEB differentiation sequences varied in several critical ways from murine ESC models, the developmental phases of human hematopoiesis were remarkably analogous to those described for murine systems (Keller et al. 1993, Wiles and Keller, 1991).
Human ESCs were first followed during their commitment to the hematopoietic lineage with the use of surface marker and transcription factor expression, and clonogenic colony-forming cell assays in differentiating hEB. A fine kinetic analysis of hEB-derived hematopoietic cell commitment was conducted with the use of FACS and real-time quantitative PCR methods. Undifferentiated hESCs (day 0) expressed CD117, CD133, and, surprisingly, KDR/flk1, but had low or undetectable expression of CD34 and CD31. CD34 and CD31 protein expression required ∼12 to 15 days of hEB development to peak under our conditions. Furthermore, the pan-hematopoietic cell marker CD45 was expressed on 1% to 3% of hEB cells, and not until ∼1 week after onset of CD34/CD31 expression (days 15–30 of hEB development). Expression of critical hematopoietic transcriptional regulators, including SCL/TAL1, CDX4, GATA1, GATA2, EKLF, and PU.1, increased dramatically after 1 week of hEB differentiation, coinciding with a similar increase in CD31, CD34, and flk-1/KDR. These results indicated that hESC differentiate efficiently into embryoid body precursors expressing a developmental progression of hemato-endothelial surface markers and regulatory genes comparable to events described during normal vertebrate embryonic–fetal hematopoiesis.
Interestingly, day 6 to 9 hEB cells, in which the expression of SCL/TAL1, CDX4, GATA1, GATA2, CD31, and CD34 was significantly increased, gave rise to novel mesodermal-hemato-endothelial (MHE) colonies when grown in serum-free semisolid medium (Figures 2A and 3). These MHE colonies were detected as adherent clusters of cells with endothelioid morphology and an affinity for acetylated low-density lipoprotein (a hallmark of endothelial cells) from which yolk sac-type erythroblast colonies rapidly budded (Figure 2A). These erythroblasts produced distinctive brilliant red nucleated erythrocytes expressing embryonic and fetal, but not adult, hemoglobins. Large numbers of CD45+ hematopoietic cells were subsequently produced from hEB-derived MHE colonies expanding in semisolid medium with hematopoietic growth factors. Primitive, followed by definitive, hematopoiesis was next detected from hEB cells differentiated at later time points. Eryth-roid colonies derived from day 12 to 20 hEB cells possessed a “definitive” phenotype in comparison to those observed from day 7 to 12 hEB, displaying a “primitive” (yolk sac-type) erythroid phenotype. Definitive day 12 to 20 erythroid colonies had a typical morphology of BFU-e- and CFU-e-derived colonies with salmon red hemoglobinization, and expression of adult-type β globin, whereas day 7 to 12 hEB erythroid colonies displayed a bright red hemoglobinization pattern and possessed distinct nucleated erythrocytes expressing exclusively embryonic and fetal hemoglobins. Our novel protocols and assay methods were thus able to reveal two distinct waves of primitive, then definitive hEB-derived erythropoiesis, and the existence of an endothelium-like developmental intermediate (MHE colonies) temporally preceding these waves.
Figure 2.
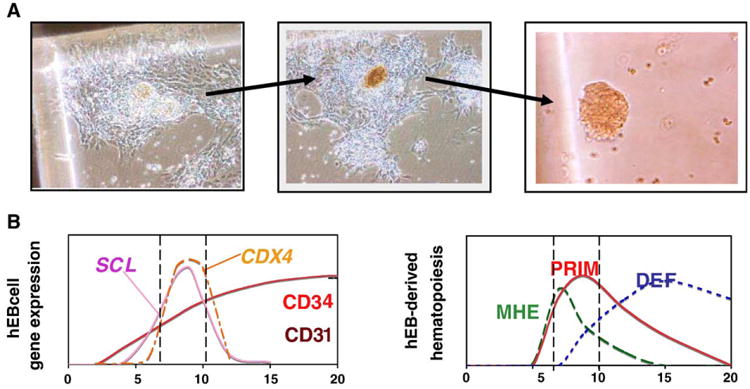
Hemogenic endothelium can be modeled with in vitro differentiation of human embryonic stem cells. Hemogenic endothelial can be modeled within novel MHE colonies from in vitro differentiated hEB cells under serum-free conditions with hematopoietic growth factors (A). Mesodermal-hemato-endothelial colonies from day 6 to 10 hEB cells first adhere to plastic dishes, then expand laterally, and eventually bud off multipotent, or hemoglobinizing erythroblastic hematopoietic cells (right panels). The adherent portion of MHE colonies (left panel) has been demonstrated to be a mixture of mesenchymal cells and mature endothelial cells capable of taking up acetylated low-density lipoprotein. Our main hypothesis, as described in the text, (B) puts in parallel, in a schematic way, the sequence of appearance of key markers of hematopoiesis/angiogenesis left) and the order of emergence of angio-hematopoietic/hematopoietic progenitor cells (right) in our hES/hEB differentiation system. We propose that mesoderm commitment to hemangioblastic precursors from hESC is correlated directly with the emergence of CD34, CD31, SCL/TAL1, and CDX4 expression in hEB cells. Reprinted with permission from Zambidis ET, Peault B, Park TS, Bunz F, and Civin C. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development.
Figure 3.
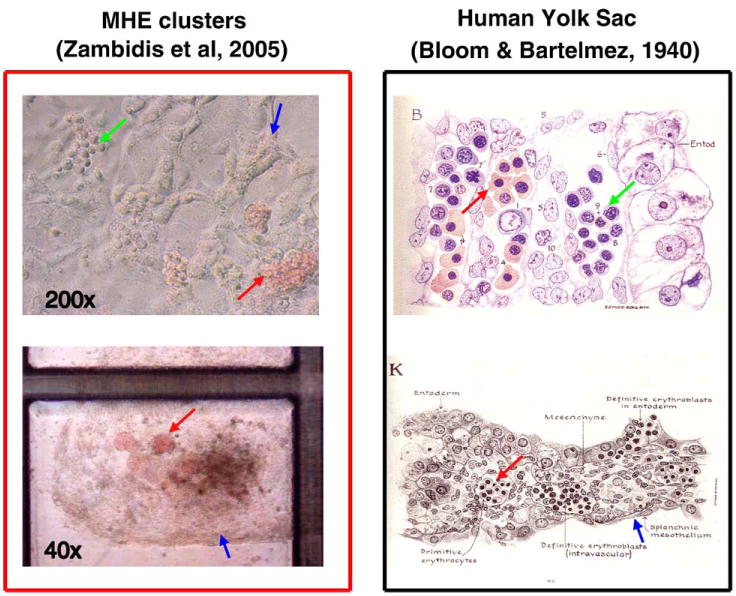
Human ESC-derived MHE colonies possess many characteristics that resemble primary human yolk sac differentiation. Day 6 to 10 hEB cells recultured in serum-free methylcellulose containing hematopoietic growth factors can give rise to rare, unique MHE colonies (top left panel), which can be shown to consist of a mixture of mature CD31+VE-cadherin+ endothelial, vimentin+ mesenchymal, and primitive hematopoietic cells. If fed with fresh medium with growth factors, these clusters can become quite prolific after 5 to 6 weeks (bottom left panel). MHE clusters possess the following features that have great similarity to classic human yolk sac histologic architecture (right panels): (1) hematopoietic progenitors (green arrows) arise in intimate association with endothelium-mesenchymal-like cells (blue arrows); (2) the predominant hematopoietic progeny of both MHE colonies and yolk sac blood islands are nucleated primitive erythroblasts expressing embryonic/fetal hemoglobins (red arrows); and (3) primitive hematopoiesis precedes definitive erythro-myelopoiesis. Reprinted with permission from Bloom W, Bartelemez GW. Hematopoiesis in young human embryos.
We further noted a morphologic similarity (Figure 3) between fully differentiated MHE clusters and classic histologic descriptions of human yolk sac by Bloom and Bartelmez (1940). These authors described hematocytoblasts, in precirculatory day 13 to 24 human yolk sac sections, as the primary hematopoietic progenitors for developing yolk sac blood islands, and arose “by direct transformation of mesenchymal cells.” In the later (day 24) yolk sac, hematocytoblasts were noted to give rise to adult-type erythrocytes, megakaryocytes, and granulocytes, thus suggesting a common origin for both primitive and definitive hematopoieses. We have noted that our hESC-derived MHE colonies possess developmental characteristics comparable to those of human yolk sac (Figure 3): (1) hematopoietic progenitors arise in intimate association with endothelium-like cells; (2) the predominant hematopoietic progeny of both MHE colonies and yolk sac blood islands are nucleated primitive erythroblasts synthesizing embryonic/fetal hemoglobins; (3) primitive hematopoiesis directly precedes definitive erythro-myelopoiesis.
To directly test the hypothesis that hEB contain early endothelial cells with hemogenic potential, we assayed purified populations of hEB cells with an endothelial (CD45–) phenotype. Because CD31 and CD34 expression preceded that of the pan-hematopoietic cell marker CD45 by ∼1 week in our system, we hypothesized that CD45−CD31+CD34+ hEB cells arising between days 7 and 15 of differentiation represent this primitive endothelial phenotype. Our results confirmed this hypothesis: sorted day 9 to 10 hEB cells with an embryonic endothelial phenotype (CD45-CD31+CD34+) indeed possess hematopoietic potential and give rise to definitive-type CD45+ erythro-myeloid cells, as well as CD56+ NK cells. Another group, with the use of slightly different hEB differentiation methods, similarly reported that day 9 to 10 hEB cells purified as CD31+, VE-cadherin+, flk-1+, and CD45− (CD45neg PFV) contain hemato-endothelial potential (Wang et al. 2004).
In summary, our hESC differentiation system provides a compelling in vitro model for human embryonic angio-hematopoiesis, with sequential expression of early hematopoietic genes and a resemblance to human yolk sac development. Our main hypothesis (summarized in Figure 2B) is that during hEB differentiation there is sequential mesoderm commitment to a hemangioblast precursor, which can be assayed within our MHE colonies. This transient precursor can give rise to primitive (PRIM) and adult-type (definitive; DEF) HSCs. We propose that incipient hemangioblastic potential is directly correlated with the onset of expression of SCL/TAL1, as it is in other species (Porcher et al. 1996, Robertson et al. 2000), but also with the homeobox-regulating factor CDX4. Although the function of the highly conserved caudalizing factor CDX4 in humans is obscure, it has been implicated in mediating mesodermal commitment to the hematopoietic cell lineage in zebrafish and mouse (Davidson et al, 2003). CDX4 also appears to play a critical role in forming engraftable HSCs from mESCs (Wang et al. 2005). We are currently attempting to correlate directly both SCL/TAL1 and CDX4 expression with the emergence of known early hematopoietic-hemangioblast markers in developing hESCs.
Conclusion
Sabin (1920) first proposed the hypothesis that blood cells can sprout from vascular endothelium, based on microscopic observations of the live chicken yolk sac. These observations fell into oblivion until the end of the century, when several investigators used cell marking, cell sorting, genetic and transgenic techniques to document the existence of blood-forming endothelial cells in the amphibian, fish, avian, and mammalian embryonic aorta (Jaffredo et al. 1998, Nishikawa et al. 1998, Ciau-Uitz et al. 2000, North et al. 2002, Oberlin et al. 2002, Bollerot et al. 2005, Gering and Patient, 2005). Recent availability of first-month human embryos to experimentation, as well as the development of reliable and sensitive assays for human angiogenesis and lymphohematopoiesis, has permitted the precise analysis of incipient angio-hematopoiesis in human ontogeny (Figure 1). Our studies have revealed that the multipotent HSC that found definitive, adult-type human hematopoiesis emerge in the truncal intraembryonic arteries and not in the yolk sac, as previously assumed (Tavian et al. 1996, 1999, 2001). We have further shown that these HSC likely arise from hematogenous endothelial cells (Oberlin et al. 2002), as is the case in all vertebrate animal models investigated so far. Intriguingly though, such hematogenous endothelial cells were also detected in other human embryonic and fetal organs, such as the yolk sac, liver, and bone marrow, suggesting that blood cell generation from vessel walls may be more widespread than initially thought and confirming Sabin's pioneering observations. These unexpected results raise the question as to whether hematogenous endothelial cells may also persist in the adult bone marrow. The observation that endothe-lial cells in chronic myeloid leukemia patients bear the bcr-abl mutation, the hallmark of leukemic cells in this malignancy, may indirectly suggest such affiliation between endothelial cells and hematopoietic cells in the adult (Gunsilius et al. 2000). We also have preliminary data indicating that vascular endothelial cells stringently sorted by flow cytometry from the human adult bone marrow can generate, albeit at a very low frequency, hematopoietic cells in culture. Moreover, we have recently documented the existence in diverse adult human tissues of vessel wall-associated multipotent stem cells that might account for the persistence of such nonclassical hematogenous cells at postnatal and adult stages (Tavian et al., 2005, and manuscripts in preparation). It is finally of major significance that we have been able to mimic a transition between endothelial cells and blood cells with the use of models of hESC differentiation in culture. We have indeed observed the development in these conditions of mesodermal/hematopoietic/endothelial cell colonies that should be developmentally equivalent to the hemangioblastic cell clusters in transition to angio-hematopoiesis, prior to blood island emergence, as seen in the early yolk sac. Although we have currently demonstrated yolk sac-type hematopoiesis from differentiated hESCs, our current efforts focus on the ability of hESC-derived hematogenous endothelial cells to produce definitive, long-term engrafting HSC, similar to those derived from the large vessels of the human embryo.
Acknowledgments
The authors are grateful to Dr. Curt I. Civin for his mentorship, guidance, and support in some of these studies, and to Roseanne Perry for assistance in the preparation of the manuscript.
Contributor Information
Elias T. Zambidis, Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, USA
Estelle Oberlin, Inserm U506, Hopital Paul Brousse, Villejuif, France.
Manuela Tavian, Inserm U506, Hopital Paul Brousse, Villejuif, France.
Bruno Péault, Children's Hospital, Pittsburgh, PA, USA; Hopital Paul Brousse, Villejuif, France.
References
- Bloom W, Bartelmez GW. Hematopoiesis in young human embryos. Am J Anat. 1940;67:21–53. [Google Scholar]
- Bollerot K, Romero S, Dunon D, Jaffredo T. Core binding factor in the early avian embryo: cloning of cbfbeta and combinational expression patterns with Runx-1. Gene Expr Patterns. 2005;6(1):29–39. doi: 10.1016/j.modgep.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Brown LA, Rodaway AR, Schilling TF, et al. Insight into early vasculogenesis revealed by expression of the ETS-domain transcription factor Fli-1 in wild-type and mutant zebrafish embryos. Mech Dev. 2000;90:237–252. doi: 10.1016/s0925-4773(99)00256-7. [DOI] [PubMed] [Google Scholar]
- Choi K, Kennedy M, Kazarov A, et al. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- Ciau-Uitz A, Walmsley M, Patient R. Distinct origins of adult and embryonic blood in Xenopus. Cell. 2000;102:787–796. doi: 10.1016/s0092-8674(00)00067-2. [DOI] [PubMed] [Google Scholar]
- Cortes F, Debacker C, Péault B, Labastie MC. Differential expression of KDR/VEGFR-2 and CD34 during mesoderm development of the early human embryo. Mech Dev. 1999;83:161–164. doi: 10.1016/s0925-4773(99)00030-1. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Ernst P, Wang Y, et al. cdx4 mutants fail to specify blood progenitors and can be rescued by multiple hox genes. Nature. 2003;425:300–306. doi: 10.1038/nature01973. [DOI] [PubMed] [Google Scholar]
- Dzierzak E. Ontogenic emergence of definitive hematopoietic stem cells. Curr Opin Hematol. 2003;10:229. doi: 10.1097/00062752-200305000-00006. [DOI] [PubMed] [Google Scholar]
- Gering M, Patient R. Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Dev Cell. 2005;8:389–400. doi: 10.1016/j.devcel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Godin I, Cumano A. Of birds and mice: hematopoietic stem cell development. Int J Dev Biol. 2005;49:251–257. doi: 10.1387/ijdb.041945ig. [DOI] [PubMed] [Google Scholar]
- Gunsilius E, Duba HC, Petzer AL, et al. Evidence from a leukaemia model for maintenance of vascular endothelium by bone-marrow-derived endothelial cells. Lancet. 2000;355:1688–1691. doi: 10.1016/S0140-6736(00)02241-8. [DOI] [PubMed] [Google Scholar]
- Huber TL, Kouskoff V, Fehling HJ, et al. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- Jaffredo T, Gautier R, Eichmann A, Dieterlen-Lievre F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development. 1998;125:4575–4583. doi: 10.1242/dev.125.22.4575. [DOI] [PubMed] [Google Scholar]
- Kabrun N, Buhring HJ, Choi K, et al. Flk-1 expression defines a population of early embryonic hematopoietic precursors. Development. 1997;124:2039–2048. doi: 10.1242/dev.124.10.2039. [DOI] [PubMed] [Google Scholar]
- Keller G, Kennedy M, Papayannopoulou T, Wiles MV. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993;13:473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- Kyba M, Perlingeiro RC, Hoover RR, et al. Enhanced hematopoietic differentiation of embryonic stem cells conditionally expressing Stat5. Proc Natl Acad Sci U S A. 2003;100:11904–11910. doi: 10.1073/pnas.1734140100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labastie MC, Cortés F, Roméo PH, et al. Molecular identity of hematopoietic precursor cells emerging in the human embryo. Blood. 1998;92:3624–3635. [PubMed] [Google Scholar]
- Le Douarin NM, Houssaint E, Jotereau FV, Belo M. Origin of hemopoietic stem cells in embryonic bursa of Fabricius and bone marrow studied through interspecific chimeras. Proc Natl Acad Sci U S A. 1975;72:2701–2705. doi: 10.1073/pnas.72.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath K, Palis J. Hematopoiesis in the yolk sac: more than meets the eye. Exp Hematol. 2005;33:1021–1028. doi: 10.1016/j.exphem.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Murray PDF. The development ‘invitro’ of blood of the early chick embryo. Proc Roy Soc London. 1932;11:497–521. [Google Scholar]
- Nishikawa SI, Nishikawa S, Kawamoto H, et al. In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity. 1998;8:761–769. doi: 10.1016/s1074-7613(00)80581-6. [DOI] [PubMed] [Google Scholar]
- North TE, de Bruijn MF, Stacy T, et al. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16:661–672. doi: 10.1016/s1074-7613(02)00296-0. [DOI] [PubMed] [Google Scholar]
- Oberlin E, Tavian M, Blazsek I, Péault B. Blood-forming potential of vascular endothelium in the human embryo. Development. 2002;129:4147–4457. doi: 10.1242/dev.129.17.4147. [DOI] [PubMed] [Google Scholar]
- Palis J, Robertsin S, Kennedy M, et al. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- Porcher C, Swat W, Rockwell K, et al. The T cell leukemia oncoprotein SCL/tal1 is essential for development of all hematopoietic lineages. Cell. 1996;86:47–57. doi: 10.1016/s0092-8674(00)80076-8. [DOI] [PubMed] [Google Scholar]
- Robertson SM, Kennedy M, Shannon JM, Keller G. A transitional stage in the commitment of mesoderm to hematopoiesis requiring the transcription factor SCL/tal-1. Development. 2000;127:2447–2459. doi: 10.1242/dev.127.11.2447. [DOI] [PubMed] [Google Scholar]
- Sabin FR. Studies on the origin of blood vessels and of red blood corpuscles as seen in the living blastoderm of checks during the second day of incubation. Vol. 9. Contrib Embryol, Carnegie Institute; Washington: 1920. p. 214. Pub. no. 272. [Google Scholar]
- Tavian M, Péault B. Embryonic development of the human hematopoietic system. Int J Dev Biol. 2005;49:243–250. doi: 10.1387/ijdb.041957mt. [DOI] [PubMed] [Google Scholar]
- Tavian M, Coulombel L, Luton D, et al. Aorta-associated CD34+ hematopoietic cells in the early human embryo. Blood. 1996;87:67–72. [PubMed] [Google Scholar]
- Tavian M, Hallais MF, Péault B. Emergence of intraembryonic hematopoietic precursors in the pre-liver human embryo. Development. 1999;126:793–803. doi: 10.1242/dev.126.4.793. [DOI] [PubMed] [Google Scholar]
- Tavian M, Robin C, Coulombel L, Péault B. The human embryo, but not its yolk sac, generates lympho-myeloid stem cells. mapping multipotent hematopoietic cell fate in intraembryonic mesoderm. Immunity. 2001;15:487–495. doi: 10.1016/s1074-7613(01)00193-5. [DOI] [PubMed] [Google Scholar]
- Tavian M, Zheng B, Oberlin E, et al. The vascular wall as a source of stem cells. Ann N Y Acad Sci. 2005;1044:41–50. doi: 10.1196/annals.1349.006. [DOI] [PubMed] [Google Scholar]
- Vittet D, Prandini MH, Berthier R, et al. Embryonic stem cells differentiate in vitro to endothelial cells through successive maturation steps. Blood. 1996;88(9):3424–3431. [PubMed] [Google Scholar]
- Wang L, Li L, Shojaei F, et al. Endothelial and hematopoietic cell fate of human embryonic stem cells originates from primitive endothelium with hemangioblastic properties. Immunity. 2004;20:31–41. doi: 10.1016/j.immuni.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yates F, Naveiras O, et al. Embryonic stem cell-derived hematopoietic stem cells. Proc Natl Acad Sci. 2005;102:19081–19086. doi: 10.1073/pnas.0506127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiles MV, Keller G. Multiple hematopoietic lineages develop from embryonic stem (ES) cells in culture. Development. 1991;111:259–267. doi: 10.1242/dev.111.2.259. [DOI] [PubMed] [Google Scholar]
- Zambidis ET, Péault B, Park TS, et al. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hemato-endothelial, primitive, and definitive stages resembling human yolk sac development. Blood. 2005;106:860–870. doi: 10.1182/blood-2004-11-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]


