Abstract
Background and Purpose
Unrecognized or unreported stroke-like symptoms, called covert symptoms, occur in persons free of clinical stroke. Whether covert symptoms are associated with subclinical brain infarcts (SBI) is unknown. This study examined the association between covert stroke-like symptoms and SBI/stroke in persons with no history of stroke or TIA.
Methods
1881 Atherosclerosis Risk in Communities (ARIC) participants free of clinical stroke or transient ischemic attack (TIA) (40% male, 50% African-American, 47–70y) were queried for covert symptoms and underwent cerebral MRI during baseline MRI visit. Symptoms were reassessed after 3 years at Visit 4 (n=1001; 39% male, 50% African-American), and approximately 10 years with a follow-up MRI (n=1006; 40% male, 50% African-American, 61–83y).
Results
Covert symptoms were associated with prevalent SBI (OR=1.94, [95% CI 1.21, 3.11], p=0.006). Baseline MRI visit symptoms were not associated with SBI at follow-up MRI visit. In participants without SBI at baseline, symptoms at Visit 4(OR=2.96, [1.23, 7.13], p=0.016) and symptoms at follow-up MRI visit (OR=4.29, [2.51, 7.33], p<0.001) were associated with a combined outcome of new SBI on follow-up MRI/clinical stroke. Covert symptoms at follow-up MRI visit were also associated with having new SBI (OR=2.26, (1.18, 4.32), p=0.014) on the follow-up MRI that were not seen on the baseline MRI.
Conclusions
Covert neurological symptoms were associated with prevalent SBI, and when ascertained at time of follow-up MRI, with new SBI. Covert symptoms may reflect heightened risk for future infarcts.
Keywords: subclinical brain infarcts, brain imaging, lacunar infarcts, epidemiology
Introduction
Stroke-like neurologic symptoms are not uncommon in persons free of a clinical history of stroke and transient ischemic attacks (TIA) and thus may be considered “covert.” In the Atherosclerosis Risk in Communities (ARIC) study, 6% of participants without a history of stroke or TIA reported symptoms at baseline evaluation1, as did 17.8% in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study.2 In both studies, symptoms, which we hereafter refer to as “covert” symptoms, have been associated with cerebrovascular risk factors3,4 and increased risk of incident clinical stroke.5,6 These associations may be explained by subclinical cerebrovascular disease. However, this is not known since the relationship between symptoms and signs of subclinical disease on brain imaging has not been reported.
Neurological symptoms in persons with no history of clinical stroke could be due to silent or subclinical brain infarcts (SBI) that represent occult cerebrovascular disease from chronic ischemic processes or hypoperfusion.7 SBI are common, increase with age, and are associated with incident stroke, dementia, cognitive decline,8–12 and falls.13 This study examined the association of covert neurological symptoms with SBI or a combined outcome of SBI or clinical stroke on follow-up visits in ARIC participants free of clinical stroke or TIA at the baseline MRI visit. Symptoms indicative of increased risk of subclinical cerebrovascular disease could provide a simple, clinical risk stratification tool for identifying individuals for whom imaging and preventive therapy may be warranted.
Methods
ARIC, a longitudinal study of cardiovascular diseases, enrolled a probability sample cohort of 15,792 men and women (45–64 yrs) at four US sites: Forsyth County, North Carolina; Jackson, Mississippi (African-Americans only); Minneapolis, Minnesota; and Washington County, Maryland. Eligible participants underwent baseline clinical examination in 1987–1989 and subsequent triennial visits through 1998, as previously described.14 The current study uses data from visit 3 (1993–95), hereafter called the baseline MRI visit; visit 4 (1996–98), and an ancillary study visit (2004–2006), hereafter called the follow-up MRI visit. Since the MRI was only conducted at Forsyth County and Jackson as previously described,15 this analysis is limited to participants at these sites. Institutional review boards approved study protocols; all participants provided informed consent.
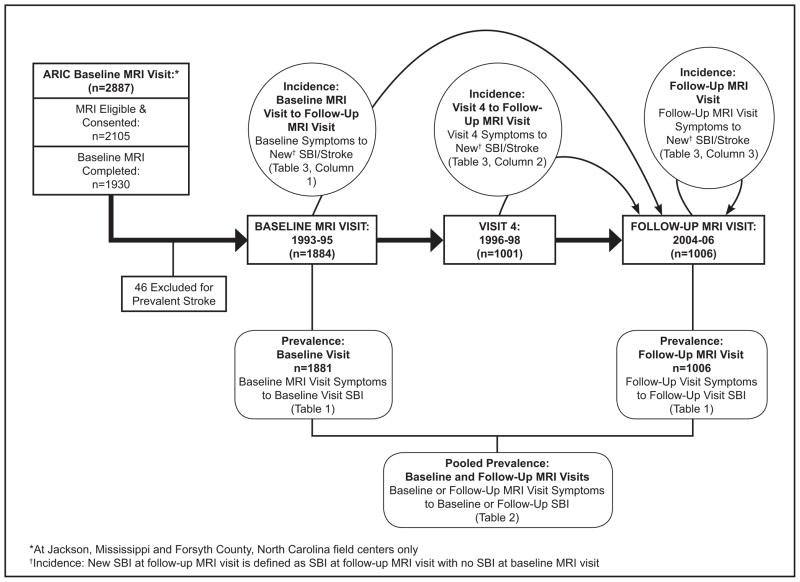
Of 2887 participants ≥55 years at the Forsyth County and Jackson sites, 1881 had MRI and symptoms questionnaire data at baseline. Usual safety exclusion criteria for MRI excluded 2% of women and 6% of men. Of the 1881 at baseline MRI visit, 1001 had visit 4 data; 1006 had follow-up MRI visit data, including the symptoms questionnaire, physical examination, and repeat MRI. Figure 1 shows study flow and related prevalence vs. incidence analytic frameworks.
Figure 1.
Data collection and analyses
The TIA/Stroke Symptoms Questionnaire, used in nearly identical fashion in the Asymptomatic Carotid Atherosclerosis Study clinical trial and validated against neurologists’ ascertainment,16 was administered at each visit by certified interviewers. Participants were asked if a physician had ever told them that they have had a stroke or TIA. They subsequently were asked whether they had experienced a sudden episode of any of six neurologic trigger symptoms since the previous ARIC visit: 1) speech dysfunction; 2) loss of vision; 3) double vision; 4) weakness or paralysis; 5) numbness or tingling; or 6) dizziness or loss of balance. For endorsed symptoms, additional questions explored occurrence, mode of onset, duration, frequency, nature, and concomitant symptoms to exclude non-cerebrovascular symptoms. The algorithm defining stroke-like symptoms has been previously described and validated using an ARIC study clinician’s diagnosis based on the clinician’s review of symptoms and evaluation of participants.1 Participants were asked if they sought medical attention and, if so, what diagnosis was given. Comparisons were made between the algorithm, ARIC study clinician, and the participant’s reported diagnosis if available. Only symptoms of sudden onset lasting ≥30 seconds and determined to be of cerebrovascular origin were considered positive. Covert symptoms were defined as symptomatic on the TIA/Stroke Symptoms Questionnaire in persons free of clinical TIA/stroke.
Clinical stroke was defined using cohort examinations, annual follow-up interviews, and community surveillance, including medical record reviews of potential stroke cases as previously described.17 Potential cases were reviewed separately using a computerized algorithm and a physician reviewer. Both drew upon data from radiologic tests, lumbar puncture, hospital discharge summaries, autopsy findings, other clinical information, and medical records to evaluate neurological deficits. Final classification was established when the computer algorithm and reviewer agreed or when a second physician reviewer resolved discordant classification.
ARIC cerebral MRI protocols were identical to those in the Cardiovascular Health Study.15, 18 Briefly, 1.5T magnetic resonance scanners (GE and Picker) were used to obtain 5mm axial MR images angled parallel to the anterior commissure-posterior commissure line. Although interval MRI scanner upgrades occurred between visits, follow-up scans were matched as closely as possible to baseline visit reference levels for signal-to-noise ratio, resolution, and contrast-weighting. The resulting digitized images, evaluated at the ARIC MRI Reading Center by certified neuroradiologists, were subject to routine quality control checks. Methods for scoring scans were identical at both visits; new readers were tested on a sample of earlier scans to ensure continuity.
MR images obtained at the two visits were read independently, and each image was read by two neuroradiologists, with discrepant infarct findings subject to adjudication. Lesions 3mm in size and visible on both T1- and proton-density/T2-weighted images were classified as infarcts. In the basal ganglia or cortical gray matter, lesions were considered infarcts regardless of T1-weighted image intensity.
The primary outcome, SBI, was defined as infarcts on MRI in persons free of clinical stroke or TIA at the same or a prior visit. Participants with prevalent clinical stroke at baseline MRI visit were excluded. Prevalent SBI at baseline MRI visit was defined as an infarct on baseline MRI. Prevalent SBI at the follow-up MRI was defined as an infarct on the follow-up MRI among those without clinical stroke at or prior to the follow-up MRI visit. Incident SBI was defined as an infarct on follow-up MRI and no visible infarcts on baseline MRI in persons free of clinical stroke at any visit. The combined outcome incident SBI/stroke included anyone with incident SBI at follow-up MRI or who had experienced clinical stroke after baseline MRI visit. Figure 1 shows data flow for these definitions.
Hypertension was defined by self-report, taking anti-hypertensive medication, or blood pressure ≥140/90. Diabetes was defined by self-report, taking medications for diabetes, or having fasting glucose ≥140 mg/dl except at follow-up MRI visit, when only self-report was available. Smoking status was categorized as never, former, or current smoker. These covariates were chosen based on the Framingham Stroke Risk Factors (FSRF) with minimal modifications. SBP and medication use were included in the definition of hypertension. We chose not to use other FSRF due to reductions in the sample size, low prevalence, and the fact that analyses using FSRF in the smaller sample showed similar but stronger associations. Therefore, the associations reported are conservative (data not shown; results available from corresponding author).
Statistical Analysis
Univariate comparisons were conducted using t-tests for continuous data and chi-square tests for categorical data. Concurrent (same visit) associations between symptoms and prevalent outcomes were estimated using marginal multilevel logistic regression models fit with generalized estimating equations19 to account for within-subject associations. Robust variance estimates are reported.
Prevalence Analyses
Potential differences in relationships between prevalent SBI and symptoms across visits were examined using interaction terms between symptom and visit variables in sensitivity analyses (http://stroke.ahajournals.org, Table S1). Associations between symptoms and SBI at the same visit were examined. Prevalence analyses were pooled since no substantial time-by-visit effect modifications were found.
Incidence Analyses
Associations between symptoms and incident SBI or combined incident SBI/Stroke outcomes were estimated using logistic regression.
All multivariate regression models included terms for age, sex, BMI, education, hypertension, diabetes, smoking, and a three-level race-site variable (African-American—Forsyth; African-American—Jackson; White—Forsyth). This race-site variable was used to account for both race and site effects while avoiding the zero cell issue, since the Jackson cohort was entirely African-American. Sensitivity analyses were conducted to examine effects of missingness using weighted GEE techniques20; nearly identical results were obtained.
Results
Participants without clinical stroke at baseline MRI (n=1881; mean age 63y; 40% men; 50% African-American, of whom 88% were from Jackson site) were followed for an average of 10.5 years. Most (65%) were <65 years at baseline, i.e. mid-life; 98% were >65 years at the follow-up MRI visit. Table 1 shows participant characteristics at each visit, stratified at baseline and follow-up MRI visits by presence/absence of SBI. Associations with SBI at baseline MRI visit included age, systolic and diastolic blood pressure, black race, diabetes, hypertension, and education. Only age, systolic blood pressure, and hypertension were statistically associated with SBI at follow-up MRI. Comparison of baseline characteristics for those with and without the repeat MRI (http://stroke.ahajournals.org, Table S2) showed that participants who did not return were slightly older, had higher systolic blood pressure, BMI, and higher prevalence of hypertension, diabetes and smoking. Most differences were of questionable clinical significance.
Table 1.
Participant characteristics stratified at baseline and follow-up MRI visits by presence/absence of Subclinical Brain Infarcts (SBI)
| Baseline (1993–95) | Visit 4 (1996–98) | Follow-up MRI (2004–06) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| All (n=1881) | No SBI (n=1661) | SBI (n=220) | p-value* | All (n=1001) | All (n=1006) | No SBI (n=786) | SBI (n=220) | p-value* | |
|
| |||||||||
| Age (years) | 62.9 (4.5) | 62.6 (4.5) | 64.6 (4.4) | <0.0001 | 65.3 (4.4) | 72.8 (4.4) | 73.8 (4.4) | <0.001 | <0.001 |
|
| |||||||||
| Systolic BP mmHg | 128 (21) | 127 (20) | 137 (26) | <0.001 | 129 (19) | 133 (19) | 132 (19) | 135 (18.9) | 0.040 |
|
| |||||||||
| Diastolic BP mmHg | 72 (11) | 72 (11) | 75 (13) | 0.002 | 71 (10) | 68 (10.8) | 68 (11) | 68 (11) | 0.820 |
|
| |||||||||
| BMI | 28.0 (5.2) | 28 (5.2) | 28 (5.5) | 0.662 | 28.1 (4.9) | 28.7 (5.3) | 29 (5.0) | 29 (5.9) | 0.150 |
|
| |||||||||
| Male | 743 (40%) | 658 (40%) | 85 (39%) | 0.883 | 392 (39%) | 390 (39%) | 317 (40%) | 73 (33%) | 0.060 |
|
| |||||||||
| Blacks | 933 (50%) | 794 (48%) | 139 (64%) | † | 505 (50%) | 505 (50%) | 391 (50%) | 114 (52%) | † |
|
| |||||||||
| Jackson site | 820 (44%) | 694 (42%) | 126 (58%) | † | 462 (46%) | 459 (46%) | 58 (7%) | 101 (46%) | † |
|
| |||||||||
| Hypertension | 898 (48%) | 71 (7%) | 142 (16%) | <0.001 | 501 (50%) | 690 (71%) | 519 (68%) | 171 (81%) | <0.001 |
|
| |||||||||
| Diabetes | 268 (14%) | 224 (14%) | 44 (20%) | 0.007 | 145 (15%) | 243 (24%) | 182 (23%) | 61 (28%) | 0.150 |
|
| |||||||||
| Education | |||||||||
|
| |||||||||
| <12 years | 506 (27%) | 426 (26%) | 80 (37%) | 0.003 | 216 (21%) | 215 (21%) | 169 (21%) | 46 (21%) 79 (36%) | 0.940 |
| 12–16 years | 636 (34%) | 570 (34%) | 66 (31%) | 347 (35%) | 353 (35%) | 275 (35%) | 93 (43%) | ||
| >16 years | 728 (39%) | 658 (40%) | 70 (32%) | 441 (44%) | 435 (43%) | 342 (44%) | |||
|
| |||||||||
| Atrial fibrillation | 4 (0.22%) | 3 (0.19%) | 1 (0.47%) | ||||||
|
| |||||||||
| Left ventricular hypertrophy | 77 (4%) | 65 (4%) | 12 (6%) | ||||||
|
| |||||||||
| Coronary heart disease | 102 (6%) | 85 (5%) | 17 (8%) | ||||||
|
| |||||||||
| Hypertension medications | 800 (43%) | 663 (40%) | 137 (63%) | 678 (46%) | |||||
|
| |||||||||
| ≥1 symptom | 54 (2.9%) | 42 (2.5%) | 12 (5.5%) | 0.023 | 24 (2.4%) | 51 (5.1%) | 31 (4.0%) | 20 (9.1%) | 0.005 |
| Double Vision (n=1341) | 3 (0.2%) | 3 (0.2%) | 0 (0%) | 0.711 | 2 (0.2%) | 9 (0.9%) | 6 (0.8%) | 3 (1.4%) | 0.420 |
| Dizziness (n 1342) | 10 (0.5%) | 8 (0.5%) | 2 (0.9%) | 0.634 | 4 (0.4%) | 2 (0.2%) | 1 (0.1%) | 1 (0.5%) | 0.390 |
| Numbness (n=1342) | 11 (0.6%) | 11 (0.7%) | 0 (0%) | 1.000 | 5 (0.5%) | 10 (1.0%) | 4 (0.5%) | 6 (2.7%) | 0.010 |
| Paralysis (n=1340) | 3 (0.2%) | 3 (0.2%) | 0 (0%) | 1.000 | 1 (0.1%) | 5 (0.5%) | 1 (0.1%) | 4(1.8%) | 0.009 |
| Speech (n=1344) | 13 (0.7%) | 7 (0.4%) | 6 (2.8%) | 0.008 | 8 (0.8%) | 8 (0.8%) | 5 (0.6%) | 3 (1.4%) | 0.380 |
| Vision (n=1347) | 21 (1.1%) | 17 (1.0%) | 4 (1.8%) | 0.169 | 8 (0.8%) | 28 (2.8%) | 18 (2.3%) | 10 (4.6%) | 0.100 |
p-value for test of difference between SBI and No SBI; t tests for continuous variables, Fisher’s exact or Chi-Square for categorical
126 of 139 African-Americans were from Jackson site. Therefore, comparisons by race are aliased by comparisons by site, and formal tests are not reported
BP=blood pressure; BMI=body mass index
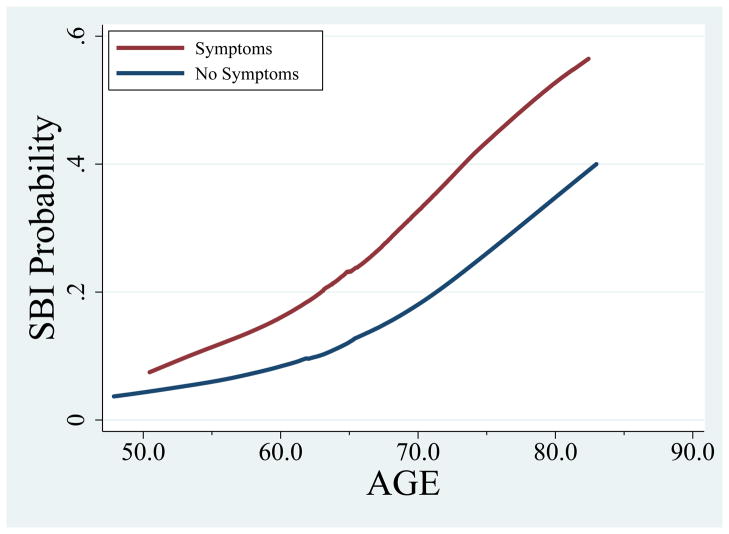
Symptoms were infrequent at all visits (2.9% at baseline, 2.4% at Visit 4, and 5.1% at follow-up MRI; Table 1), but were highly associated with SBI. Figure 2 shows the prevalence of SBI increased with age in both those with and without covert symptoms. However, at any age, SBI prevalence was higher among those with symptoms than without (e.g. among 80yo persons, 54% of those with symptoms would be expected to have SBI versus 36% without symptoms). As shown in Table 2, a nearly two-fold risk of concurrent, prevalent SBI was observed (adjusted OR=1.94; 95% CI 1.21, 3.11) for participants reporting any symptom versus no symptoms. Associations were similar at both MRI visits, with no significant symptoms-visit interaction (http://stroke.ahajournals.org, Table S1). Interaction terms between symptoms and age, hypertension, diabetes, sex and race-site in separate models did not suggest a differential relationship between symptoms and SBI by these subgroups, although power to detect differences was limited.
Figure 2.
Adjusted prevalence of SBI by symptom status across age
Table 2.
Associations between symptoms and SBI at the same visit (prevalence analyses).
| Symptoms | Odds Ratio | p Value | 95% Confidence Interval |
|---|---|---|---|
| Unadjusted | |||
| ≥ 1 Symptom | 2.39 | 0.001 | (1.56, 3.66) |
| Speech problem | 3.99 | 0.002 | (1.67, 9.51) |
| Vision problem | 2.13 | 0.018 | (1.14, 4.00) |
| Double vision | 1.75 | 0.40 | (0.47, 6.50) |
| Paralysis | 5.27 | 0.02 | (1.31, 21.19) |
| Dizziness | 1.75 | 0.40 | (0.47, 6.50) |
| Numbness | 2.10 | 0.13 | (0.81, 5.46) |
| Adjusted | |||
| ≥ 1 Symptom | 1.94 | 0.006 | (1.21, 3.11) |
| Speech problem | 3.46 | 0.013 | (1.30, 9.20) |
| Vision problem | 1.66 | 0.16 | (0.82, 3.35) |
| Double vision | 0.96 | 0.99 | (0.23, 4.18) |
| Paralysis | 3.12 | 0.07 | (0.93, 10.44) |
| Dizziness | 2.12 | 0.27 | (0.56, 7.99) |
| Numbness | 1.59 | 0.33 | (0.62, 4.04) |
Adjusted for age, sex, race-site, BMI, education, hypertension, diabetes, smoking. Estimates are from longitudinal models pooling information on within-visit associations.
Neither baseline nor Visit 4 symptoms were associated with incident SBI at follow-up MRI; however, symptoms at the follow-up MRI were associated with double the risk of incident SBI on the follow-up MRI (OR=2.26, 95% CI 1.18, 4.32; Table 3). Additionally, we considered whether persons with covert symptoms at prior visits could have developed interim clinical stroke. Their exclusion from analyses would underestimate potential associations. We therefore created an incident cerebrovascular disease outcome by combining incident SBI or clinical stroke (31 with stroke, 172 with incident SBI). Baseline symptoms (reported an average 10.5 years prior to follow-up MRI) were not associated with the incident SBI/stroke outcome (OR=0.69, 95% CI 0.23, 2.09). However, visit 4 symptoms (reported an average 7.5 years prior to follow-up MRI), were associated with a nearly three-fold greater risk of incident SBI/stroke outcome (OR=2.96, 95% CI 1.23, 7.13). Concurrent symptoms at the follow-up MRI were associated with a more than four times greater risk of incident SBI/stroke outcome (OR=4.29, 95% CI 2.51, 7.33).
Table 3.
Associations between symptoms at baseline MRI, Visit 4, and follow-up MRI visits and incident (SBI) or incident SBI/stroke at follow-up MRI. Values in table are odds ratios, p-value (95% confidence interval)
| Baseline MRI visit symptoms* | Visit 4 symptoms† | Follow-up MRI visit symptoms | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Follow-up MRI: | Follow-up MRI: | Follow-up MRI: | ||||
| New SBI | New SBI/stroke | New SBI | New SBI/stroke | New SBI | New SBI/stroke | |
|
| ||||||
| ≥ 1 Symptom | 0.62 p = 0.45 (0.18, 2.15) |
0.69 p = 0.51 (0.23, 2.09) |
2.04 p = 0.17 (0.73, 5.67) |
2.96 p = 0.016 (1.23, 7.13) |
2.26 p = 0.014 (1.18, 4.32) |
4.29 p<0.001 (2.51, 7.33) |
|
| ||||||
| Speech problem | ‡ | ‡ | 1.00 p=1.00 (0.09, 10.52) |
4.01 p=0.08 (0.85, 18.90) |
0.69 p=0.74 (0.08, 6.23) |
5.27 p=0.005 (1.66, 16.75) |
|
| ||||||
| Vision problem | 0.38 p=0.36 (0.05, 3.05) |
0.33 p=0.30 (0.04, 2.66) |
0.68 p=0.73 (0.08, 5.80) |
1.21 p=0.82 (0.24, 6.16) |
2.15 p=0.07 (0.93, 5.00) |
2.47 p=0.02 (1.14, 5.34) |
|
| ||||||
| Double vision | ‡ | ‡ | ‡ | ‡ | 2.18 p=0.29 (0.52, 9.16) |
2.42 p=0.19 (0.65, 8.99) |
|
| ||||||
| Paralysis | 3.97 p=0.33 (0.24, 64.41) |
3.76 p=0.35 (0.23, 60.99) |
‡ | ‡ | 3.97 p=0.33 (0.24, 64.41) |
3.76 p=0.35 (0.23, 60.99) |
|
| ||||||
| Dizziness | 2.20 p=0.54 (0.18, 26.62) |
3.12 p=0.29 (0.38, 25.57) |
7.74 p=0.10 (0.67, 88.94) |
9.74 p=0.05 (0.99, 96.04) |
4.52 p=0.29 (0.28, 74.18) |
20.69 p=0.005 (2.45, 174.54) |
|
| ||||||
| Numbness | ‡ | ‡ | ‡ | ‡ | 4.15 p=0.06 (0.97, 17.82) |
9.27 p<0.001 (2.78, 30.90) |
average of 10.5 years between baseline MRI visit & follow-up MRI
average of 7.5 years between visit 4 & follow-up MRI
Symptoms too infrequent for model to converge
Discussion
This study demonstrated that covert stroke-like symptoms assessed at older ages at the time of the follow-up MRI were associated with new SBI compared to the baseline MRI. Remote (nearly eight years prior) covert stroke-like symptoms were also associated with a combined outcome of clinical stroke/new SBI on a follow-up MRI. The several-year lag between symptoms and outcomes potentially present an important opportunity to implement preventive measures to reduce risk of stroke. Our findings build on those of the recent REGARDS study6 and earlier ARIC findings5 of the association between symptoms and stroke by demonstrating associations between symptoms and subclinical disease. This suggests that subclinical disease may cause “whispering” symptoms21 of cerebrovascular disease that are not routinely detected.
Both ARIC and REGARDS have shown associations between covert symptoms and stroke risk factors3,4 as well as clinical stroke.5,6 The current study fills a salient gap in knowledge regarding the association between symptoms and clinical disease in two ways. First, symptoms reported in ARIC underwent additional scrutiny before being classified as “stroke-like” which should reduce misclassification of non-cerebrovascular symptoms. This may explain the lower prevalence of symptoms in ARIC compared to the REGARDS study. Second, we report associations between symptoms and subclinical disease, which supports findings from ARIC and REGARDS linking covert symptoms to clinical disease in two populations free of clinical TIA/stroke at the time of symptoms ascertainment. Importantly, these findings provide evidence for a hypothesized pathway whereby seemingly subclinical cerebrovascular disease is visible on imaging and causes symptoms that can be elicited with directed questioning but do not seem to result in a clinical diagnosis of TIA or stroke in the community. The subclinical disease may subsequently contribute to future stroke risk. Therefore, identifying symptoms that alert clinicians to subclinical disease may present an opportunity for stroke prevention. The findings from REGARDS and ARIC are congruent, and suggest that these symptoms warrant further study as important risk factors in predicting and preventing disease.
More remote symptoms, i.e. on average 10.5 years earlier, in primarily middle-aged participants were not associated with future clinical or subclinical cerebrovascular disease assessed in late life. Some explanations could be more false positives in a younger population with lower probability of disease, or the possibility that participants with symptoms sought treatment in the inter-visit interval.
Surprisingly, many participants who reported symptoms at earlier visits did not report them at future visits (http://stroke.ahajournals.org, Table S3). Several explanations are possible: participants may not have recalled subtle, transient symptoms over the long inter-visit intervals and were misclassified as having no symptoms; symptomatic patients may have sought treatment that led to symptom resolution; participants may have developed overt symptoms that resulted in a diagnosis of TIA/stroke, thus eliminating them from analyses of incident subclinical disease. To examine the latter consideration, we evaluated 29 participants who developed incident stroke by the follow-up MRI visit. Of these, 14% reported symptoms at the previous visit compared to 2% without stroke. Thus, inquiring about symptoms closer to the time of the repeat MRI might reveal associations between symptoms and incident disease. Indeed, concurrent reporting of symptoms at the follow-up MRI was associated with a doubled risk of incident SBI, even though the symptoms being recalled had occurred over the prior eight years.
Low prevalence of symptoms and clinical stroke limited this study’s statistical power for evaluating some associations, such as relationships between individual symptoms and outcomes. Another limitation involves race-specific analyses. All participants at the Jackson, Mississippi site are African-American, and this site accounted for nearly 90% of all African-Americans in the study. Thus, observed differences may be due to race, regional differences, or other factors. We accounted for this by including a three-level race-site covariate (AA-Jackson, AA-Forsyth, White-Forsyth) instead of separate race and sex adjustors that would have extrapolated estimates to a nonexistent White-Jackson group. We believe our findings are robust given associations between symptoms and clinical stroke in REGARDS6 which also sampled blacks and whites in the south and southeastern United States.
The ARIC algorithm incorporates clinical interpretation to distinguish cerebrovascular from non-cerebrovascular symptoms, thereby providing comparability to clinical observations. Further clinical review of covert neurological symptoms similar to those assessed by the ARIC algorithm might help distinguish markers of cerebrovascular disease useful for identifying potentially high-risk individuals who warrant further evaluation and/or treatment.
Summary
Covert neurological symptoms in participants without a history of stroke or TIA are associated with increased risk for prevalent SBI and incidence of SBI and clinical stroke. These symptoms may be assessed in systematic screening to identify individuals at greater risk for stroke. Clinical trials are needed to evaluate whether preventive treatment, such as medication or education with modification of risk factors, is cost-effective or improves outcomes.
Supplementary Material
Supplemental table S1. Pooled and individual visit results from baseline and follow-up MRI visits for associations between symptoms and SBI at the same visit (prevalence analyses).
Supplemental table S2. Baseline and visit 4 characteristics of participants with and without MRI at follow-up MRI visit.
Supplemental table S3. Few symptomatic participants at baseline MRI or visit 4 continued to report symptoms on follow-up MRI visit.
Acknowledgments
Funding Sources: The ARIC Study is carried out as a collaborative study by National Heart, Lung and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Neurocognitive data is collected by U01 HL096812, HL096814, HL096899, HL096902, and HL096917 with previous brain MRI examinations funded by R01 HL70825.
The authors thank ARIC study staff and participants for their important contributions, and David Deardorff for assistance with figures and editing of the manuscript.
Footnotes
Conflicts of Interest: None.
References
- 1.Toole JF, Lefkowitz DS, Chambless LE, Wijnberg L, Paton CC, Heiss G. Self-reported transient ischemic attack and stroke symptoms: Methods and baseline prevalence: The ARIC study, 1987–1989. Am J Epidemiol. 1996;144:849–856. doi: 10.1093/oxfordjournals.aje.a009019. [DOI] [PubMed] [Google Scholar]
- 2.Howard VJ, McClure LA, Meschia JF, Pulley L, Orr SC, Friday GH. High prevalence of stroke symptoms among persons without a diagnosis of stroke or transient ischemic attack in a general population: The Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Arch Intern Med. 2006;166:1952–1958. doi: 10.1001/archinte.166.18.1952. [DOI] [PubMed] [Google Scholar]
- 3.Chambless LE, Shahar E, Sharrett AR, Heiss G, Wijnberg L, Paton CC, et al. Association of transient ischemic attack/stroke symptoms assessed by standardized questionnaire and algorithm with cerebrovascular risk factors and carotid artery wall thickness: The ARIC study, 1987–1989. Am J Epidemiol. 1996;144:857–866. doi: 10.1093/oxfordjournals.aje.a009020. [DOI] [PubMed] [Google Scholar]
- 4.Wadley VG, McClure LA, Howard VJ, Unverzagt FW, Go RC, Moy CS, et al. Cognitive status, stroke symptom reports, and modifiable risk factors among individuals with no diagnosis of stroke or transient ischemic attack in the REasons for Geographic and Racial Differences in Stroke (REGARDS)Study. Stroke. 2007;38:1143–1147. doi: 10.1161/01.STR.0000259676.75552.38. [DOI] [PubMed] [Google Scholar]
- 5.Chambless LE, Toole JF, Nieto FJ, Rosamond W, Paton C. Association between symptoms reported in a population questionnaire and future ischemic stroke: the ARIC study. Neuroepidemiology. 2004;23:33–37. doi: 10.1159/000073972. [DOI] [PubMed] [Google Scholar]
- 6.Kleindorfer D, Judd S, Howard VJ, McClure L, Safford MM, Cushman M, et al. Self-reported stroke symptoms without a prior diagnosis of stroke or transient ischemic attack: a powerful new risk factor for stroke. Stroke. 2011;42:3122–3126. doi: 10.1161/STROKEAHA.110.612937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer JS, Kawamura J, Terayama Y. White matter lesions in the elderly. J Neurol Sci. 1992;110:1–7. doi: 10.1016/0022-510x(92)90002-3. [DOI] [PubMed] [Google Scholar]
- 8.Bernick C, Kuller L, Dulberg C, Longstreth WT, Jr, Manolio T, Beauchamp N, et al. Silent MRI infarcts and the risk of future stroke: The Cardiovascular Health Study. Neurology. 2001;57:1222–1229. doi: 10.1212/wnl.57.7.1222. [DOI] [PubMed] [Google Scholar]
- 9.Carey CL, Kramer JH, Josephson SA, Mungas D, Reed BR, Schuff N, et al. Subcortical lacunes are associated with executive dysfunction in cognitively normal elderly. Stroke. 2008;39:397–402. doi: 10.1161/STROKEAHA.107.491795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gold G, Kovari E, Herrmann FR, Canuto A, Hof PR, Michel J-P, et al. Cognitive consequences of thalamic, basal ganglia, and deep white matter lacunes in brain aging and dementia. Stroke. 2005;36:1184–1188. doi: 10.1161/01.STR.0000166052.89772.b5. [DOI] [PubMed] [Google Scholar]
- 11.Knopman DS, Mosley TH, Catellier DJ, Coker LH. Fourteen-year longitudinal study of vascular risk factors, ApoE genotype, and cognition: The ARIC MRI study. Alzheimers Dement. 2009;5:207–214. doi: 10.1016/j.jalz.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 12.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MMB. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 13.Srikanth V, Beare R, Blizzard L, Phan T, Stapleton J, Chen J, et al. Cerebral white matter lesions, gait, and the risk of incident falls: A prospective population-based study. Stroke. 2009;40:175–180. doi: 10.1161/STROKEAHA.108.524355. [DOI] [PubMed] [Google Scholar]
- 14.The Atherosclerosis Risk in Communities (ARIC) study: Design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 15.Bryan RN, Cai J, Burke G, Hutchinson RG, Liao D, Toole JF, et al. Prevalence and anatomic characteristics of infarct-like lesions on MR images of middle-aged adults: The Atherosclerosis Risk in Communities Study. Am J Neuroradiol. 1999;20:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- 16.Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 17.Rosamond WD, Folsom AR, Chambless LE, Wang C-H, McGovern PG, Howard G, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 18.Bryan RN, Manolio TA, Schertz LD, Jungreis C, Poirier VC, Elster AD, et al. A method for using MR to evaluate the effects of cardiovascular disease on the brain: The Cardiovascular Health study. Am J Neuroradiol. 1994;15:1625–1633. [PMC free article] [PubMed] [Google Scholar]
- 19.Diggle P, Heagerty P, Liang K-Y, Zeger SL. Analysis of longitudinal data. Oxford University Press; 2002. [Google Scholar]
- 20.Robins JM, Rotnitzky A, Zhao LP. Analysis of semiparametric regression models for repeated outcomes in the presence of missing data. J Amer Statistical Assoc. 1995;90:106–121. [Google Scholar]
- 21.Tanne D, Levine SR. Capturing the scope of stroke: Silent, whispering, and overt. Arch Neurol. 2009;66:819–820. doi: 10.1001/archneurol.2009.103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental table S1. Pooled and individual visit results from baseline and follow-up MRI visits for associations between symptoms and SBI at the same visit (prevalence analyses).
Supplemental table S2. Baseline and visit 4 characteristics of participants with and without MRI at follow-up MRI visit.
Supplemental table S3. Few symptomatic participants at baseline MRI or visit 4 continued to report symptoms on follow-up MRI visit.