To the Editor:
Metabolic acidosis is a clinical disturbance characterized by low pH in body tissues and blood and a variety of neuromuscular and cardiorespiratory responses.1 Besides treating the initial disorder, the main goal for patients with acidosis is to increase the systemic pH with alkalizing agents, such as bicarbonates2; however, adverse reactions linked to the administration of sodium bicarbonate (eg, metabolic alkalosis, edema due to sodium overload, and congestive heart failure) could limit its use in the treatment of metabolic acidosis.3
Hydrogen functions as an important physiologic regulatory factor with antioxidant, anti-inflammatory, and antiapoptotic protective effects on cells and organs and the ability to mitigate a variety of diseases.4 Oral intake of liquid that contains hydrogen represents a novel, easily translatable, and safe method of delivering hydrogen to humans, with hydrogen-rich water (HRW) exhibiting high pH, low dissolved oxygen, high dissolved hydrogen, and significant negative redox potential values. Although the production of HRW by electrolysis, reaction of water with hydrogen-producing minerals, or direct contact with hydrogen gas is an elemental and well-established process, its use in human nutrition is rather new, with HRW being marketed as a nutritional aid for humans accompanied by claims of acidity-lowering, antioxidant, and antiaging effects. These claims extrapolate the findings from animal studies5 but have not been substantiated in humans. Specifically, because of its high pH and significant negative redox potential values, oral use of HRW as an alkalizing agent in the treatment of metabolic acidosis could be of particular interest to humans experiencing an increase in plasma acidity.4 The main aims of this study were to investigate whether daily oral administration of 2 L of HRW for 7 days affected baseline arterial pH and the rate of acidosis induced by exercise in young healthy men and to determine how many participants experienced adverse effects at follow-up after this treatment.
Nineteen healthy male participants aged 20 to 26 years received 2 L of HRW daily (with approximately 1.1 mM/L of hydrogen dissolved in a drink, an oxidation-reduction potential of approximately 400 mV, and a mean ± SD pH of 9.3±0.3) for 7 days, with participants instructed to sip the fluid throughout the day. The HRW was generated when the magnesium tablet (NORP Inc, San Diego, CA) was dissolved in drinking water before consumption (Mg + 2H2O → Mg[OH]2 + H2). Participants were asked to maintain their usual dietary intake and to not change their physical activity patterns during the study. All procedures were performed in accordance with the Declaration of Helsinki, and the study was approved by the local institutional review board. All participants gave their informed consent regarding their voluntary participation in the study. Participants underwent blood sampling and endurance running at the start (day 0) and end (day 7) of the intervention period. Arterial blood samples were collected after an overnight fast and after exercise, and blood pH was determined by the direct method with a pH meter equipped with a microglass electrode. Participants were instructed to report on adverse effects of supplementation through an open-ended questionnaire at the end of the intervention week. The baseline and postexercise arterial blood pH concentrations after 1 week of intervention with HRW were compared with the baseline values by paired t test (P<.05).
At the preintervention stage, the mean ± SD fasting blood pH was 7.42±0.01, whereas the postexercise pH was 7.29±0.06. Intake of HRW significantly increased fasting arterial blood pH by 0.04 (95% confidence interval, 0.01-0.09) and postexercise pH by 0.05 (95% confidence interval, 0.01-0.10) after 7 days of intervention (Figure). No volunteers withdrew before the end of the study, and no participant reported any adverse effects of supplementation.
FIGURE.
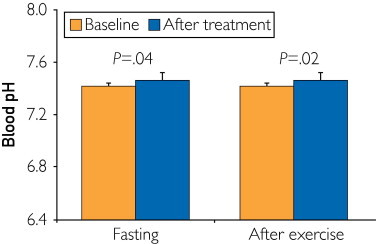
Effect of hydrogen-rich water (2 L daily) on fasting and postexercise blood pH (mean ± SD) in healthy volunteers (n=19). P values are for comparisons with the start of the intervention period (paired t test).
Intake of HRW formulation for 1 week increased fasting and postexercise blood pH in healthy volunteers with no adverse effects reported. Evidence confirmed previous animal studies that suggested that HRW may provide some benefits as a neutralizing agent.5 However, the size of the elevation is much smaller in healthy volunteers compared with those receiving bicarbonates. Oral sodium bicarbonates significantly increased baseline blood pH by up to 2% and postexercise pH by up to 3%, whereas the overall incidence of significant adverse effects after bicarbonate administration is approximately 25%.3
To my knowledge, no data have been published showing the effect of HRW on fasting and postexercise blood pH in healthy volunteers. Although this study used a pre-post design without a placebo group, the findings suggest that the pH-elevating potential of HRW in healthy individuals is not nearly of the same order compared with bicarbonate intervention. On the other hand, lower incidence of adverse effects after ingestion of HRW compared with bicarbonates may affirm potential application of HRW as an alkalizing agent in individuals with exercise-induced metabolic acidosis. However, because this is a short-term trial and the adherence to the long-term use of the intervention may be poor, the estimation of adverse effect incidence should be carefully interpreted with a small number of young healthy individuals treated for only a short period. Another concern regarding HRW administration is the variation in the hydrogen content and/or fluid pH across suppliers. Most products have been standardized to a hydrogen concentration of 0.55 to 0.65 mM/L, whereas in research studies liquid hydrogen is usually administered at a dose of approximately 1.0 mM/L.
It would be premature to conclude that HRW has a blood-alkalizing effect in all individuals because no other published studies exist on HRW in the field of biochemistry or nutrition. It seems that HRW may not be of much use for treatment of many conditions of organic metabolic acidosis but may have a role in exercise-induced metabolic acidosis, yet the use of base to improve exercise capacity is uncertain. Dosage and duration of ingestion, hydrogen content of the intervention, and the health status of individuals may affect the efficacy of HRW administration. Longer administration protocol, a higher dosage of the formulation, proof of bioavailability, and monitoring other buffering indicators may be necessary to determine whether HRW has a considerable alkalizing effect. Although this study examined healthy individuals, the appropriate treatment of acute metabolic acidosis (in particular the organic form of acidosis, such as exercise-induced acidosis) has been controversial2; therefore, further studies are needed on the use of HRW as a potential antiacidic treatment strategy and its safe application in clinical patients.
Acknowledgments
This study was supported in part by the Serbian Ministry of Science (grant 175037).
References
- 1.Kellum J.A. Disorders of acid-base balance. Crit Care Med. 2007;35(11):2630–2636. doi: 10.1097/01.CCM.0000286399.21008.64. [DOI] [PubMed] [Google Scholar]
- 2.Adrogué H.J., Madias N.E. Management of life-threatening acid-base disorders: second of two parts. N Engl J Med. 1998;338(2):107–111. doi: 10.1056/NEJM199801083380207. [DOI] [PubMed] [Google Scholar]
- 3.Kaehny W.D., Anderson R.J. Bicarbonate therapy of metabolic acidosis. Crit Care Med. 1994;22(10):1525–1527. [PubMed] [Google Scholar]
- 4.Armaroli N., Balzani V. The hydrogen issue. ChemSusChem. 2011;4(1):21–36. doi: 10.1002/cssc.201000182. [DOI] [PubMed] [Google Scholar]
- 5.Abol-Enein H., Gheith O.A., Barakat N., Nour E., Sharaf A.E. Ionized alkaline water: new strategy for management of metabolic acidosis in experimental animals. Ther Apher Dial. 2009;13(3):220–224. doi: 10.1111/j.1744-9987.2009.00659.x. [DOI] [PubMed] [Google Scholar]


