Abstract
Levamisole is an immunomodulatory agent that was used to treat various cancers before being withdrawn from the United States market in 2000 because of adverse effects. Levamisole is currently approved as an antihelminthic agent in veterinary medicine, but is also being used illicitly as a cocaine adulterant. Potential complications associated with use of levamisole-laced cocaine include neutropenia, agranulocytosis, arthralgias, retiform purpura, and skin necrosis. Treatment is primarily supportive, and skin lesions typically resolve with cessation of cocaine use. The incidence of hospitalizations related to use of levamisole-contaminated cocaine continues to increase and clinicians should be aware of the more common clinical manifestations.
Abbreviations and Acronyms: DEA, Drug Enforcement Administration; GC/MS, gas chromatography/mass spectrometry
Cocaine use and its related complications are well-known public health issues. More than 5 million Americans use cocaine regularly via insufflation (snorting/sniffing), inhalation (smoking), and injection.1 Cocaine's effects on the health care system cross multiple medical disciplines, but clinicians may be less cognizant of the problems caused by some of the adulterants added to cocaine. Recent recognition of levamisole-induced agranulocytosis, vasculitis, and other complications, from contaminated cocaine, dictate that physicians be aware of these potential problems.
Since 2006, several cases of severe agranulocytosis associated with cocaine use have been reported.2,3 Epidemiological studies of initial cases reported by the New Mexico Department of Health found evidence of levamisole, an antihelminthic agent, in drug paraphernalia of cocaine users.2 Levamisole was also detected using gas chromatography/mass spectrometry (GC/MS) in a postmortem blood specimen from a cocaine user who died of Serratia marcescens sepsis secondary to agranulocytosis. During the New Mexico investigation, public health officials in British Columbia and Alberta, Canada, also reported similar findings in cocaine users presenting with leukopenia.3 These findings were reported to the Centers for Disease Control and Prevention Epi-X and poison control centers. Subsequently, similar cases of severe agranulocytosis were reported in Colorado and Washington. All patients admitted to recent cocaine use or tested positive for cocaine on a standard urine drug screen.2
Concurrent with clinical reports of cocaine-related agranulocytosis, technical reports of levamisole-adulterated cocaine have appeared in the literature since 2002 (first reported by the United States Drug Enforcement Administration [DEA]).4 Outside of North America, published reports of levamisole detected by GC/MS in cocaine users appeared in Luxembourg and the United Kingdom in 2005.5,6 Similar findings in drug enforcement–seized cocaine shipments were reported around that time in the United States, Canada, Italy, and Australia.3,6
The concentration of levamisole in cocaine has steadily increased since it was first detected. The concentration was less than 1% in 2001,8 and in 2009, levamisole comprised approximately 10% of each cocaine sample.8 By July 2009, the DEA reported that 69% of cocaine entering the United States contained levamisole,9 while in the United Kingdom, levamisole was found in over 50% of cocaine samples tested.7 In an analysis of cocaine users in Seattle, Washington, approximately 80% of users who tested positive for cocaine also tested positive for levamisole.4 Levamisole is also used to adulterate other illicit substances; seized heroin supplies in 2008 and 2009 were found to contain trace amounts of levamisole.2,10
Methods
We performed a PubMed search for the keywords levamisole, cocaine, vasculitis, pseudovasculitis, agranulocytosis, and retiform purpura. We reviewed all literature published between 1940 to 2011. Case series and reviews were given priority for inclusion in this review. Case reports were examined, but were only included in this article if they presented novel findings.
What is Levamisole?
Levamisole is a synthetic imidazothiazole derivative used for its immunomodulatory properties. Since the 1970s, it has been used for treatment of rheumatoid arthritis as a disease-modifying antirheumatic drug.11-13 In 1990, the United States Food and Drug Administration approved it for use with 5-fluorouracil for colon cancer treatment. However, levamisole was subsequently withdrawn from the United States and Canadian markets in 2000 and 2003, respectively, because of reports of agranulocytosis. Currently, it is primarily used as an antihelminthic medication in veterinary sciences. Outside of the United States, pediatric nephrologists use it as a steroid-sparing agent in childhood steroid-dependent nephrotic syndrome.14-16
Levamisole acts as an immunomodulator and immunoenhancer by increasing macrophage chemotaxis and T-cell lymphocyte function.17 It has also been shown to stimulate neutrophil chemotaxis, up-regulate toll-like receptors, and enhance dendritic cell maturation.18-22 Notable adverse effects of levamisole include severe agranulocytosis, retiform purpura, and seizures.23-25
Levamisole's exact physiologic effect when used in combination with cocaine is unclear. One theory is that it potentiates the nicotinic acetylcholinergic effects of the central nervous system, thus prolonging cocaine-induced euphoria.26 Other studies have also found that levamisole is metabolized into aminorex, a substrate for serotonin transporters, thus possibly acting as an indirect serotonin agonist.27,28 Aminorex is known to cause pulmonary hypertension, although this adverse effect has not been definitively linked to levamisole-laced cocaine.
Levamisole's physical similarity to cocaine also allows it to act as a cutting or bulking agent, increasing the total weight of the sample and making the drug appear purer. Levamisole adulteration often occurs as part of the refining process of cocaine production. Levamisole additives are usually of pharmaceutical-grade quality, although impure levamisole has also been infrequently reported by DEA laboratories.9
Characteristics of Levamisole Toxicity
Patients with levamisole toxicity typically present with cutaneous manifestations consisting of large, painful hemorrhagic bullae and/or necrosis. The face is commonly affected, especially the bilateral helixes and cheeks (Figures 1 and 2). Similar bullae can present elsewhere on the body, with case reports documenting involvement of the abdomen, chest, lower back, buttocks, and legs. Retiform purpura with or without bullae may also be prominent.3,23,24 The pathologic mechanism of these symptoms is unclear, as some patients present with true vasculitis while others present with pseudovasculitis. Among the cases presenting as a true vasculitis, direct immunofluorescence findings suggest an immune complex–mediated vasculitis with vascular deposits of IgM, IgA, IgG, and C3, and vascular staining for fibrin.23,24,29,30 In addition to characteristic cutaneous manifestations, the majority of patients complain of arthralgias, most commonly involving the large joints. Ear, nose, and throat complaints are also common, as are feelings of generalized malaise and fatigue.31
FIGURE 1.
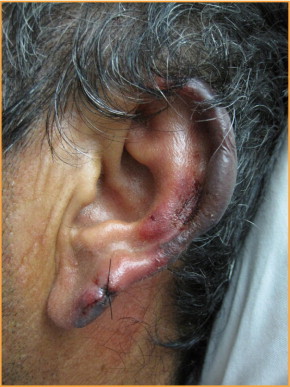
Left helix with hemorrhagic bullae. Suture on upper ear lobe is biopsy site.
FIGURE 2.
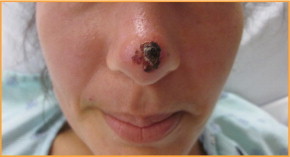
Nasal tip with necrotic, hemorrhagic crust due to reexposure to levamisole-laced cocaine.
Laboratory findings include leukopenia, neutropenia, agranulocytosis, positive antinuclear antibody titers, positive anti-proteinase 3 titers, and positive perinuclear or cytoplasmic staining patterns for antineutrophil cytoplasmic antibodies. Absolute neutrophil counts ranging from 0 to 3000 have been reported, and these patients are at high risk of infection.32 Bone marrow biopsies from such patients have shown markedly decreased production of all cell lines. When positive, antinuclear antibody is often mildly or moderately elevated, most commonly in a speckled pattern. Anti–double-stranded DNA and lupus anticoagulant findings are also inconsistently positive in various case reports and series.23,24,32-36 Complement studies typically yield normal results, although mildly decreased levels of C4 and/or C3 have been reported. Urine toxicology studies are usually positive for cocaine and occasionally for levamisole.24,37,38 Levamisole may also be found on drug paraphernalia such as pipes, paper currency, or other tools used for smoking or snorting cocaine.
Antihuman elastase antibody is both sensitive and specific for levamisole-induced vasculitis.33 Hyponatremia has also been reported,39 and pulmonary hemorrhage and renal failure can also occur.31 Biopsy of cutaneous lesions reveals leukocytoclastic vasculitis, thrombotic microangiopathy, panniculitis, and/or gross necrosis. On renal biopsy of patients with acute kidney failure, pauci-immune focal necrotizing crescentic glomerulonephritis is found (Table).31
TABLE.
Common Clinical Manifestations and Laboratory Findings Associated With Levamisole Toxicity
| Generalized malaise,23 fatigue,24,31 arthralgias31 |
| Pulmonary hemorrhage,31 renal failure,31 seizures |
| Skin necrosis,43 (hemorrhagic) bullae,23 retiform purpura33 (Figures 1 and 2) |
| Leukopenia,28 neutropenia,29,36,37 agranulocytosis,33 hyponatremia39 |
| Positive results for ANA,34 PR3,40,43 ANCAs,30,40 dsDNA,34 lupus anticoagulant,34 antihuman elastase antibody33 |
| Urine toxicology studies positive for cocaine; occasionally positive for levamisole24,37,38 |
| Detection of levamisole in urine with GC/MS41 |
ANA = antinuclear antibody; ANCA = antineutrophil cytoplasmic antibody; dsDNA = double-stranded DNA; PR3 = anti-proteinase 3; GC/MS = gas chromatography/mass spectrometry.
The natural progression of this condition is generally benign, as most symptoms will resolve without intervention. Complete cessation of cocaine use is absolutely necessary because symptoms can recur on rechallenge (Figure 2). Systemic infection in those with agranulocytosis may require hospitalization.
Public Awareness
Since 2007, clinical awareness of levamisole toxicity among clinicians has been increasing. Case reports and reviews of levamisole-induced agranulocytosis and vasculitis have appeared in internal medicine,40,41 emergency medicine,42 wound care,43 rheumatology,32,38 dermatology,30 allergy/immunology,22 nephrology,25 and cardiology27 journals. Improved detection methods using GC/MS on urine samples is being employed at some institutions. Utilizing GC/MS, levamisole was found in 68% of patients who tested positive for cocaine on standard urine toxicology screen.41
Challenges
Levamisole-induced vasculitis and agranulocytosis may not be initially suspected if patients are not asked about illicit drug use or deny drug use when questioned. An astute clinician suspicious of this denial should always perform a urine drug toxicology screen because cocaine remains in the urine for 48 to 72 hours following use. Even with a positive cocaine screen, a definitive connection to levamisole may be difficult to establish because levamisole is rapidly absorbed and has a short half-life (5.5-6 hours).44 Alternatively, clinicians can test samples from drug paraphernalia (plastic bags previously containing cocaine/heroin, pipes, currency used for insufflation).33 Gas chromatography/mass spectrometry or liquid chromatography/mass spectrometry testing of patients' urine can also be used, although these techniques are expensive, time-consuming, and limited in availability.45
Future Directions
With increasing amounts of levamisole contaminating worldwide cocaine supplies, clinicians may start to see levamisole-associated vasculitis and agranulocytosis more frequently. Because of the small number of cases reported, the medical community still lacks a definitive agreement on appropriate laboratory tests to perform for diagnosis, as well as appropriate treatment strategies. Clinicians suspecting levamisole-induced vasculitis should first order a urine toxicology screen to evaluate for cocaine or heroin. Other pertinent laboratory studies include complete blood cell count with differential to evaluate for leukopenia; renal and liver function tests to evaluate for evidence of renal and liver failure, respectively; antineutrophil cytoplasmic antibodies, antiphospholipid antibodies, cryoglobulins, and lupus anticoagulants to evaluate for evidence of other autoimmune diseases; and antihuman elastase antibody, which is both sensitive and specific for levamisole-induced vasculitis (Figure 3). Some patients have been treated with skin grafts, wound debridement, filgrastim, cyclosporine, mycophenolate, and observation with drug rehabilitation.31,37,38,46
FIGURE 3.
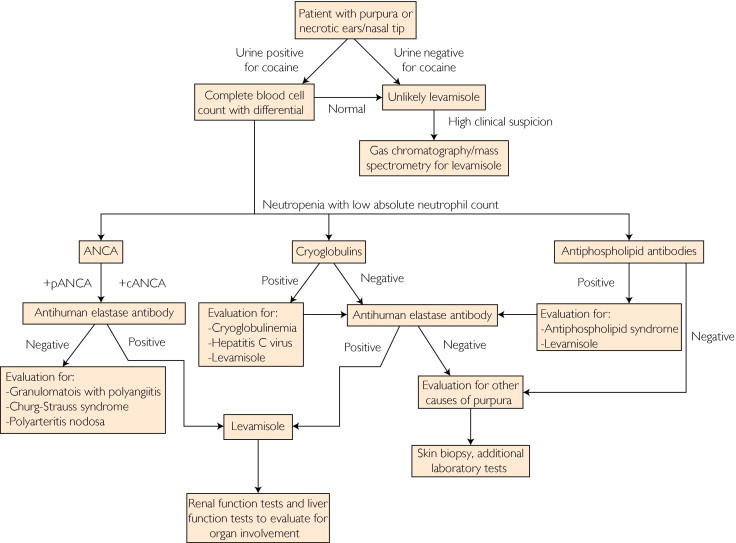
Algorithm for diagnosis and evaluation of levamisole-induced vasculitis. ANCA = antineutrophil cytoplasmic antibody; +cANCA = ANCA with positive cytoplasmic staining pattern; +pANCA = ANCA with positive perinuclear staining pattern.
Improved reporting of cases to state public health departments and the Centers for Disease Control and Prevention will help us to understand the true incidence and prevalence of this condition among cocaine users. Additionally, it is still unclear why only some cocaine users develop levamisole toxicity. Of those who develop this condition, some present in more severe forms with widespread vasculitis and necrosis, while others present with only mild cutaneous symptoms and arthralgias. Possible genetic risk factors influencing levamisole metabolism could play a role, as well as total volume of levamisole consumed.
Conclusion
Increased patient and physician awareness is essential to ensuring proper diagnosis and treatment of complications associated with the use of levamisole-contaminated cocaine. Misdiagnosis, or lack of diagnosis, often leads to unnecessary tests and health care expenses. Because cocaine and heroin supplies are likely to continually contain levamisole, it is important for clinicians and social workers caring for illicit drug users to recognize this recently characterized condition.40
Article Highlights.
-
■
Over 5 million Americans abuse cocaine via insufflation (snorting), inhalation (smoking), and injection. The majority of cocaine in the United States is believed to be tainted with levamisole.
-
■
Levamisole is an immunomodulatory agent previously used to treat rheumatoid arthritis and various cancers before being withdrawn from the United States market in 2000.
-
■
Levamisole is currently approved as an antihelminthic agent in veterinary medicine, but is also being used illicitly as a cocaine adulterant or bulking agent, given its physical similarity to cocaine.
-
■
Levamisole toxicity is characterized by arthralgias, retiform purpura, and skin necrosis. Laboratory findings include neutropenia, positive perinuclear or cytoplasmic staining patterns for antineutrophil cytoplasmic antibodies, and positive antihuman elastase antibody.
-
■
Treatment of levamisole toxicity is primarily supportive, and skin lesions typically resolve with cessation of cocaine use.
References
- 1.SAMHSA, Center for Behavioral Health Statistics and Quality (formerly the Office of Applied Studies), National Survey on Drug Use and Health, 2009 and 2010 Table 1.1A - Types of Illicit Drug Use in Lifetime, Past Year, and Past Month Among Persons Aged 12 or Older: Numbers in Thousands, 2009 and 2010: Substance Abuse and Mental Health Services Administration Web site. http://www.samhsa.gov/data/NSDUH/2k10ResultsTables/Web/HTML/Sect1peTabs1to46.htm#Tab1.1A Accessed December 12, 2011.
- 2.Centers for Disease Control and Prevention (CDC) Agranulocytosis associated with cocaine use—four states, March 2008-November 2009. MMWR Morb Motal Wkly Rep. 2009;58(49):1381–1385. [PubMed] [Google Scholar]
- 3.Knowles L., Buxton J.A., Skuridina N. Levamisole tainted cocaine causing severe neutropenia in Alberta and British Columbia. Harm Reduct J. 2009;6:30. doi: 10.1186/1477-7517-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu N.Y., Legatt D.F., Turner A.R. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med. 2009;150(4):287–289. doi: 10.7326/0003-4819-150-4-200902170-00102. [DOI] [PubMed] [Google Scholar]
- 5.Schneider S., Meys F. Analysis of illicit cocaine and heroin samples seized in Luxembourg from 2005-2010. Forensic Sci Int. 2011;212(1-3):242–246. doi: 10.1016/j.forsciint.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 6.Morley S.R., Hall J.C., Forrest A.R.W., Galloway J.H. Levamisole as a contaminant of illicit cocaine. J Clandestine Lab Invest Chemists Assoc. 2006;16(4):11. [Google Scholar]
- 7.Erowid Crew Cocaine adulterated with levamisole on the rise: Status as of September 2009. Oct 1, 2009. http://www.erowid.org/cocaine/cocaine_article2.shtml Accessed October 14, 2011.
- 8.US Department of Justice, National Drug Intelligence Center National Drug Threat Assessment 2010: Impact of Drugs on Society: US Department of Justice, National Drug Intelligence Center Web site. http://www.justice.gov/ndic/pubs38/38661/drugImpact.htm Accessed December 12, 2011.
- 9.Casale J.F., Corbeil E.M., Patrick A.H. Identification of levamisole impurities found in illicit cocaine exhibits. Microgram J. 2008;6(3-4):82–89. [Google Scholar]
- 10.Casale E.M., Casale J.F. Identification of levamisole and lidocaine acetylation reaction impurities found in heroin exhibits. Microgram J. 2011;8(1):16–23. [Google Scholar]
- 11.Popovic M., Stefanovic D., Pejnovic N. Comparative study of the clinical efficacy of four DMARDs (leflunomide, methotrexate, cyclosporine, and levamisole) in patients with rheumatoid arthritis. Transplant Proc. 1998;30(8):4135–4136. doi: 10.1016/s0041-1345(98)01370-0. [DOI] [PubMed] [Google Scholar]
- 12.Sany J. Immunological treatment of rheumatoid arthritis. Clin Exp Rheumatol. 1990;8(suppl 5):81–88. [PubMed] [Google Scholar]
- 13.Olsen N., Halberg P., Halskov O., Bentzon M.W. Scintimetric assessment of synovitis activity during treatment with disease modifying antirheumatic drugs. Ann Rheum Dis. 1988;47(12):995–1000. doi: 10.1136/ard.47.12.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyer O., Moulder J.K., Grandin L., Somers M.J. Short- and long-term efficacy of levamisole as adjunctive therapy in childhood nephrotic syndrome. Pediatr Nephrol. 2008;23(4):575–580. doi: 10.1007/s00467-007-0708-7. [DOI] [PubMed] [Google Scholar]
- 15.Durkan A., Hodson E.M., Willis N.S., Craig J.C. Non-corticosteroid treatment for nephrotic syndrome in children. Cochrane Database Syst Rev. 2005;(2):CD002290. doi: 10.1002/14651858.CD002290.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Davin J.C., Merkus M.P. Levamisole in steroid-sensitive nephrotic syndrome of childhood: the lost paradise? Pediatr Nephrol. 2005;20(1):10–14. doi: 10.1007/s00467-004-1615-9. [DOI] [PubMed] [Google Scholar]
- 17.Smith C.M., Reynard A.M. Saunders; Philadelphia, PA: 1992. Textbook of Pharmacology. [Google Scholar]
- 18.Stein A.N., Diez R.A., Sen L., Estévez M.E. Chemotactic function of polymorphonuclear leukocytes from patients with recurrent infections: partial correction by levamisole “in vitro.”. Allergol Immunopathol (Madr) 1985;13(2):127–134. [PubMed] [Google Scholar]
- 19.Hogan N.A., Hill H.R. Enhancement of neutrophil chemotaxis and alteration of levels of cellular cyclic nucleotides by levamisole. J Infect Dis. 1978;138(4):437–444. doi: 10.1093/infdis/138.4.437. [DOI] [PubMed] [Google Scholar]
- 20.Rabson A.R., Anderson R., Glover A. Defective neutrophil motility and recurrent infection: in vitro and in vivo effects of levamisole. Clin Exp Immunol. 1978;33(1):142–149. [PMC free article] [PubMed] [Google Scholar]
- 21.Wright D.G., Kirkpatrick C.H., Gallin J.I. Effects of levamisole on normal and abnormal leukocyte locomotion. J Clin Invest. 1977;59(5):941–950. doi: 10.1172/JCI108716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson R., Glover A., Koornhof H.J., Rabson A.R. In vitro stimulation of neutrophil motility by levamisole: maintenance of cGMP levels in chemotactically stimulated levamisole-treated neutrophils. J Immunol. 1976;117(2):428–432. [PubMed] [Google Scholar]
- 23.Lee K.C., Culpepper K., Kessler M. Levamisole-induced thrombosis: literature review and pertinent laboratory findings. J Am Acad Dermatol. 2011;65(4):e128–e129. doi: 10.1016/j.jaad.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins J., Babu K., Hsu-Hung E., Robinson-Bostom L., Kroumpouzos G. ANCA-positive necrotizing vasculitis and thrombotic vasculopathy induced by levamisole-adulterated cocaine: a distinctive clinicopathologic presentation. J Am Acad Dermatol. 2011;65(1):e14–e16. doi: 10.1016/j.jaad.2010.09.778. [DOI] [PubMed] [Google Scholar]
- 25.Aberastury M.N., Silva W.H., Vaccarezza M.M., Maxit C., Agosta G. Epilepsia partialis continua associated with levamisole. Pediatr Neurol. 2011;44(5):385–388. doi: 10.1016/j.pediatrneurol.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 26.Martin R.J., Verma S., Levandoski M. Drug resistance and neurotransmitter receptors of nematodes: recent studies on the mode of action of levamisole. Parasitology. 2005;131(suppl):S71–S84. doi: 10.1017/S0031182005008668. [DOI] [PubMed] [Google Scholar]
- 27.Karch SB, Mari F, Bartolini V, Bertol E. Aminorex poisoning in cocaine abusers [published online ahead of print July 15, 2011]. Int J Cardiol. PMID: 21764154 [DOI] [PubMed]
- 28.Bertol E., Mari F., Milia M.G., Politi L., Furlanetto S., Karch S.B. Determination of aminorex in human urine samples by GC-MS after use of levamisole. J Pharm Biomed Anal. 2011;55(5):1186–1189. doi: 10.1016/j.jpba.2011.03.039. [DOI] [PubMed] [Google Scholar]
- 29.Jacob R.S., Silva C.Y., Powers J.G. Levamisole-induced vasculopathy: a report of 2 cases and a novel histopathologic finding. Am J Dermatopathol. 2012;34(2):208–213. doi: 10.1097/DAD.0b013e31821cc0bf. [DOI] [PubMed] [Google Scholar]
- 30.Chung C., Tumeh P.C., Birnbaum R. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2011;65(4):722–725. doi: 10.1016/j.jaad.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGrath M.M., Isakova T., Rennke H.G., Mottola A.M., Laliberte K.A., Niles J.L. Contaminated cocaine and antineutrophil cytoplasmic antibody-associated disease. Clin J Am Soc Nephrol. 2011;6(12):2799–2805. doi: 10.2215/CJN.03440411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ullrich K., Koval R., Koval E., Bapoje S., Hirsh J.M. Five consecutive cases of a cutaneous vasculopathy in users of levamisole-adulterated cocaine. J Clin Rheumatol. 2011;17(4):193–196. doi: 10.1097/RHU.0b013e31820e6822. [DOI] [PubMed] [Google Scholar]
- 33.Waller J.M., Feramisco J.D., Alberta-Wszolek L., McCalmont T.H., Fox L.P. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63(3):530–535. doi: 10.1016/j.jaad.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 34.Graf J., Lynch K., Yeh C.L. Purpura, cutaneous necrosis, and antineutrophil cytoplasmic antibodies associated with levamisole-adulterated cocaine. Arthritis Rheum. 2011;63(12):3998–4001. doi: 10.1002/art.30590. [DOI] [PubMed] [Google Scholar]
- 35.de la Hera I., Sanz V., Cullen D. Necrosis of ears after use of cocaine probably adulterated with levamisole. Dermatology. 2011;223(1):25–28. doi: 10.1159/000329436. [DOI] [PubMed] [Google Scholar]
- 36.Powell J., Grech H., Holder J. A boy with cutaneous necrosis occurring during treatment with levamisole. Clin Exp Dermatol. 2002;27(1):32–33. doi: 10.1046/j.0307-6938.2001.00944.x. [DOI] [PubMed] [Google Scholar]
- 37.Mouzakis J., Somboonwit C., Lakshmi S. Levamisole induced necrosis of the skin and neutropenia following intranasal cocaine use: a newly recognized syndrome. J Drugs Dermatol. 2011;10(10):1204–1207. [PubMed] [Google Scholar]
- 38.Herms B., Kaplon M., Baumann M. Agranulocytosis in cocaine users in Ohio: suspected levamisole taint. Leuk Res. 2011;35(9):e173–e174. doi: 10.1016/j.leukres.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Friend K, Milone MC, Perrone J. Hyponatremia associated with levamisole-adulterated cocaine use in emergency department patients [published online ahead of print September 27, 2011]. Ann Emerg Med. PMID: 21958733 [DOI] [PubMed]
- 40.Muirhead T.T., Eide M.J. Images in clinical medicine: toxic effects of levamisole in a cocaine user. N Engl J Med. 2011;364(24):e52. doi: 10.1056/NEJMicm1008722. [DOI] [PubMed] [Google Scholar]
- 41.Buchanan J.A., Heard K., Burbach C., Wilson M.L., Dart R. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305(16):1657–1658. doi: 10.1001/jama.2011.531. [DOI] [PubMed] [Google Scholar]
- 42.Chai P.R., Bastan W., Machan J., Hack J.B., Babu K.M. Levamisole exposure and hematologic indices in cocaine users. Acad Emerg Med. 2011;18(11):1141–1147. doi: 10.1111/j.1553-2712.2011.01202.x. [DOI] [PubMed] [Google Scholar]
- 43.Ching J.A., Smith D.J., Jr Levamisole-induced necrosis of skin, soft tissue, and bone: case report and review of literature. J Burn Care Res. 2012;33(1):e1–e5. doi: 10.1097/BCR.0b013e318233fc64. [DOI] [PubMed] [Google Scholar]
- 44.Edwards G., Breckenridge A.M. Clinical pharmacokinetics of anthelmintic drugs. Clin Pharmacokinet. 1988;15(2):67–93. doi: 10.2165/00003088-198815020-00001. [DOI] [PubMed] [Google Scholar]
- 45.Lynch K.L., Dominy S.S., Graf J., Kral A.H. Detection of levamisole exposure in cocaine users by liquid chromatography-tandem mass spectrometry. J Anal Toxicol. 2011;35(3):176–178. doi: 10.1093/anatox/35.3.176. [DOI] [PubMed] [Google Scholar]
- 46.Click J. Levamisole-induced retiform purpura. J Drugs Dermatol. 2011;10(2):217. [PubMed] [Google Scholar]


